Abstract
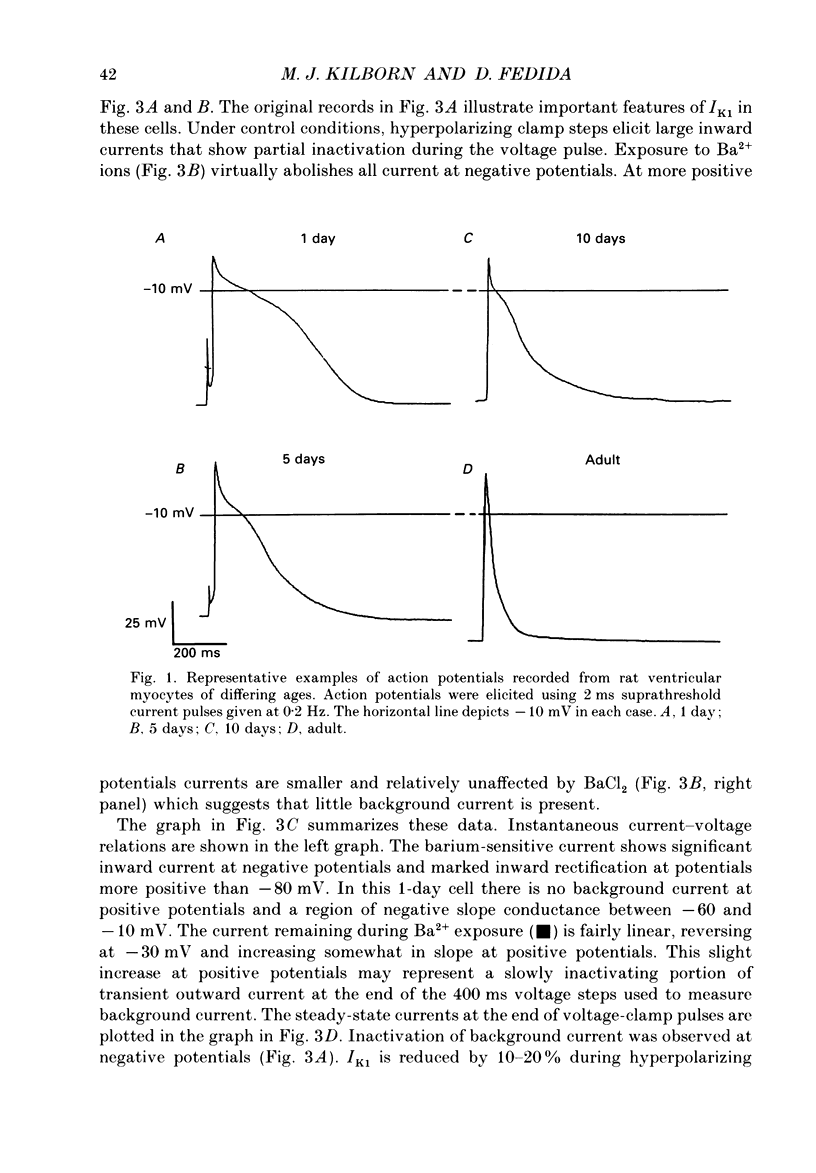
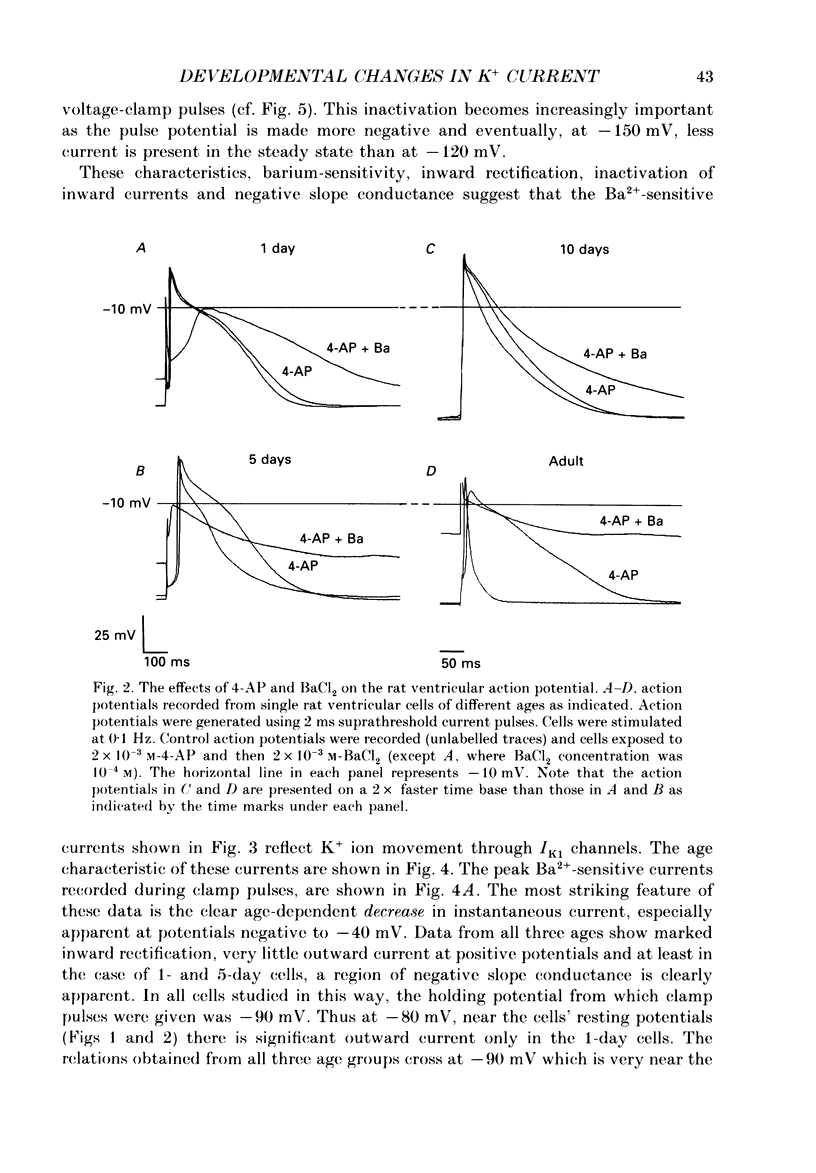
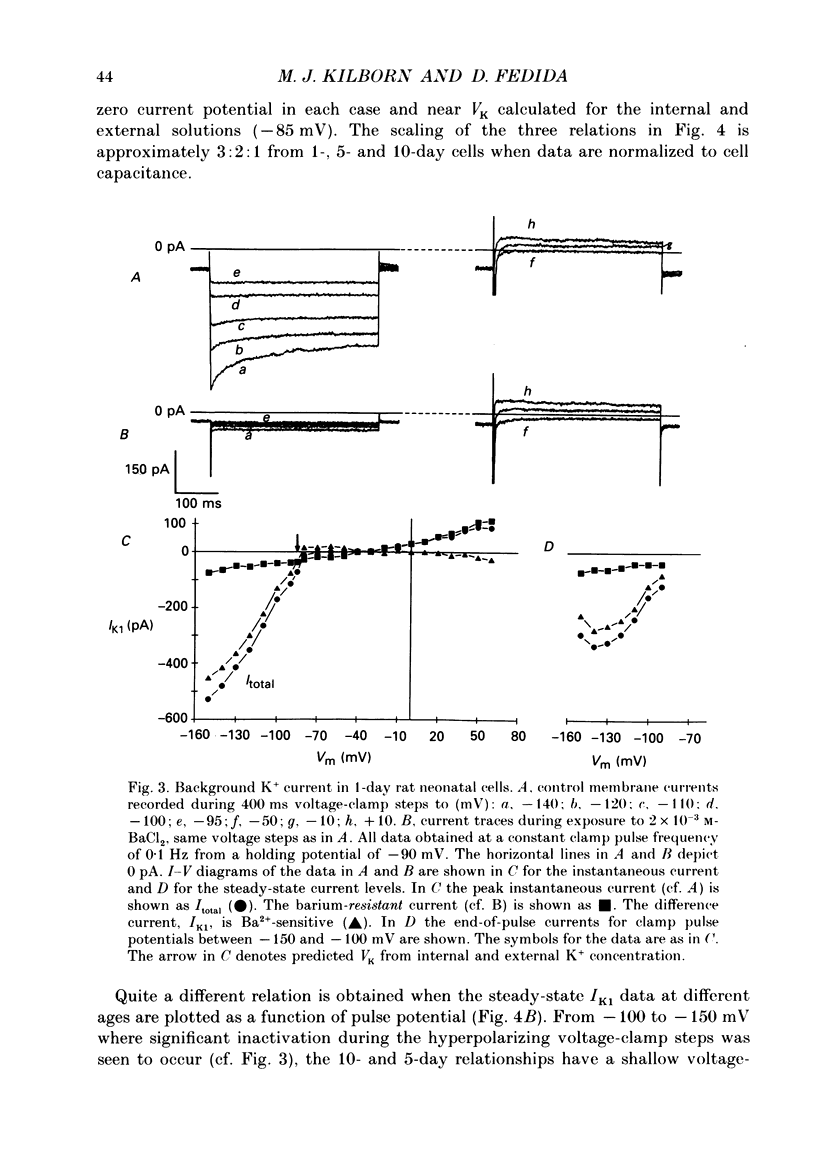
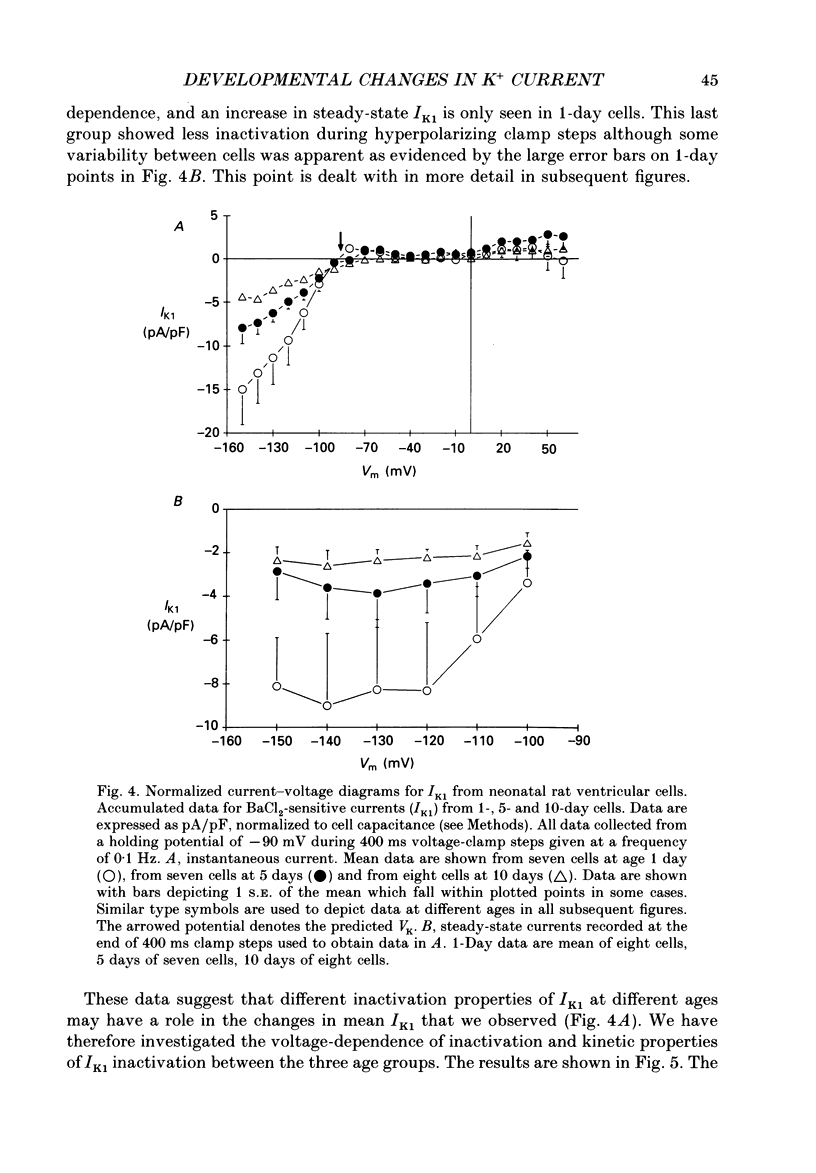
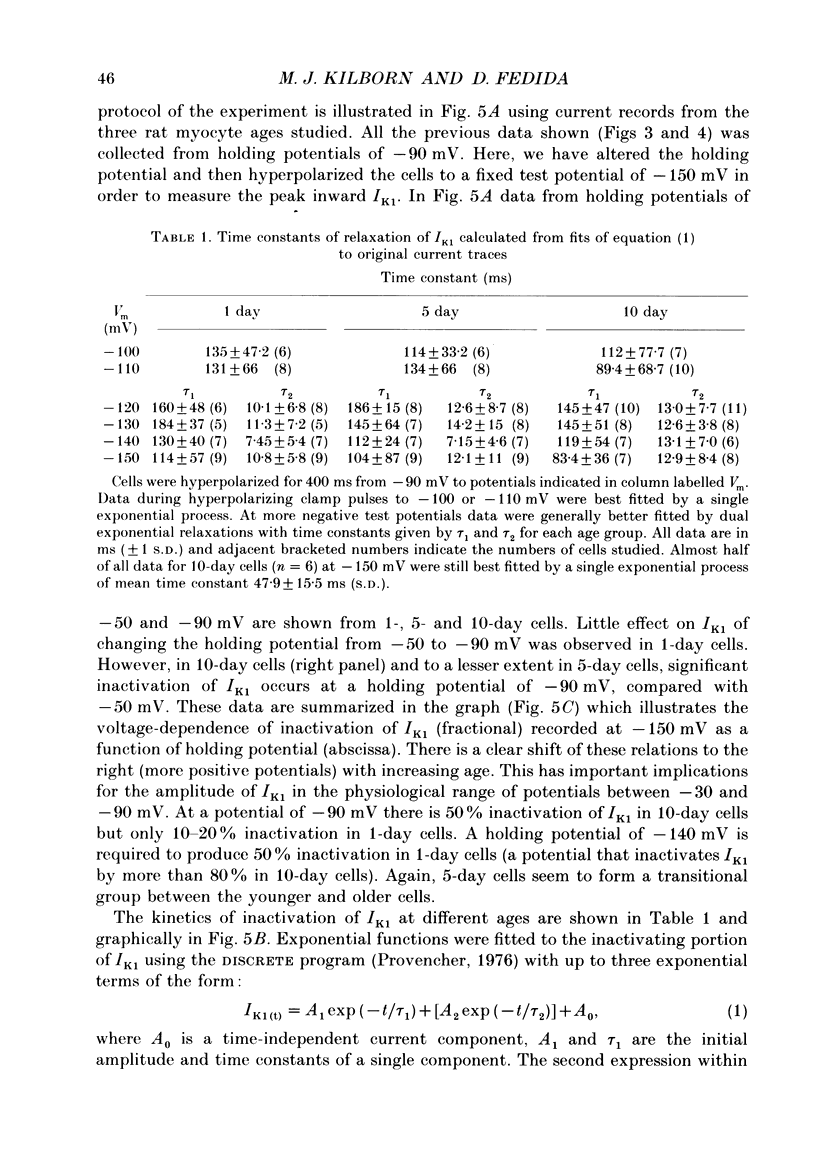
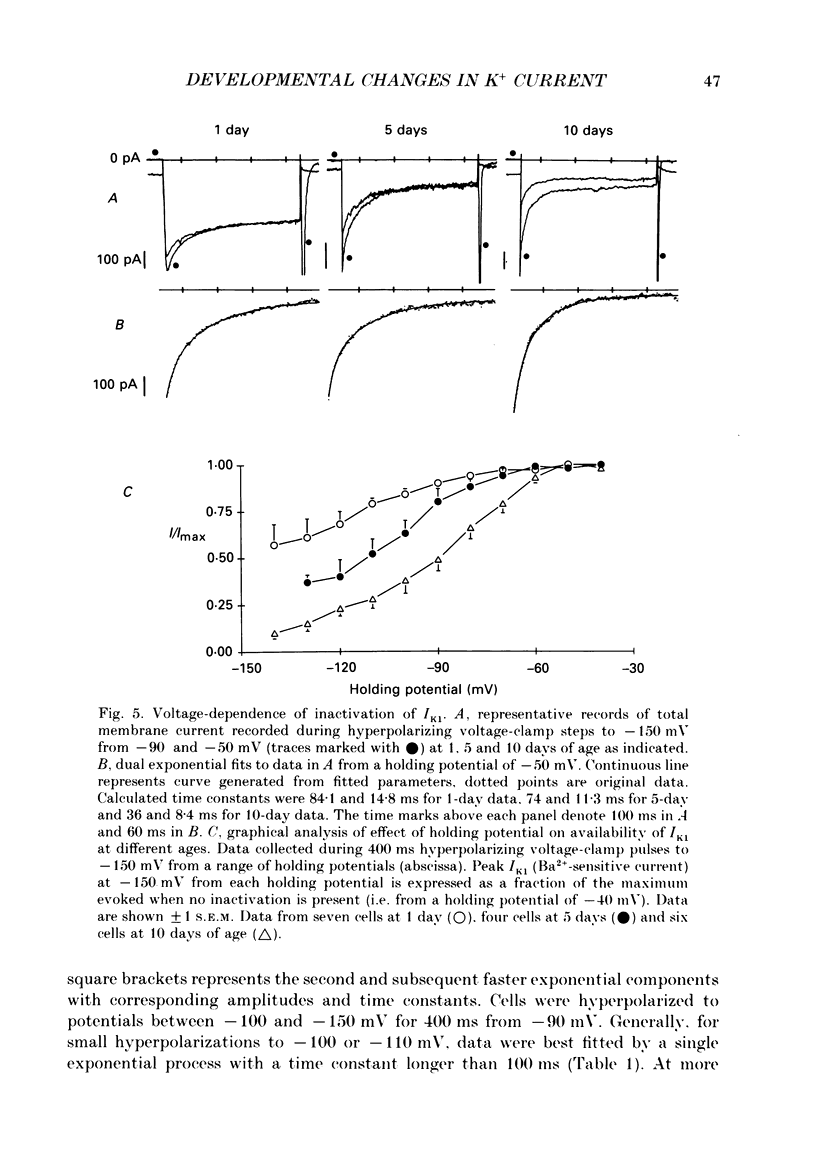
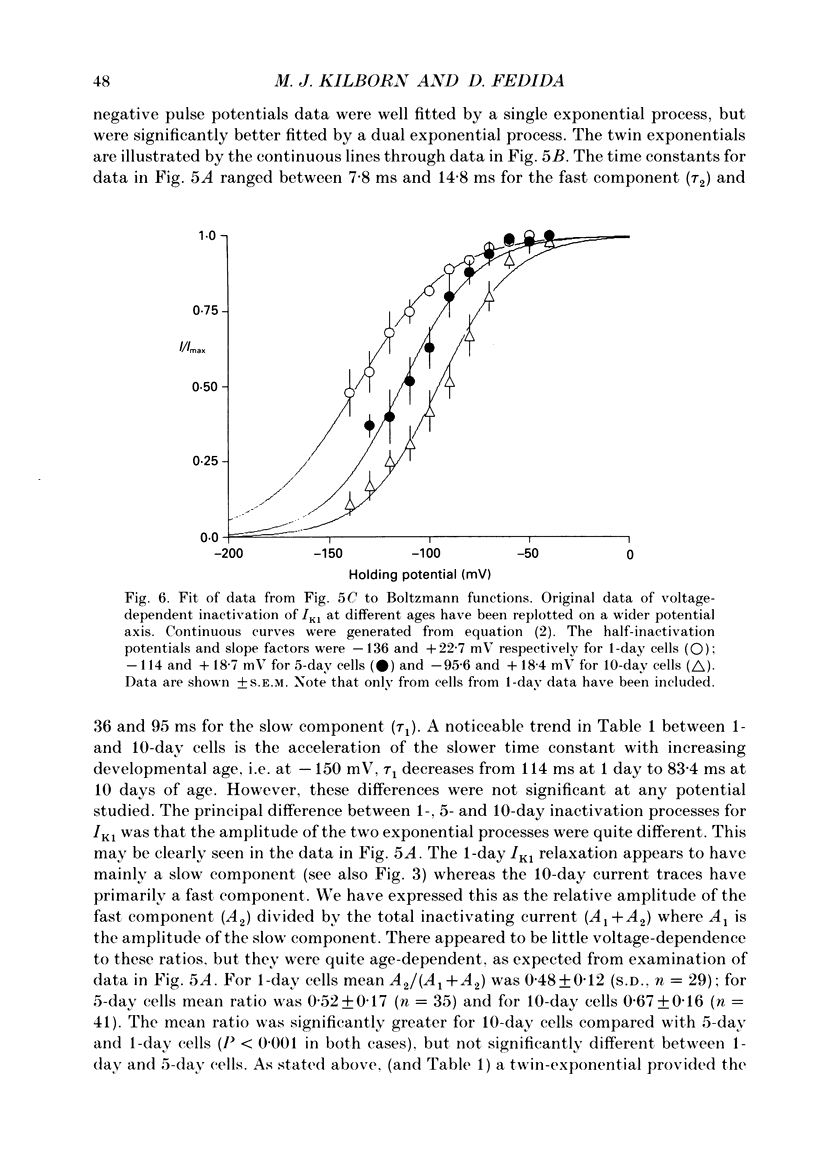
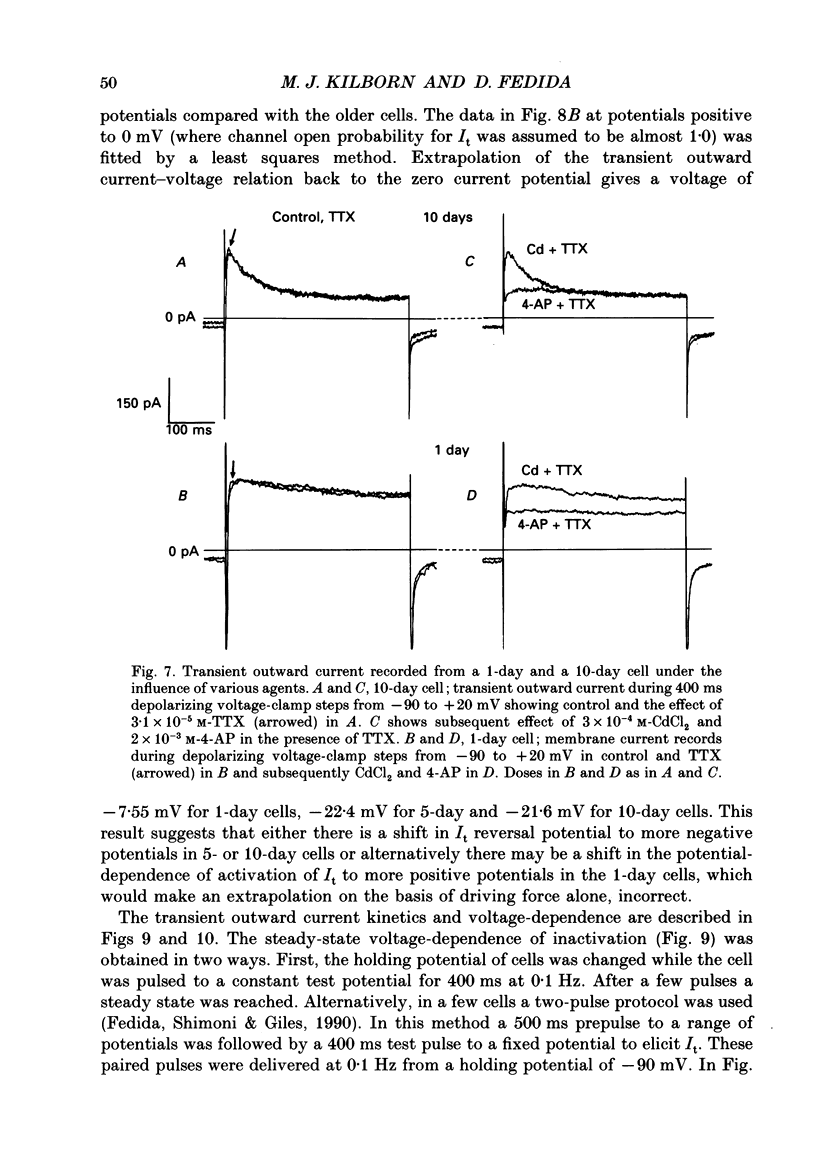
1. In ventricular muscle of rat heart, the action potential undergoes a major developmental change in shape in the days and weeks immediately after birth. Potassium (K+) currents which may affect the shape of the action potential have been studied using a whole-cell voltage-clamp technique with single cells from the ventricles of rats aged 1-10 days. All recordings were made at 22-23 degrees C. 2. Three discrete ages were chosen. 1-day (cells isolated within 24 h of birth), 5-day and 10-day rats. These parallel the developmental action potential shortening from neonatal towards adult type. Action potentials of single myocytes were initially of long duration at 1 day with a prominent plateau phase, but had shortened somewhat by 10 days of age. The 5-day group exhibited an action potential transitional in character between the earlier and later groups of cells. 