Abstract
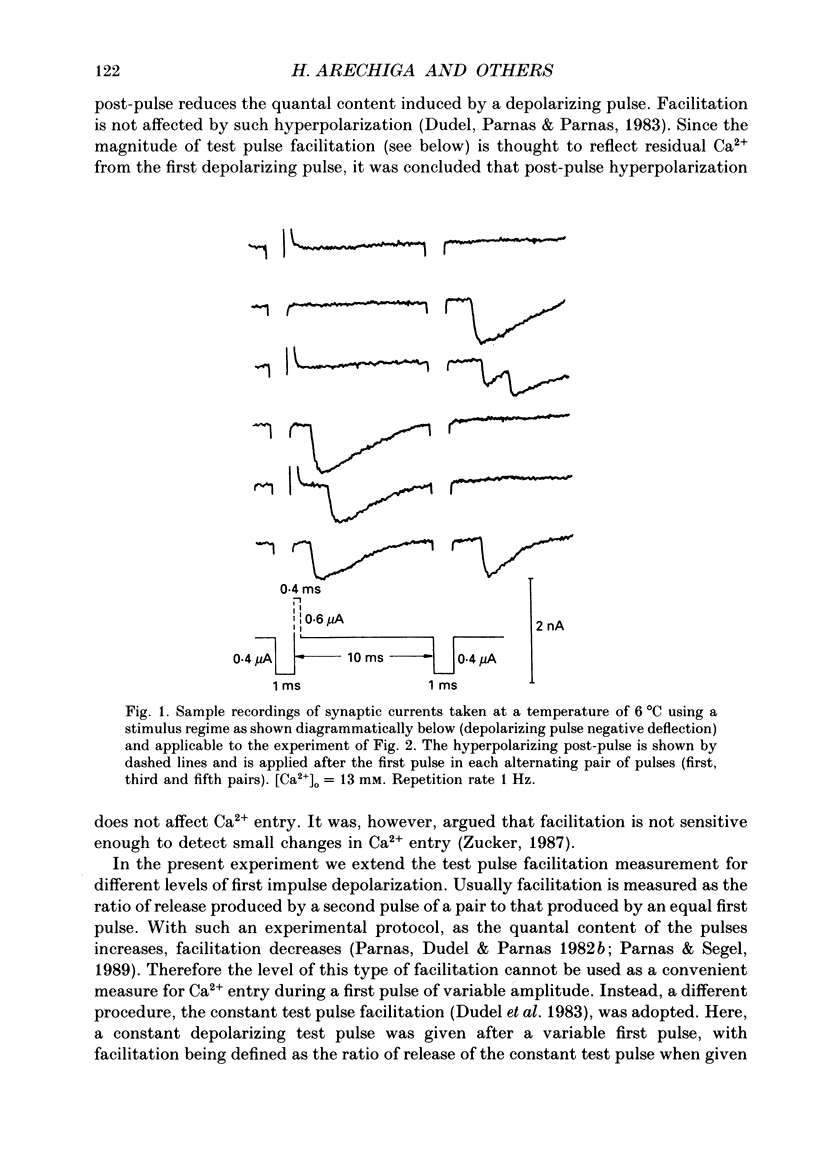
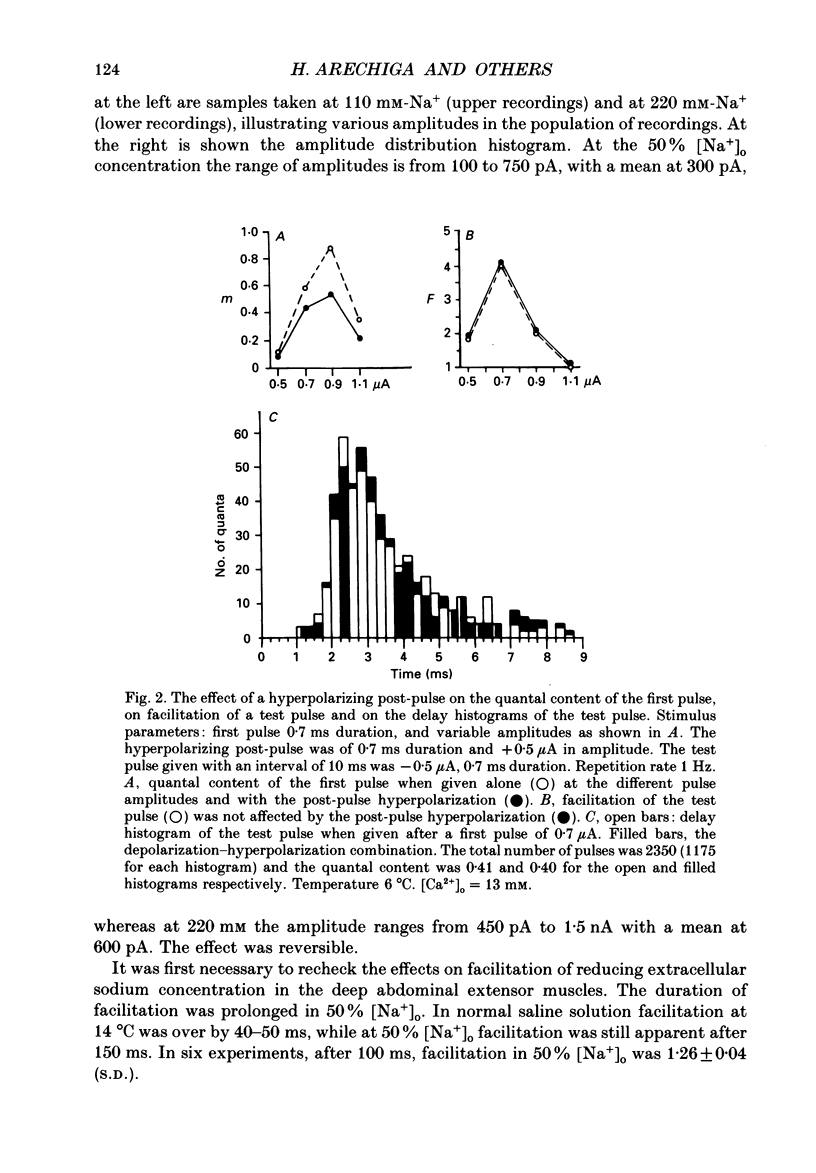
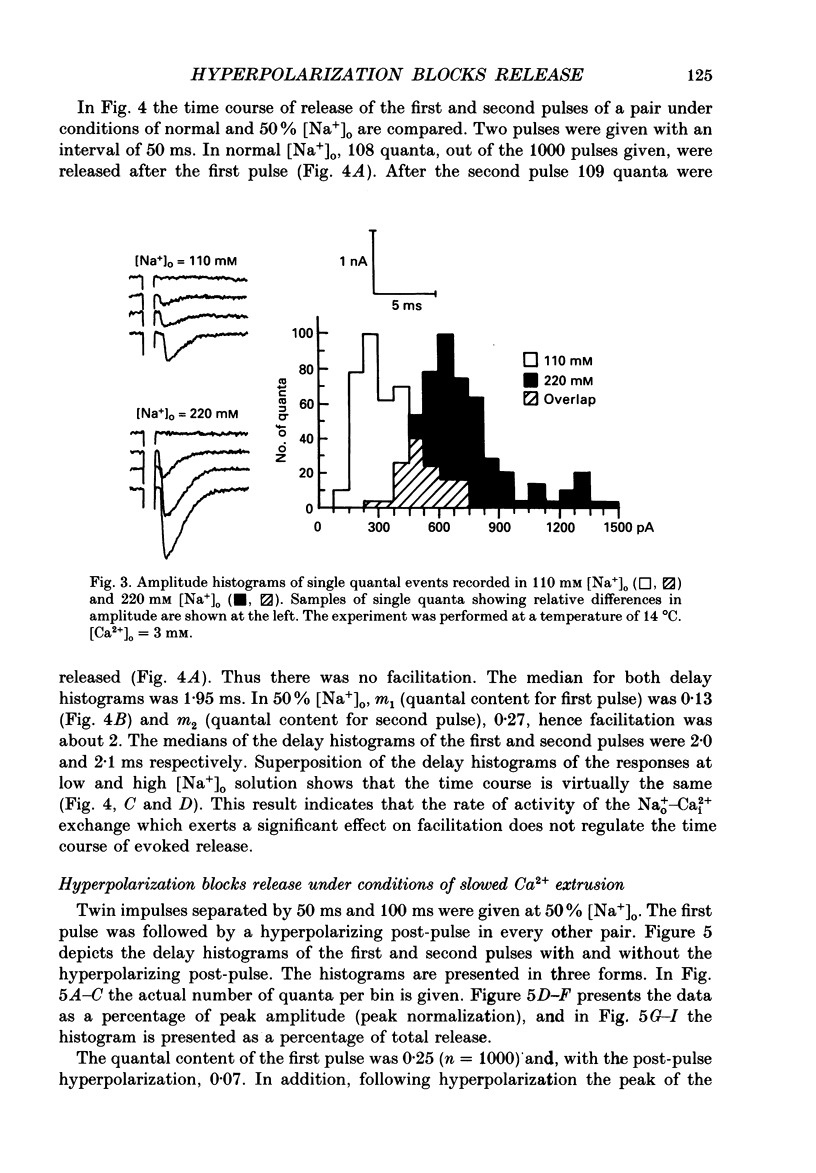
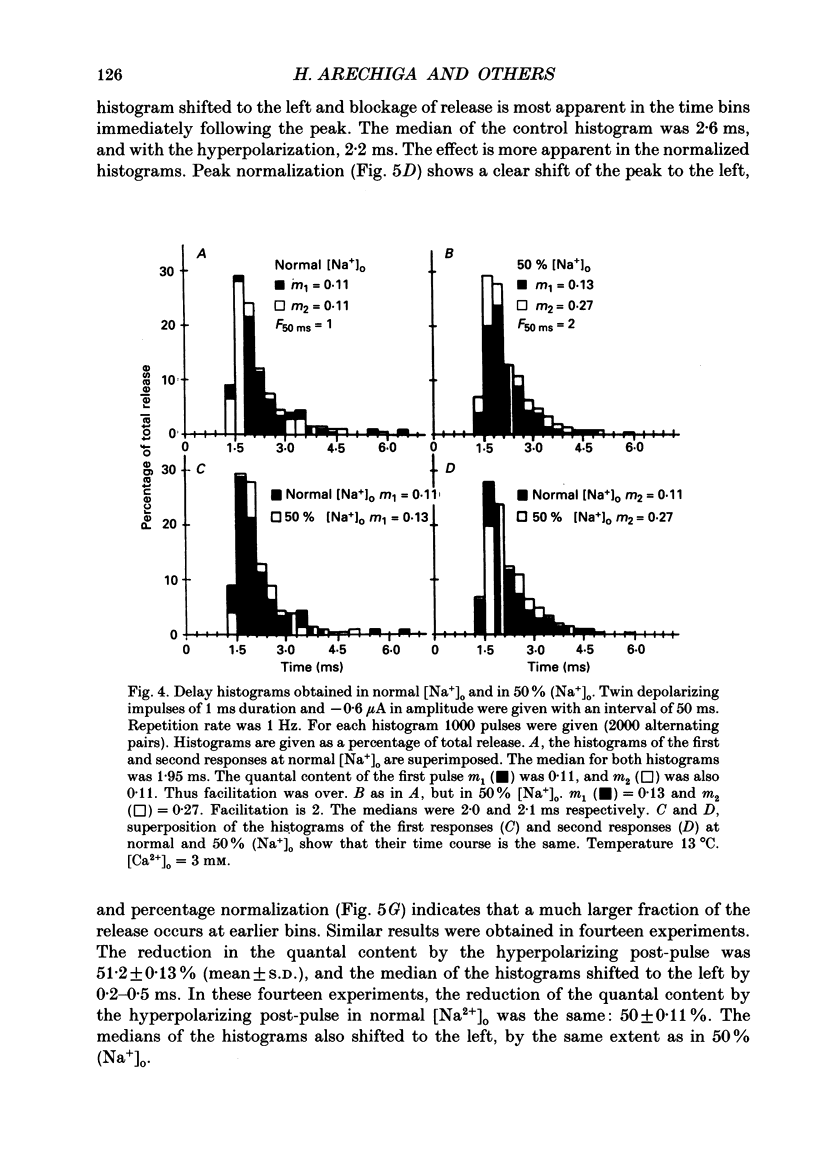
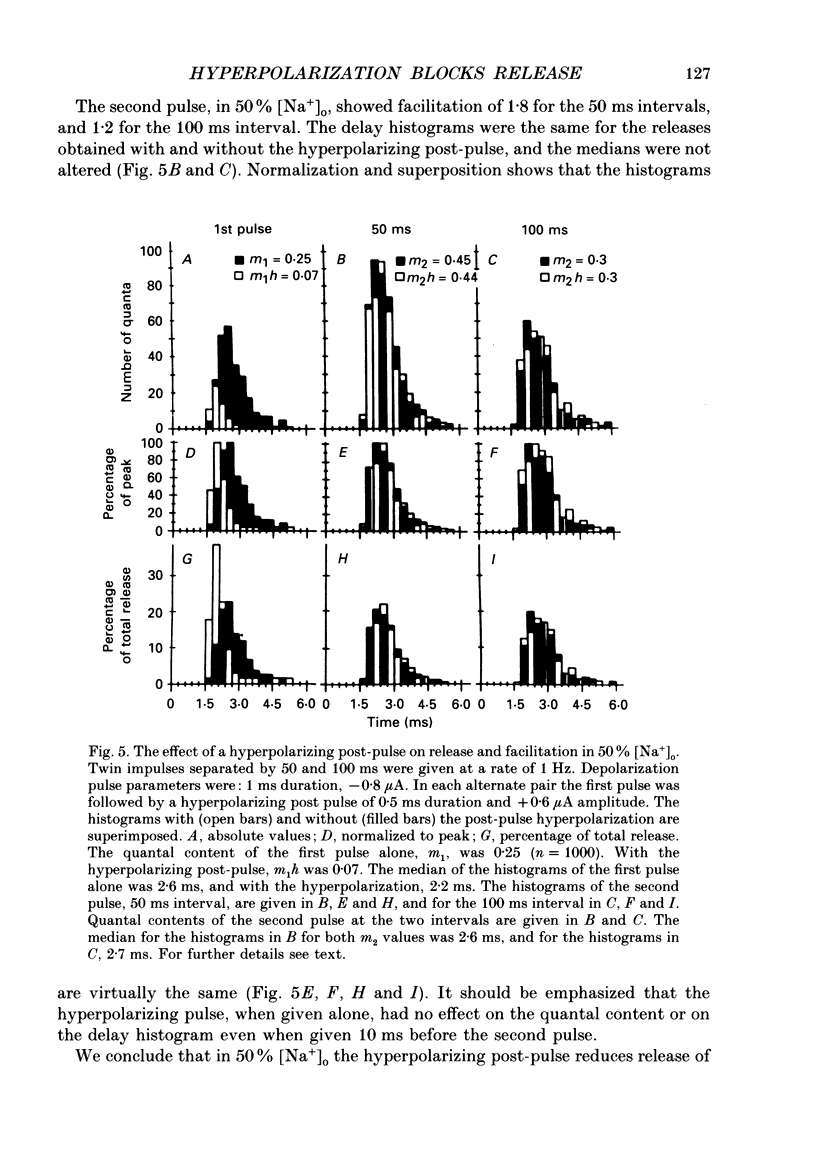
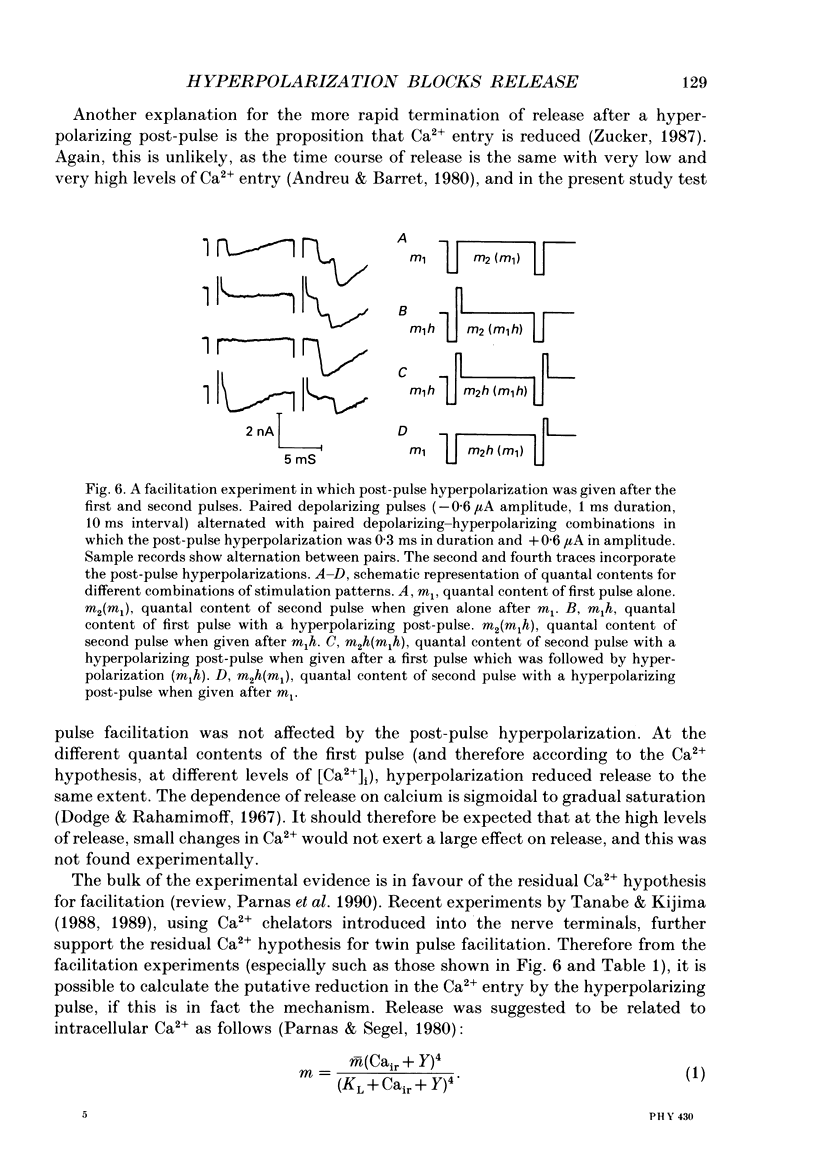
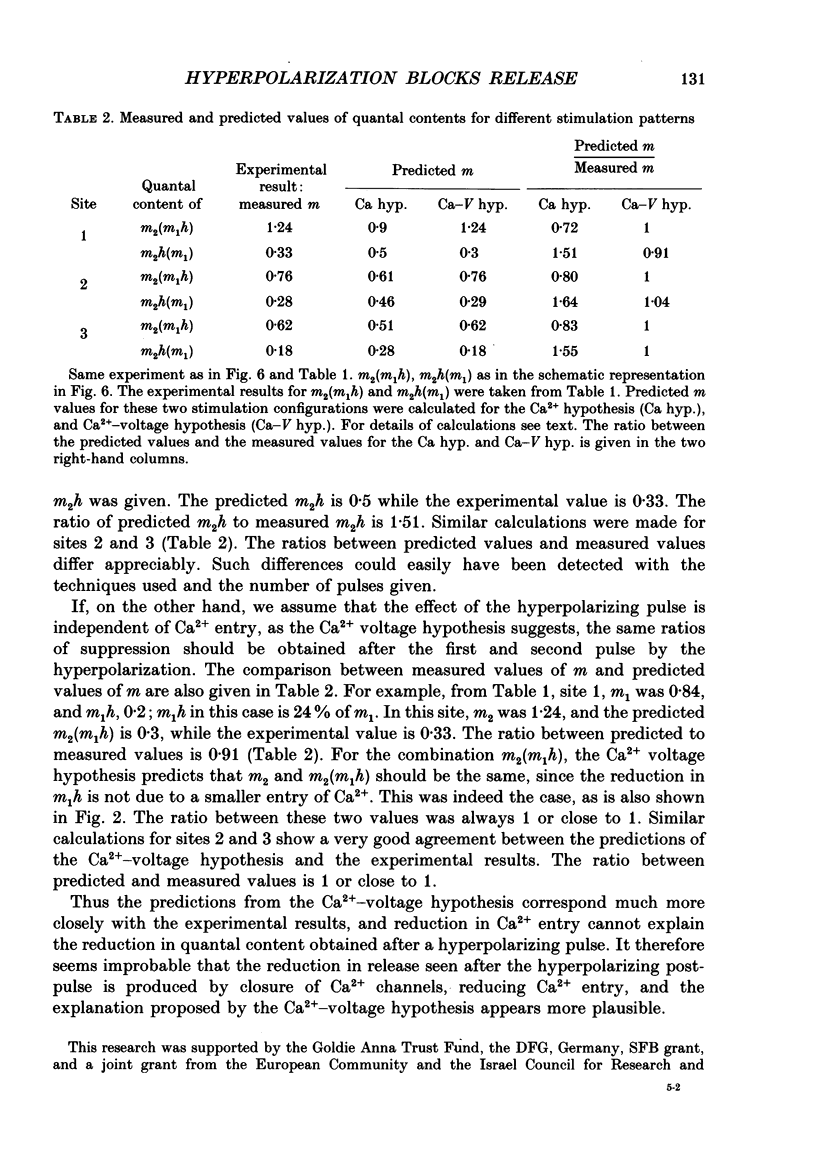
1. Synaptic currents were evoked at the neuromuscular junction of the deep extensor abdominal muscle of the crayfish by direct depolarization of motor nerve endings. 2. Quantal content and time course of neurotransmitter release were determined from delay histograms of unitary release events recorded with a macropatch clamp technique. 3. Synaptic facilitation was elicited by pairing depolarizing pulses at intervals ranging from 10 to 200 ms. At 14 degrees C the duration of facilitation was about 50 ms. Reducing activity of the Nao(+)-Cai2+ exchange by lowering [Na+]o by 50% resulted in prolonged facilitation, which lasted approximately 150 ms. 4. Normalized synaptic delay histograms at normal [Na+]o and 50% [Na+]o were the same for the first and the facilitated second response, indicating that activity of the Na(+)-Ca2+ exchange does not determine the time course of release. 