Abstract
1. Potassium currents were characterized in tall hair cells of the chick's cochlea. Outward potassium currents were found to flow through two distinct classes of channels. 2. Individual hair cells were isolated from 200 microns long segments of the apical half of the chick's cochlea. Whole-cell voltage-clamp and current-clamp recordings were made from these cells. 3. Voltage responses to injected current ranged from high-frequency (100-250 Hz) oscillations in some cells, to slowly repetitive Ca2+ action potentials or slow oscillations (5-20 Hz) in others. 4. Ionic currents recorded in voltage clamp also varied in different hair cells. Cells with high-frequency voltage oscillations had rapidly activating Ca2(+)-dependent outward K+ current, IK(Ca). Cells that generated action potentials had slow delayed rectifier outward K+ current, IK, and inward rectifier current, IIR. All hair cells had inward Ca2+ current. 5. IK(Ca) activated positive to -45 mV. Tail currents reversed at the K+ equilibrium potential. This current was eliminated in Ca2(+)-free solutions, or when exposed to 10 mM-TEA. This outward current was fully activated within 1-3 ms at 0 mV. The whole-cell current was noisy and ensemble variance analysis suggested a single-channel conductance of 63 pS near 0 mV. 6. IK activated positive to -50 mV. Tail currents reversed at the K+ equilibrium potential. This current was not eliminated in Ca2(+)-free solutions, and was relatively resistant to external TEA. IK activated slowly, reaching peak values in 10-20 ms at 0 mV. This current showed little variance and the average single-channel conductance based on macroscopic noise near 0 mV was 8 pS. 7. External tetraethylammonium (TEA) or Ca2(+)-free saline eliminated the high-frequency voltage oscillations seen in many basal cells. In contrast TEA had little effect on slow action potentials (or low-frequency oscillations) seen in cells with IK. 8. IK(Ca) was prominent in