3. Potassium current blocking agents were used to assess the importance of the various outward K+ currents for the action potential waveform at different ages. 4-Aminopyridine (4-AP; 2 x 10(-3) M) which preferentially blocks voltage-activated transient outward currents affected action potentials at all ages, but increases in duration were most pronounced in the 10-day group. Only a small prolongation of the initial phase of repolarization of 1-day action potentials was seen. Extracellular barium chloride, 0.1-2 x 10(-3) M, a blocker of inwardly rectifying potassium channels, had a marked slowing effect on repolarization in all the three age groups. Resting membrane depolarization was also produced by barium. 4. Developmental changes in the inwardly rectifying background current (IK1) and the cardiac transient outward current, It, were investigated. IK1 was recorded as the current sensitive to 2 x 10(-3) M-BaCl2 during voltage-clamp steps from a holding potential of -90 mV. It was found to decrease in magnitude, approximately by a factor of three, from 15 to 5 pA/pF during the first ten postnatal days. This reduction can explain the maturational slowing of repolarization during the final phase of the action potential in rat heart. 5. Current-voltage relations for IK1 from the three age groups crossed at the zero current potential at approximately -90 mV, near the calculated VK for the pipette filling solution and an external bath K+ concentration of 5 x 10(-3) M. This suggests that IK1 channels in these cells are quite selective for K+ ions and that developmental changes in the potassium selectivity are not responsible for changes in IK1.