5. The application of a hyperpolarizing post-pulse after the first depolarizing stimulus reduced release and altered its time course to a similar extent both in normal and in 50% [Na+]o. However, it did not affect the level and the time course of release of the facilitated response. 6. A hyperpolarizing post-pulse given after the first and second pulses of a pair reduced release to the same extent for the two depolarizing pulses. 7. These results indicate that whereas manipulations thought to increase [Ca2+]i (i.e. reducing activity of the Nao(+)-Cai2+ exchange or facilitation) affect the quantal content, they do not influence the time course of release. However, changes of membrane potential do affect the quantal content, and more importantly the time course of release, thus suggesting a contributory role of membrane potential in the control of synaptic release.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu R., Barrett E. F. Calcium dependence of evoked transmitter release at very low quantal contents at the frog neuromuscular junction. J Physiol. 1980 Nov;308:79–97. doi: 10.1113/jphysiol.1980.sp013463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium action in synaptic transmitter release. Annu Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Calcium transport and buffering in neurons. Trends Neurosci. 1988 Oct;11(10):438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan C. S., Connor J. A., Kater S. B. Electrically and chemically mediated increases in intracellular calcium in neuronal growth cones. J Neurosci. 1987 Nov;7(11):3588–3599. doi: 10.1523/JNEUROSCI.07-11-03588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Kretz R., Shapiro E. Calcium levels measured in a presynaptic neurone of Aplysia under conditions that modulate transmitter release. J Physiol. 1986 Jun;375:625–642. doi: 10.1113/jphysiol.1986.sp016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Wadman W. J., Hockberger P. E., Wong R. K. Sustained dendritic gradients of Ca2+ induced by excitatory amino acids in CA1 hippocampal neurons. Science. 