hair cells originating 1.0-2.0 mm from the cochlear apex. IK and IIR dominated the membrane conductance of tall hair cells originating within 0.5 mm of the cochlear apex. 9. The frequency of voltage oscillation in apical cells was temperature-dependent, nearly doubling for each 10 degrees C rise in temperature. 10. IIR activated at membrane potentials negative to -75 mV. The average time constant of activation at -100 mV was 2 ms. Tail currents reversed at the K+ equilibrium potential and did not depend on the external Na+ concentration. IIR was blocked by 5 mM-Cs+ or 100 microM-Ba2+ in the external saline.
Full text
PDF

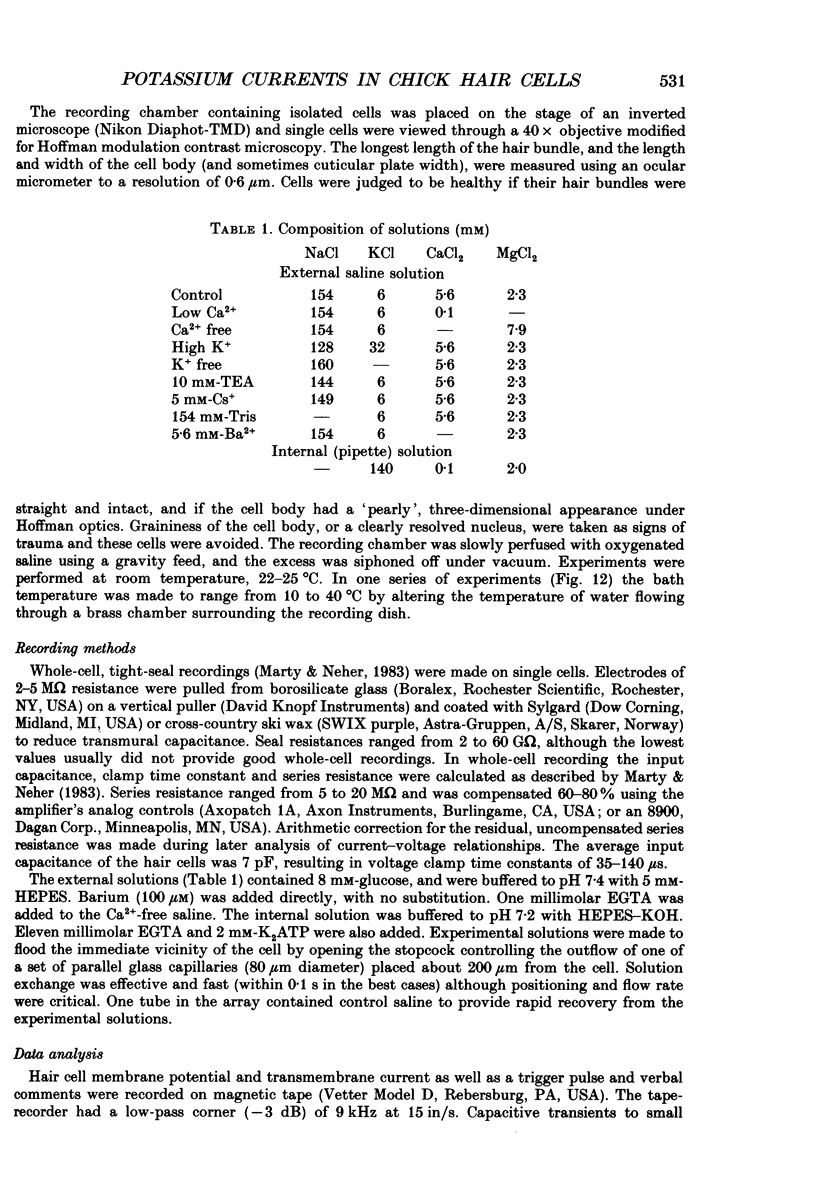

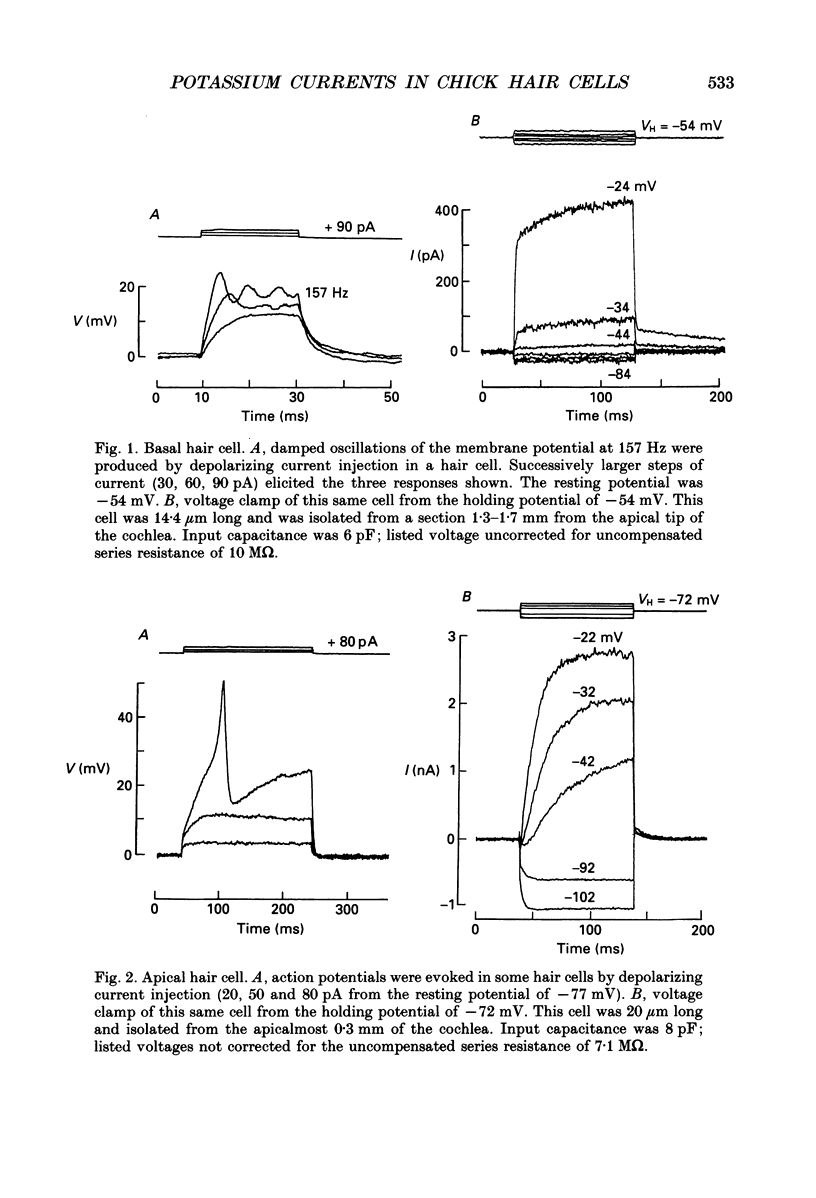
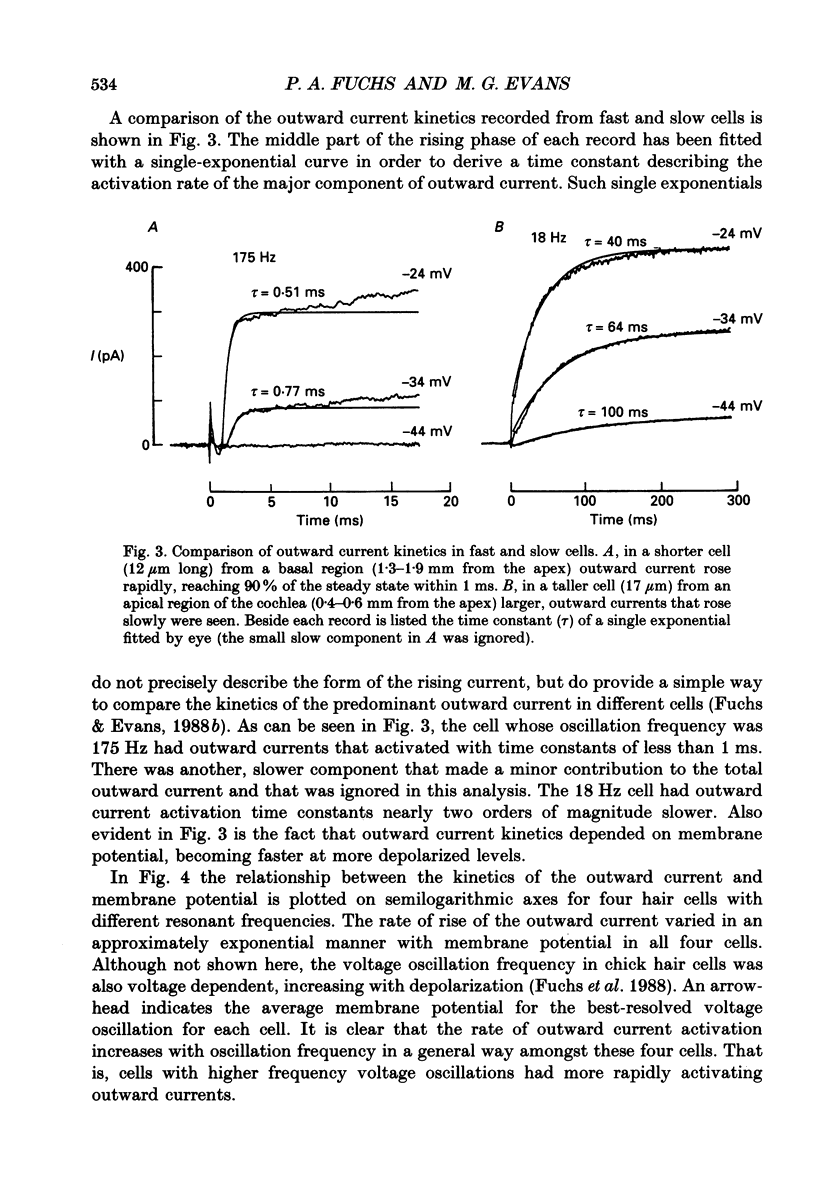







Selected References
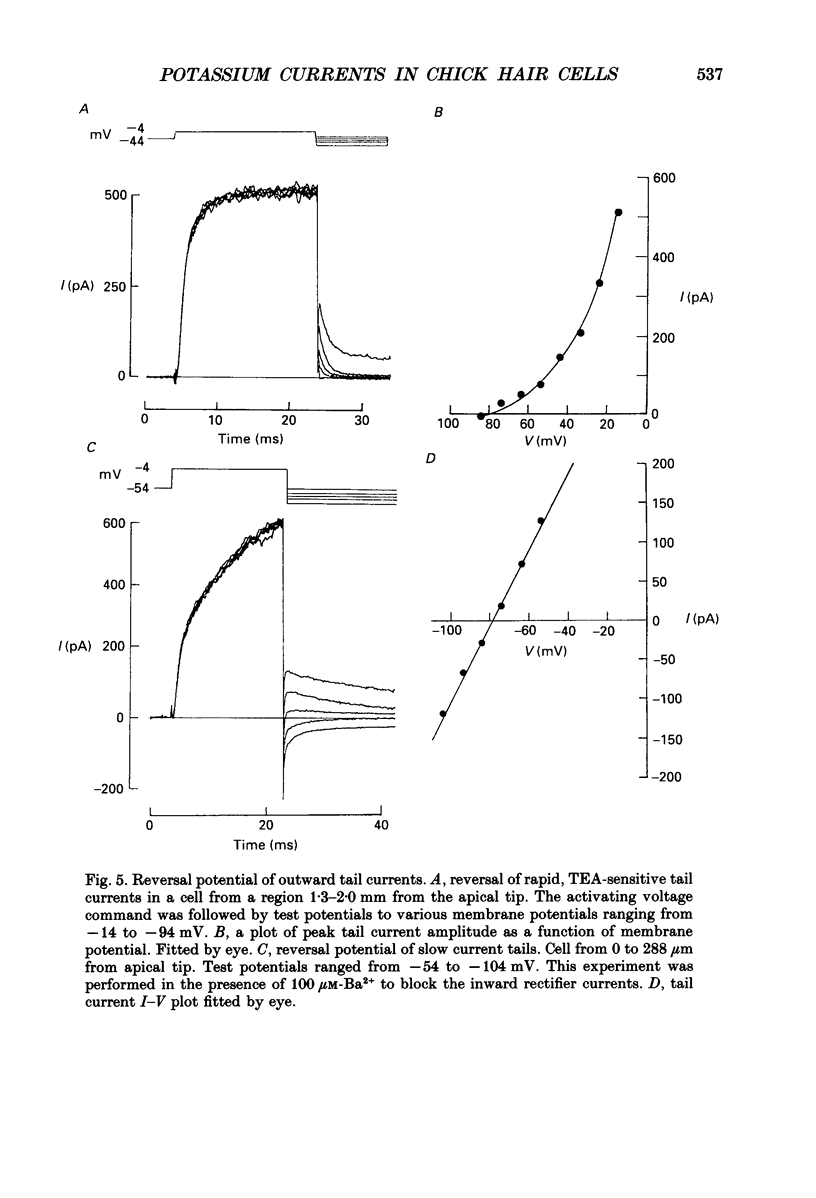
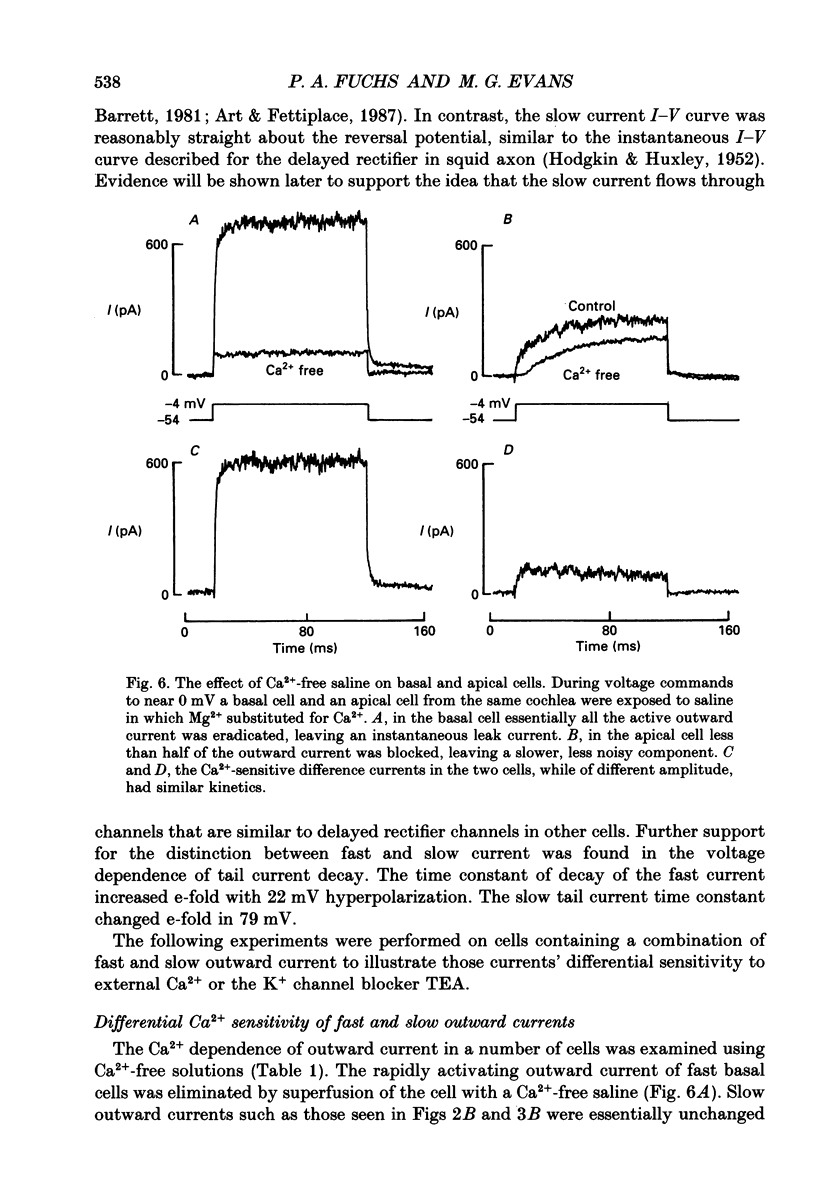
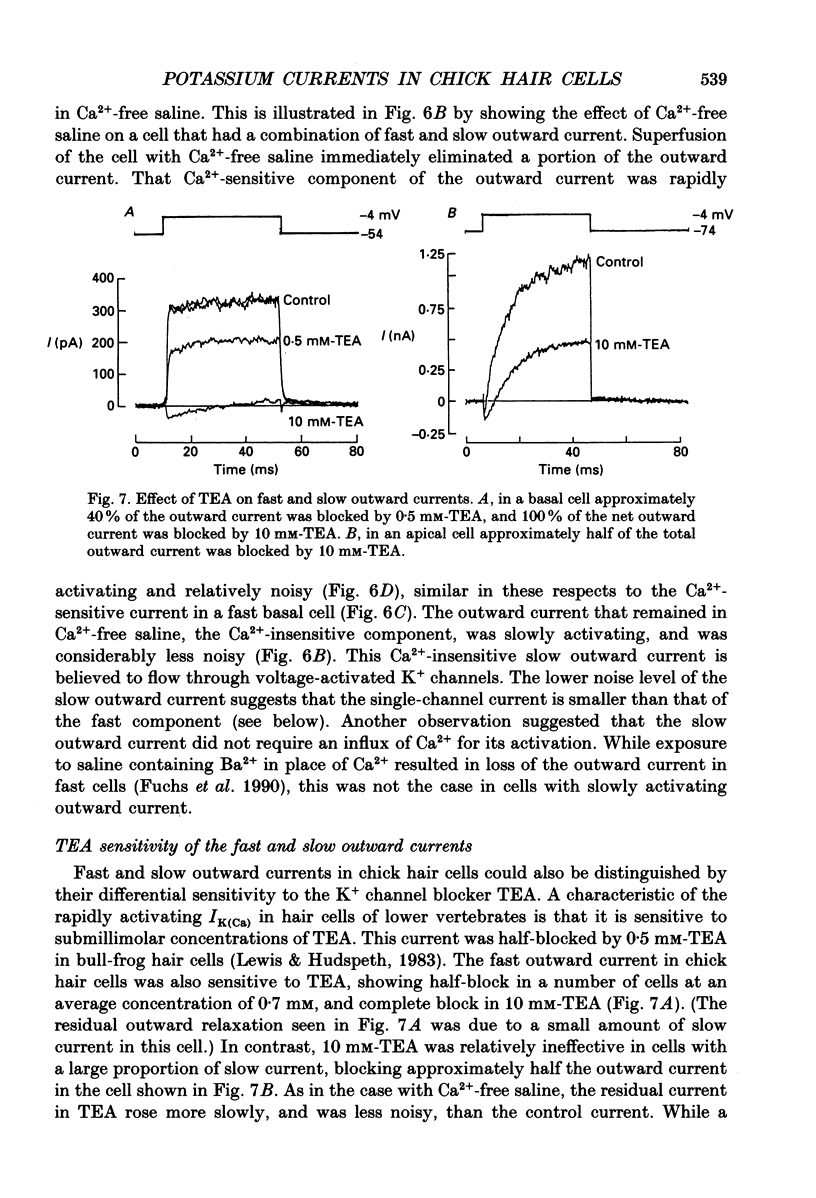
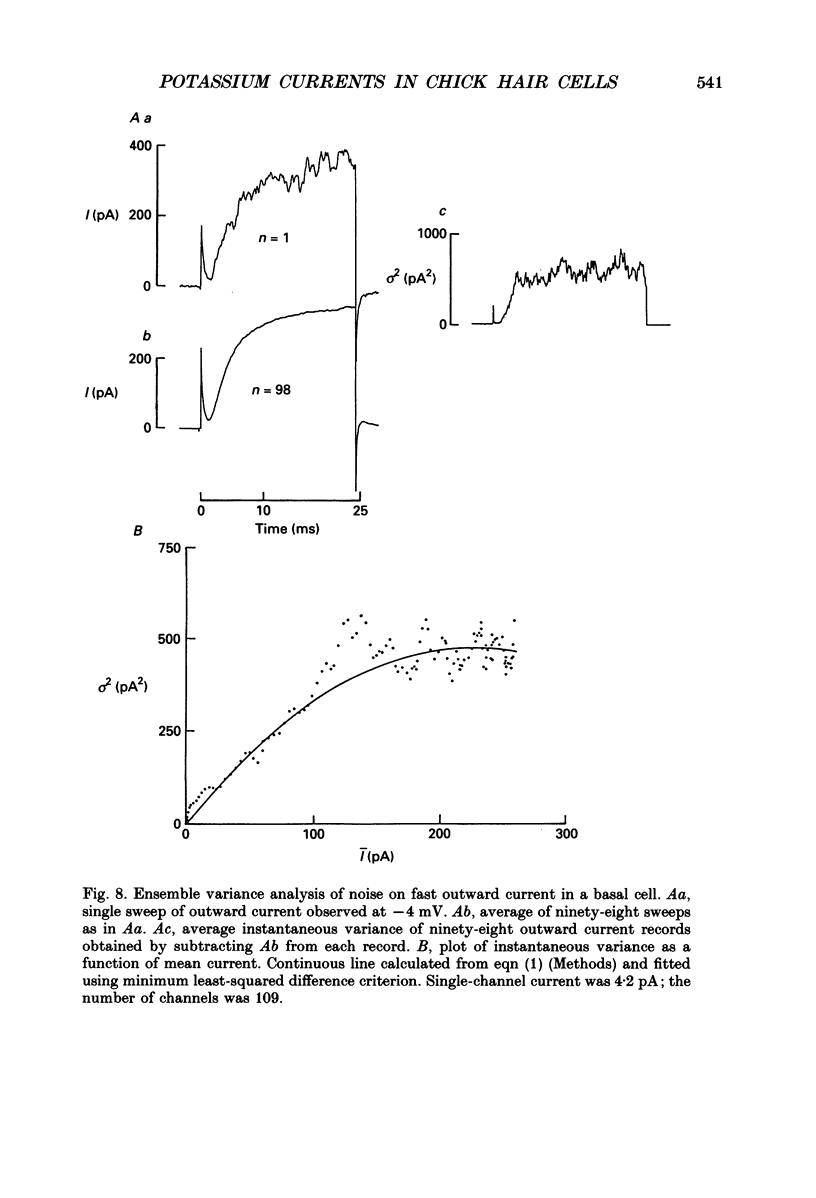
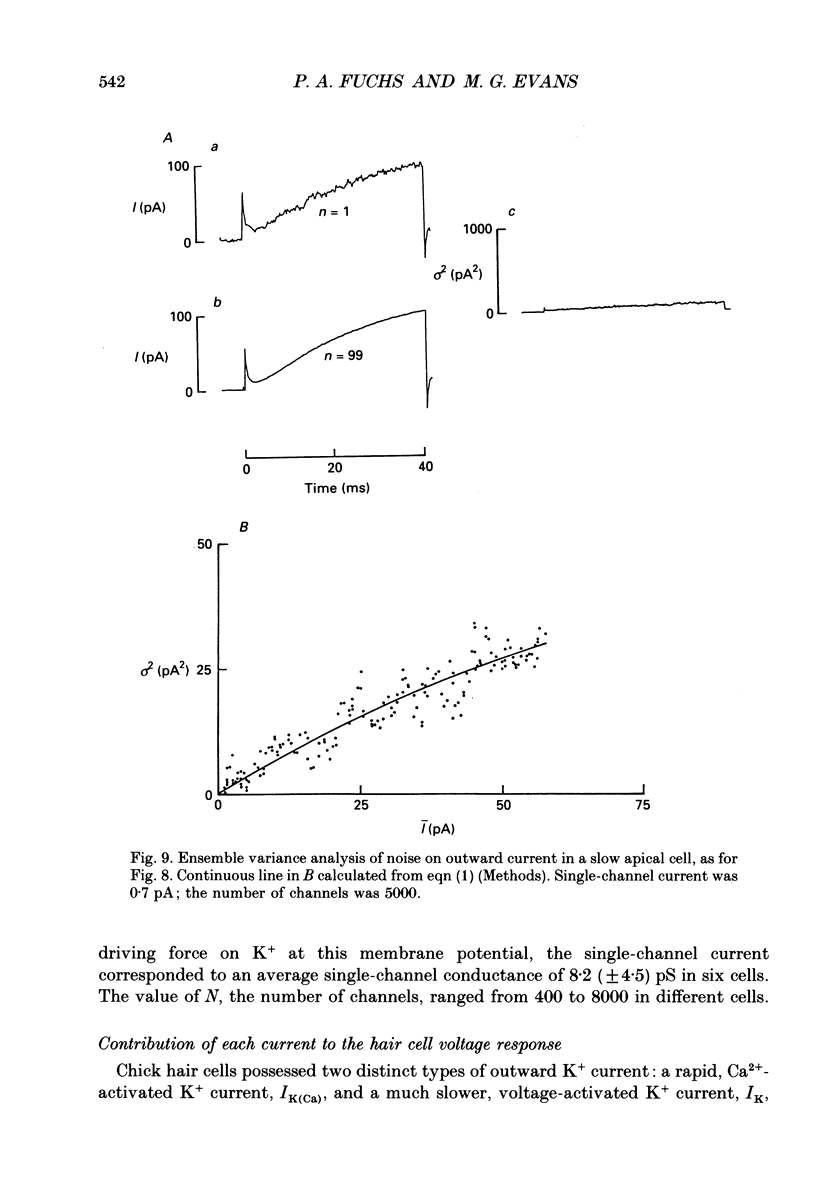