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





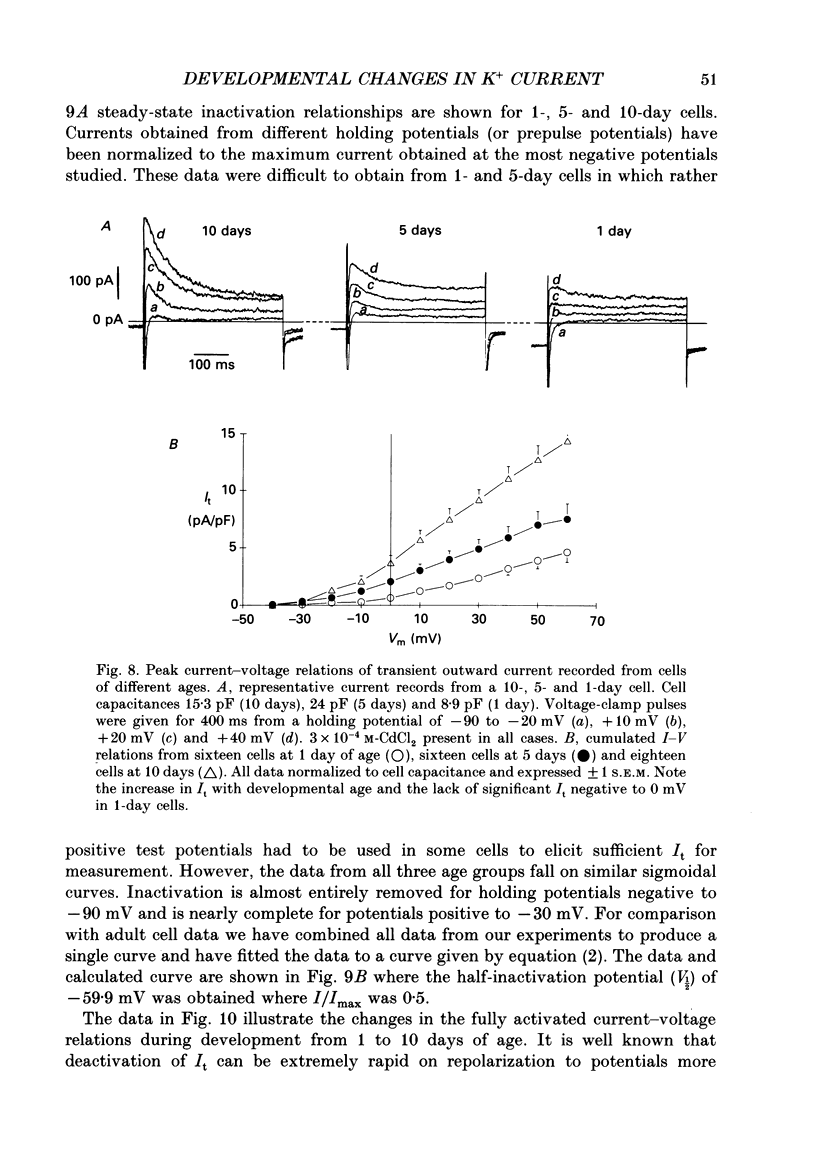
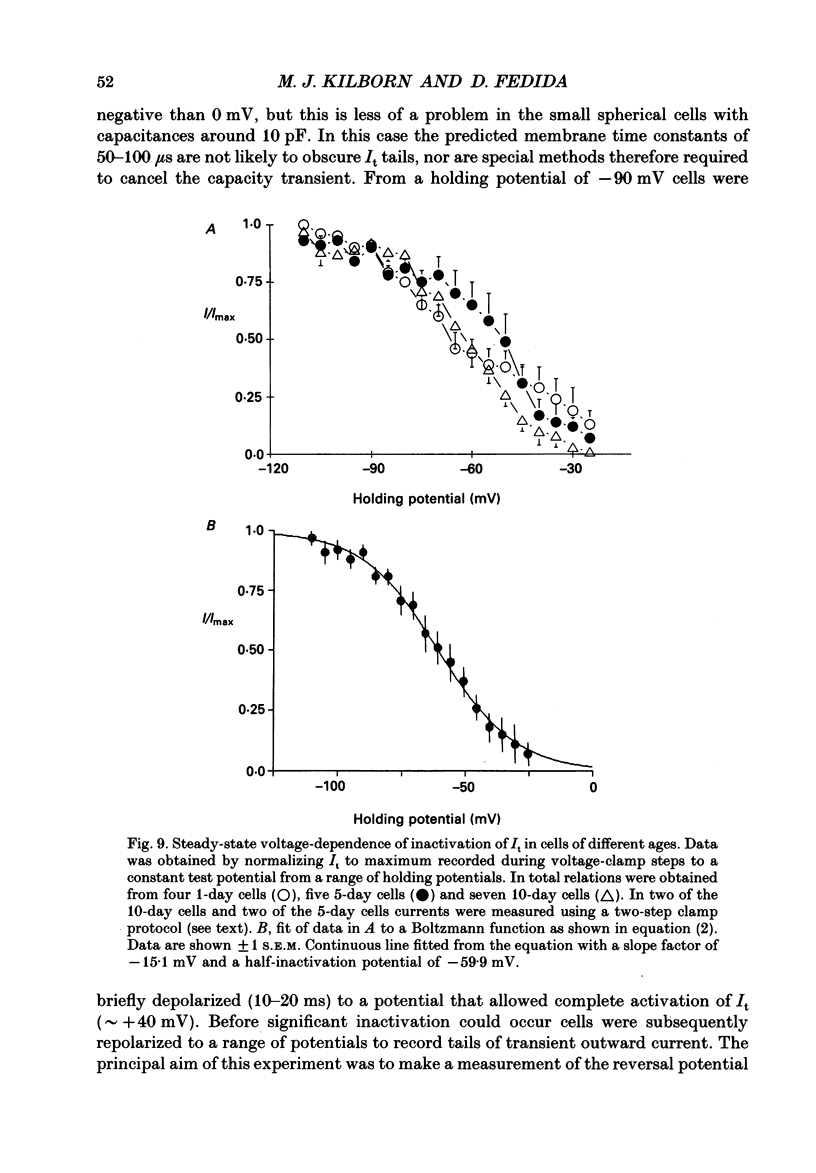
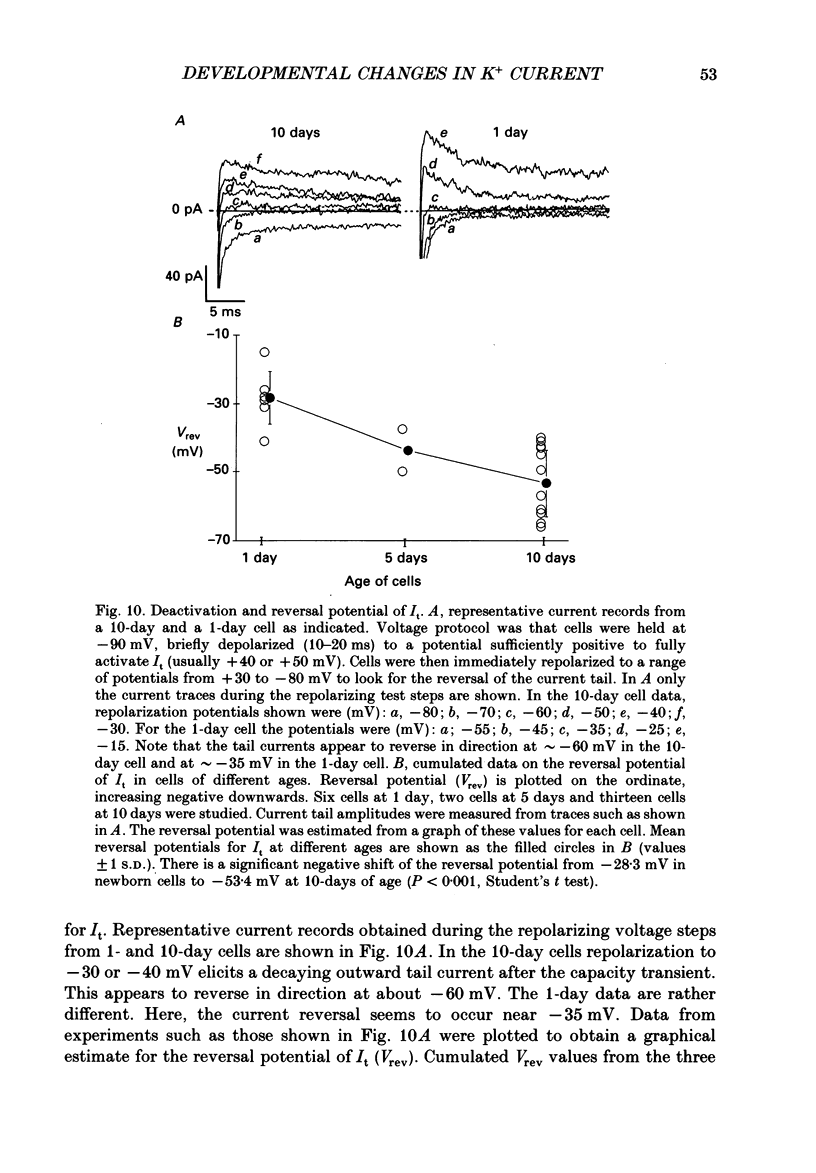







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biermans G., Vereecke J., Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflugers Arch. 1987 Dec;410(6):604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Cavalié A., McDonald T. F., Pelzer D., Trautwein W. Temperature-induced transitory and steady-state changes in the calcium current of guinea pig ventricular myocytes. Pflugers Arch. 1985 Oct;405(3):294–296. doi: 10.1007/BF00582574. [DOI] [PubMed] [Google Scholar]
- Clay J. R., Shrier A. Developmental changes in subthreshold pace-maker currents in chick embryonic heart cells. J Physiol. 1981 Mar;312:491–504. doi: 10.1113/jphysiol.1981.sp013640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. M., Lederer W. J. Changes in the calcium current of rat heart ventricular myocytes during development. J Physiol. 1988 Dec;406:115–146. doi: 10.1113/jphysiol.1988.sp017372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch J. R., West T. C., Hoff H. E. Development of the action potential of the prenatal rat heart. Circ Res. 1969 Jan;24(1):19–31. doi: 10.1161/01.res.24.1.19. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A., Visentin S. Barium-induced blockade of the inward rectifier in calf Purkinje fibres. Pflugers Arch. 1984 Dec;402(4):446–453. doi: 10.1007/BF00583946. [DOI] [PubMed] [Google Scholar]
- Escande D., Loisance D., Planche C., Coraboeuf E. Age-related changes of action potential plateau shape in isolated human atrial fibers. Am J Physiol. 1985 Oct;249(4 Pt 2):H843–H850. doi: 10.1152/ajpheart.1985.249.4.H843. [DOI] [PubMed] [Google Scholar]
- Fedida D., Shimoni Y., Giles W. R. Alpha-adrenergic modulation of the transient outward current in rabbit atrial myocytes. J Physiol. 1990 Apr;423:257–277. doi: 10.1113/jphysiol.1990.sp018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W. R., Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988 Nov;405:123–145. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Hiraoka M. Barium-induced automatic activity in isolated ventricular myocytes from guinea-pig hearts. J Physiol. 1988 Jan;395:455–472. doi: 10.1113/jphysiol.1988.sp016929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerter J., Mazet F., Vassort G. Perinatal growth of the rabbit cardiac cell: possible implications for the mechanism of relaxation. J Mol Cell Cardiol. 1981 Aug;13(8):725–740. doi: 10.1016/0022-2828(81)90255-8. [DOI] [PubMed] [Google Scholar]