1988 Apr 29;240(4852):649–653. doi: 10.1126/science.2452481. [DOI] [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel J. Control of quantal transmitter release at frog's motor nerve terminals. II. Modulation by de- or hyperpolarizing pulses. Pflugers Arch. 1984 Nov;402(3):235–243. doi: 10.1007/BF00585505. [DOI] [PubMed] [Google Scholar]
- Dudel J., Parnas I., Parnas H. Neurotransmitter release and its facilitation in crayfish muscle. VI. Release determined by both, intracellular calcium concentration and depolarization of the nerve terminal. Pflugers Arch. 1983 Sep;399(1):1–10. doi: 10.1007/BF00652515. [DOI] [PubMed] [Google Scholar]
- Dudel J. The effect of reduced calcium on quantal unit current and release at the crayfish neuromuscular junction. Pflugers Arch. 1981 Jul;391(1):35–40. doi: 10.1007/BF00580691. [DOI] [PubMed] [Google Scholar]
- Hochner B., Parnas H., Parnas I. Membrane depolarization evokes neurotransmitter release in the absence of calcium entry. Nature. 1989 Nov 23;342(6248):433–435. doi: 10.1038/342433a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Dudel J., Parnas I. Neurotransmitter release and its facilitation in crayfish. I. Saturation kinetics of release, and of entry and removal of calcium. Pflugers Arch. 1982 Mar;393(1):1–14. doi: 10.1007/BF00582384. [DOI] [PubMed] [Google Scholar]