These references are in PubMed. This may not be the complete list of references from this article.
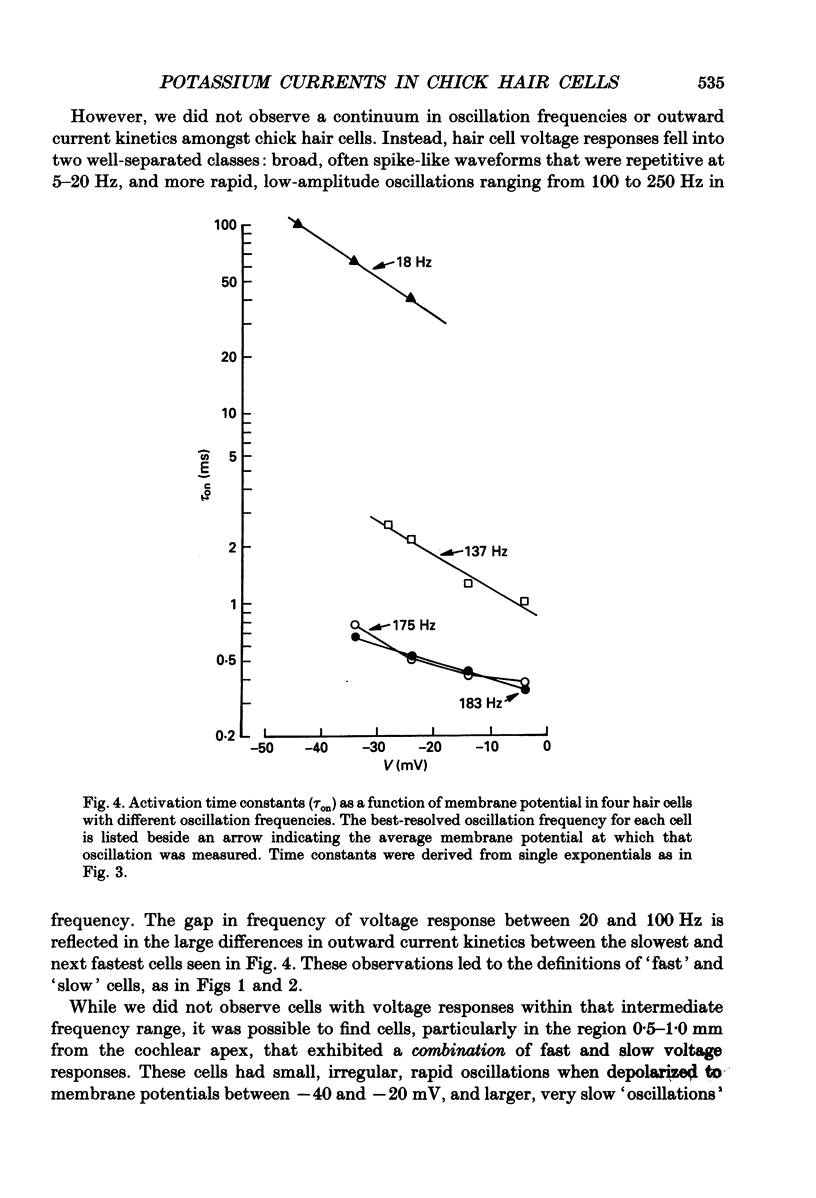
- Adrian R. H., Chandler W. K., Hodgkin A. L. Slow changes in potassium permeability in skeletal muscle. J Physiol. 1970 Jul;208(3):645–668. doi: 10.1113/jphysiol.1970.sp009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
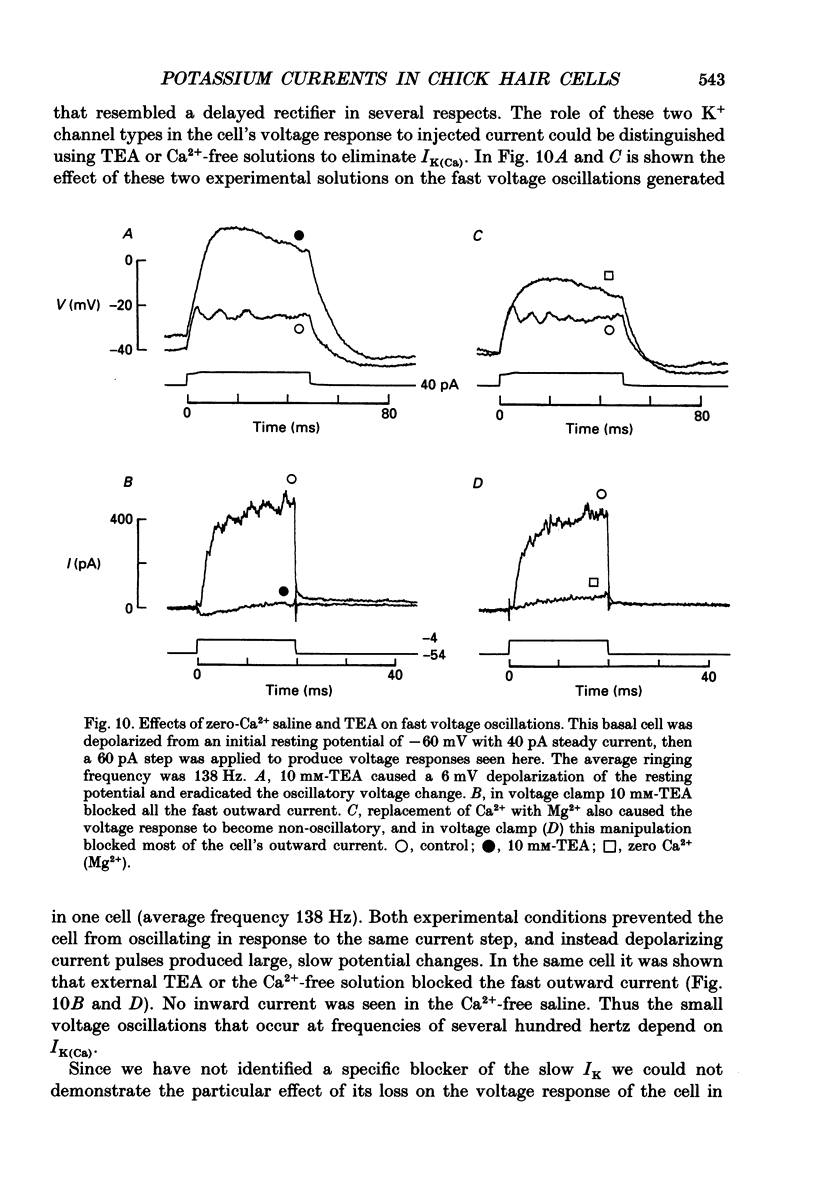
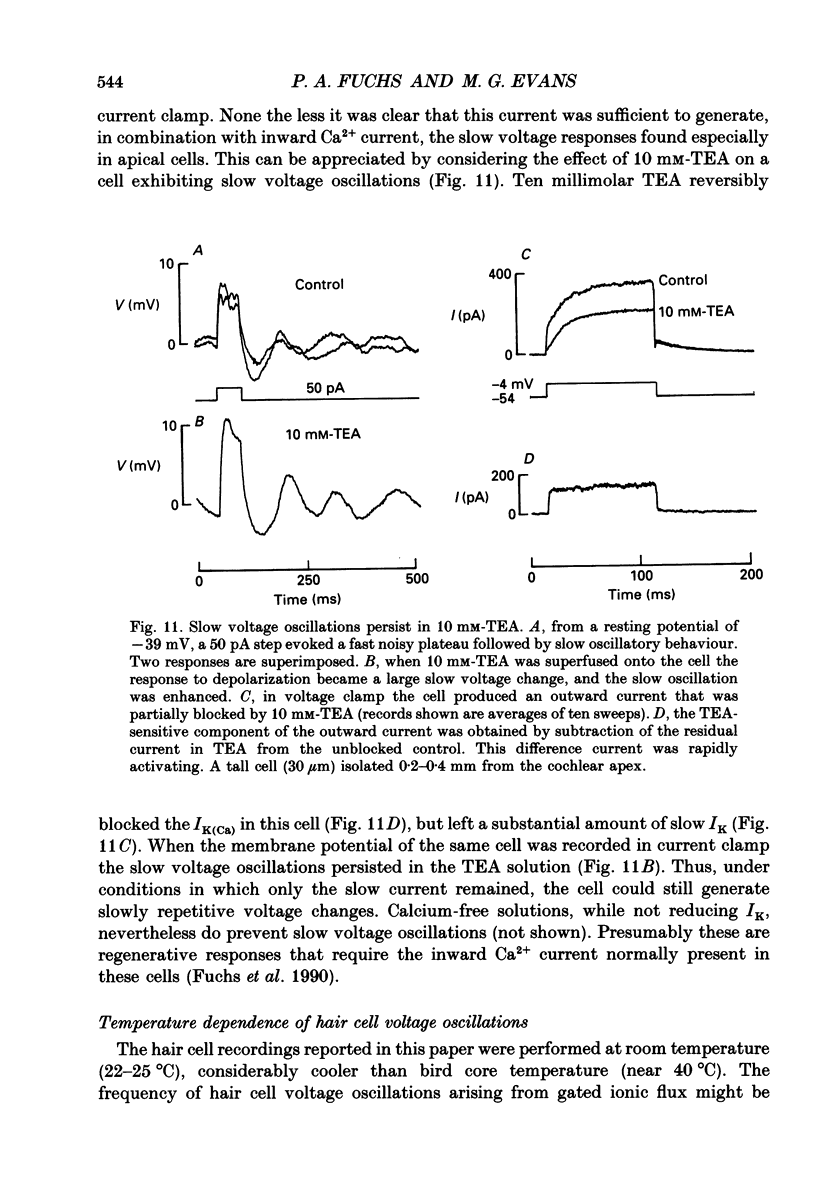
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Inactivation of delayed outward current in molluscan neurone somata. J Physiol. 1979 Jun;291:507–530. doi: 10.1113/jphysiol.1979.sp012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art J. J., Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol. 1987 Apr;385:207–242. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
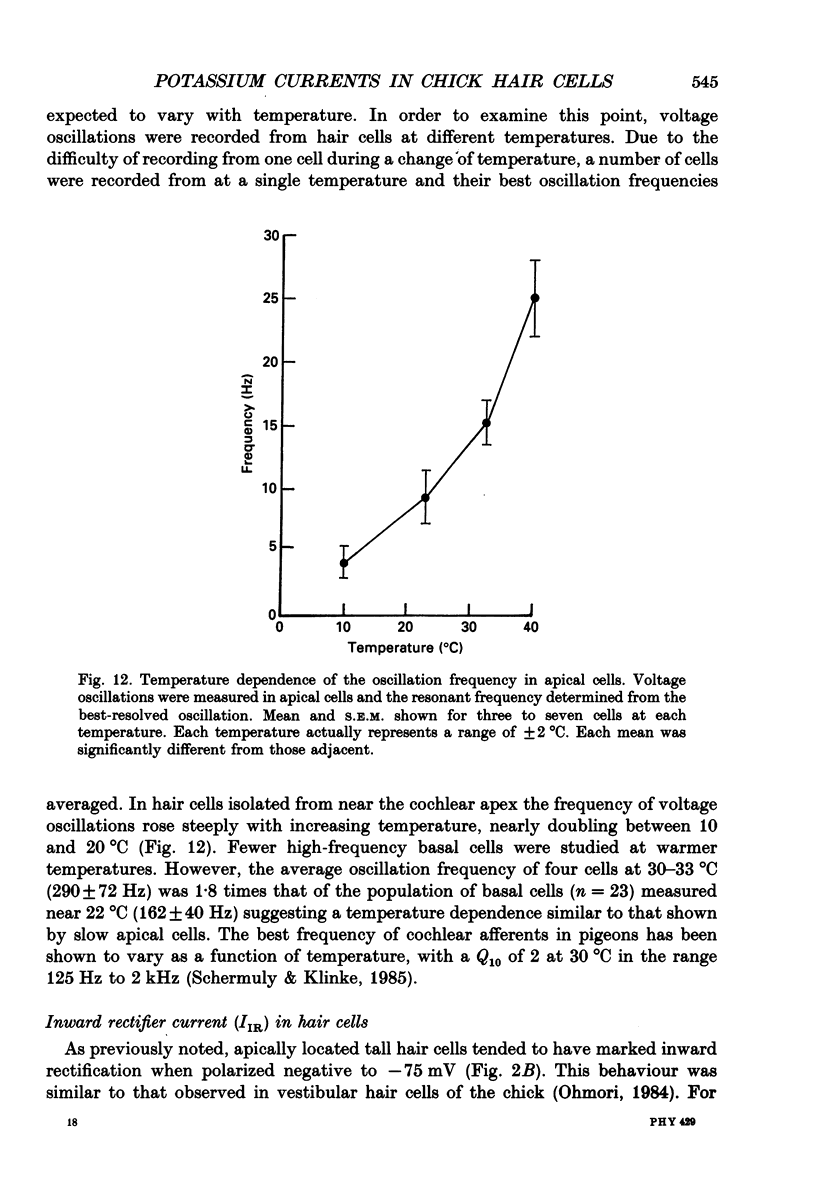
- Ashmore J. F. Frequency tuning in a frog vestibular organ. Nature. 1983 Aug 11;304(5926):536–538. doi: 10.1038/304536a0. [DOI] [PubMed] [Google Scholar]