- Hopkins S. F., Jr, McCutcheon E. P., Wekstein D. R. Postnatal changes in rat ventricular function. Circ Res. 1973 Jun;32(6):685–691. doi: 10.1161/01.res.32.6.685. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Brown A. M. Inwardly rectifying single-channel and whole cell K+ currents in rat ventricular myocytes. J Membr Biol. 1986;94(1):19–35. doi: 10.1007/BF01901010. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. Early outward current in rat single ventricular cells. Circ Res. 1984 Feb;54(2):157–162. doi: 10.1161/01.res.54.2.157. [DOI] [PubMed] [Google Scholar]
- Jourdon P., Sperelakis N. Electrical properties of cultured heart cell reaggregates from newborn rat ventricles: comparison with intact non-cultured ventricles. J Mol Cell Cardiol. 1980 Dec;12(12):1441–1458. doi: 10.1016/0022-2828(80)90127-3. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Brady A. J., Tan S. T., Serena D. Correlation of the glycoside response, the force staircase, and the action potential configuration in the neonatal rat heart. Circ Res. 1975 Jun;36(6):744–752. doi: 10.1161/01.res.36.6.744. [DOI] [PubMed] [Google Scholar]
- Legato M. J. Ultrastructural characteristics of the rat ventricular cell grown in tissue culture, with special reference to sarcomerogenesis. J Mol Cell Cardiol. 1972 Aug;4(4):299–317. doi: 10.1016/0022-2828(72)90077-6. [DOI] [PubMed] [Google Scholar]