- Parnas H., Dudel J., Parnas I. Neurotransmitter release and its facilitation in crayfish. VII. Another voltage dependent process beside Ca entry controls the time course of phasic release. Pflugers Arch. 1986 Feb;406(2):121–130. doi: 10.1007/BF00586672. [DOI] [PubMed] [Google Scholar]
- Parnas H., Hovav G., Parnas I. Effect of Ca2+ diffusion on the time course of neurotransmitter release. Biophys J. 1989 May;55(5):859–874. doi: 10.1016/S0006-3495(89)82885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H., Parnas I., Segel L. A. On the contribution of mathematical models to the understanding of neurotransmitter release. Int Rev Neurobiol. 1990;32:1–50. doi: 10.1016/s0074-7742(08)60579-6. [DOI] [PubMed] [Google Scholar]
- Parnas H., Segel L. A. A theoretical explanation for some effects of calcium on the facilitation of neurotransmitter release. J Theor Biol. 1980 May 7;84(1):3–29. doi: 10.1016/s0022-5193(80)81035-6. [DOI] [PubMed] [Google Scholar]
- Parnas H., Segel L. A. Facilitation as a tool to study the entry of calcium and the mechanism of neurotransmitter release. Prog Neurobiol. 1989;32(1):1–9. doi: 10.1016/0301-0082(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Parnas I., Atwood H. L. Phasic and tonic neuromuscular systems in the abdominal extensor muscles of the crayfish and rock lobster. Comp Biochem Physiol. 1966 Aug;18(4):701–723. doi: 10.1016/0010-406x(66)90206-4. [DOI] [PubMed] [Google Scholar]
- Parnas I., Parnas H. Calcium is essential but insufficient for neurotransmitter release: the calcium-voltage hypothesis. J Physiol (Paris) 1986;81(4):289–305. [PubMed] [Google Scholar]
- Parnas I., Parnas H., Dudel J. Neurotransmitter release and its facilitation in crayfish. II. Duration of facilitation and removal processes of calcium from the terminal. Pflugers Arch. 1982 May;393(3):232–236. doi: 10.1007/BF00584075. [DOI] [PubMed] [Google Scholar]
- Parnas I., Parnas H., Dudel J. Neurotransmitter release and its facilitation in crayfish. VIII. Modulation of release by hyperpolarizing pulses. Pflugers Arch. 1986 Feb;406(2):131–137. doi: 10.1007/BF00586673. [DOI] [PubMed] [Google Scholar]