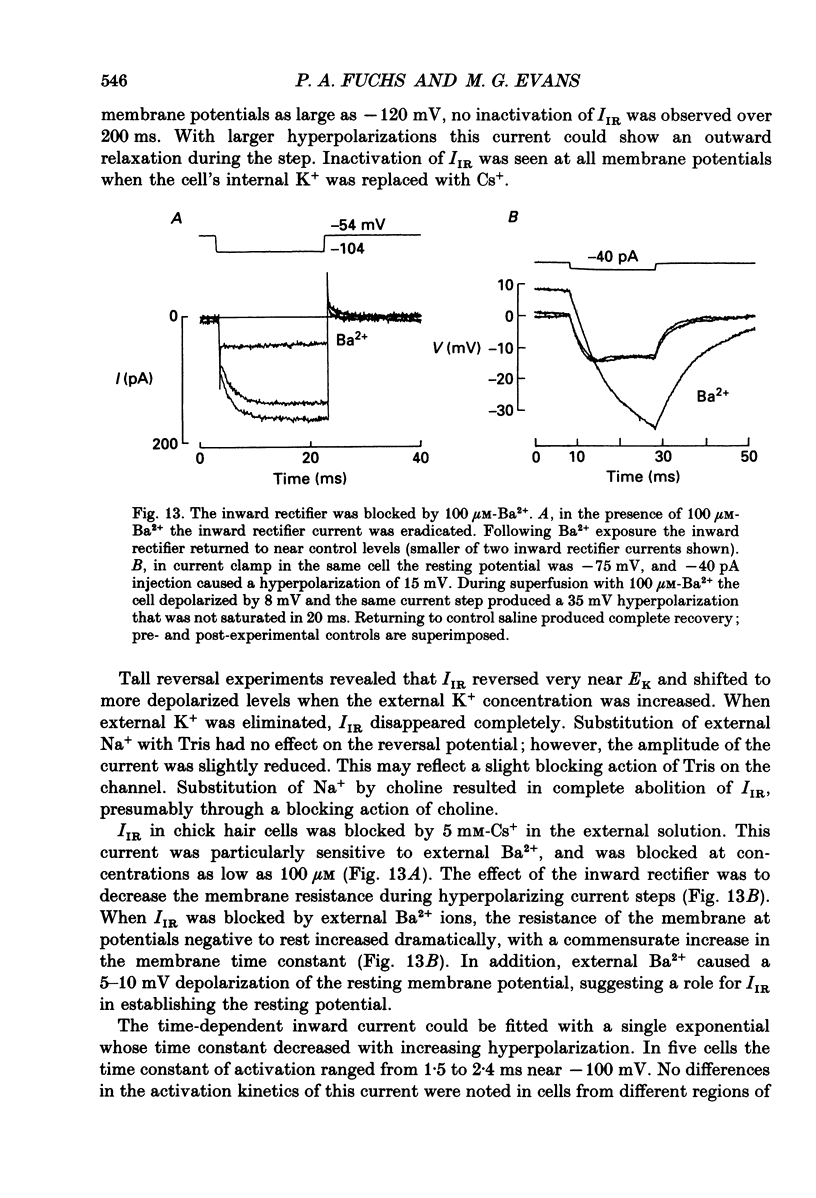
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G., Murrow B. W. Calcium currents in hair cells isolated from the cochlea of the chick. J Physiol. 1990 Oct;429:553–568. doi: 10.1113/jphysiol.1990.sp018272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. A., Evans M. G. Voltage oscillations and ionic conductances in hair cells isolated from the alligator cochlea. J Comp Physiol A. 1988 Dec;164(2):151–163. doi: 10.1007/BF00603947. [DOI] [PubMed] [Google Scholar]
- Fuchs P. A., Nagai T., Evans M. G. Electrical tuning in hair cells isolated from the chick cochlea. J Neurosci. 1988 Jul;8(7):2460–2467. doi: 10.1523/JNEUROSCI.08-07-02460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hirokawa N. The ultrastructure of the basilar papilla of the chick. J Comp Neurol. 1978 Sep 15;181(2):361–374. doi: 10.1002/cne.901810208. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. A model for electrical resonance and frequency tuning in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:275–297. doi: 10.1113/jphysiol.1988.sp017120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Lewis R. S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana. J Physiol. 1988 Jun;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Manley G. A., Gleich O., Leppelsack H. J., Oeckinghaus H. Activity patterns of cochlear ganglion neurones in the starling. J Comp Physiol A. 1985 Sep;157(2):161–181. doi: 10.1007/BF01350025. [DOI] [PubMed] [Google Scholar]
- Manley G. A. Preferred intervals in the spontaneous activity of primary auditory neurons. Naturwissenschaften. 1979 Nov;66(11):582–584. doi: 10.1007/BF00368823. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neher E. Potassium channels in cultured bovine adrenal chromaffin cells. J Physiol. 1985 Oct;367:117–141. doi: 10.1113/jphysiol.1985.sp015817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Studies of ionic currents in the isolated vestibular hair cell of the chick. J Physiol. 1984 May;350:561–581. doi: 10.1113/jphysiol.1984.sp015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Pitchford S., Ashmore J. F. An electrical resonance in hair cells of the amphibian papilla of the frog Rana temporaria. Hear Res. 1987;27(1):75–83. doi: 10.1016/0378-5955(87)90027-x. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Trube G. Calcium and delayed potassium currents in mouse pancreatic beta-cells under voltage-clamp conditions. J Physiol. 1986 May;374:531–550. doi: 10.1113/jphysiol.1986.sp016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R., Ward T. A. Properties of single potassium channels in vesicles formed from the sarcolemma of frog skeletal muscle. J Physiol. 1985 Jul;364:339–358. doi: 10.1113/jphysiol.1985.sp015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Smith C. A. Structure of the chicken's inner ear: SEM and TEM study. Am J Anat. 1978 Oct;153(2):251–271. doi: 10.1002/aja.1001530206. [DOI] [PubMed] [Google Scholar]
- Temchin A. N. Unusual discharge patterns of single fibers in the pigeon's auditory nerve. J Comp Physiol A. 1988 May;163(1):99–115. doi: 10.1007/BF00612001. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Saunders J. C. Actin filaments, stereocilia, and hair cells of the bird cochlea. I. Length, number, width, and distribution of stereocilia of each hair cell are related to the position of the hair cell on the cochlea. J Cell Biol. 1983 Mar;96(3):807–821. doi: 10.1083/jcb.96.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G., Tilney M. S. Functional organization of the cytoskeleton. Hear Res. 1986;22:55–77. doi: 10.1016/0378-5955(86)90077-8. [DOI] [PubMed] [Google Scholar]
- Whitehead M. C., Morest D. K. Dual populations of efferent and afferent cochlear axons in the chicken. Neuroscience. 1981;6(11):2351–2365. doi: 10.1016/0306-4522(81)90022-1. [DOI] [PubMed] [Google Scholar]