- Litovsky S. H., Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ Res. 1988 Jan;62(1):116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- Mark G. E., Strasser F. F. Pacemaker activity and mitosis in cultures of newborn rat heart ventricle cells. Exp Cell Res. 1966 Nov-Dec;44(2):217–233. doi: 10.1016/0014-4827(66)90427-7. [DOI] [PubMed] [Google Scholar]
- Masson-Pévet M., Jongsma H. J., De Bruijne J. Collagenase- and trypsin-dissociated heart cells: a comparative ultrastructural study. J Mol Cell Cardiol. 1976 Oct;8(10):747–757. doi: 10.1016/0022-2828(76)90082-1. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Saigusa A., Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg2+. Nature. 1987 Jan 8;325(7000):156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Moses R. L., Kasten F. H. T-tubes in cultured mammalian myocardial cells. Cell Tissue Res. 1979;203(2):173–180. doi: 10.1007/BF00237231. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payet M. D., Rousseau E., Sauvé R. Single-channel analysis of a potassium inward rectifier in myocytes of newborn rat heart. J Membr Biol. 1985;86(2):79–88. doi: 10.1007/BF01870774. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Probst I., Schwartz P., Hütter F. J., Spieckermann P. G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982 Jul;14(7):397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher S. W. A Fourier method for the analysis of exponential decay curves. Biophys J. 1976 Jan;16(1):27–41. doi: 10.1016/S0006-3495(76)85660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucelík P., Jezek K., Barták F. Postnatal development of electrophysiological manifestations of the working ventricular myocardium of albino rats. Physiol Bohemoslov. 1982;31(3):217–224. [PubMed] [Google Scholar]
- Pucelík P., Králícek P., Barták F., Jezek K. An analysis of the postnatal development of the action potential repolarization process in the working ventricular myocardium of albino rats (effect of tea, frequency, verapamil and adrenaline). Physiol Bohemoslov. 1983;32(5):419–429. [PubMed] [Google Scholar]
- Robinson R. B. Action potential characteristics of rat cardiac cells do not change with time in culture. J Mol Cell Cardiol. 1982 Jun;14(6):367–370. doi: 10.1016/0022-2828(82)90252-8. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon M. E., Safronova V. G. The rest-dependent depression of action potential duration in rabbit myocardium and the possible role of the transient outward current. A pharmacological analysis. J Physiol (Paris) 1982;78(5):461–466. [PubMed] [Google Scholar]
- Schouten V. J., ter Keurs H. E. The slow repolarization phase of the action potential in rat heart. J Physiol. 1985 Mar;360:13–25. doi: 10.1113/jphysiol.1985.sp015601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguchi M., Harding J. A., Jarmakani J. M. Developmental change in the function of sarcoplasmic reticulum. J Mol Cell Cardiol. 1986 Feb;18(2):189–195. doi: 10.1016/s0022-2828(86)80471-0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Tsien R. W. Calcium-activated transient outward current in calf cardiac Purkinje fibres. J Physiol. 1980 Feb;299:485–506. doi: 10.1113/jphysiol.1980.sp013138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C., Anderson P. A. Multiple regional differences in cellular properties that regulate repolarization and contraction in the right atrium of adult and newborn dogs. Circ Res. 1989 Dec;65(6):1594–1611. doi: 10.1161/01.res.65.6.1594. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Delbridge L. M., Bustamante J. O., McDonald T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983 Mar;52(3):280–290. doi: 10.1161/01.res.52.3.280. [DOI] [PubMed] [Google Scholar]
- van Ginneken A. C., Jongsma H. J. Slow inward current in aggregates of neonatal rat heart cells and its contribution to the steady state current-voltage relationship. Pflugers Arch. 1983 Jun 1;397(4):265–271. doi: 10.1007/BF00580259. [DOI] [PubMed] [Google Scholar]