- Parnas I., Parnas H. The 'Ca-voltage' hypothesis for neurotransmitter release. Biophys Chem. 1988 Feb;29(1-2):85–93. doi: 10.1016/0301-4622(88)87027-3. [DOI] [PubMed] [Google Scholar]
- Sanchez-Armass S., Blaustein M. P. Role of sodium-calcium exchange in regulation of intracellular calcium in nerve terminals. Am J Physiol. 1987 Jun;252(6 Pt 1):C595–C603. doi: 10.1152/ajpcell.1987.252.6.C595. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Tanabe N., Kijima H. Both augmentation and potentiation occur independently of internal Ca2+ at the frog neuromuscular junction. Neurosci Lett. 1989 Apr 24;99(1-2):147–152. doi: 10.1016/0304-3940(89)90280-2. [DOI] [PubMed] [Google Scholar]
- Tanabe N., Kijima H. Transmitter release at frog end-plate loaded with a Ca2+-chelator, BAPTA: hypertonicity and erythrosin B augment the release independently of internal Ca2+. Neurosci Lett. 1988 Sep 23;92(1):52–57. doi: 10.1016/0304-3940(88)90741-0. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. The calcium hypothesis and modulation of transmitter release by hyperpolarizing pulses. Biophys J. 1987 Aug;52(2):347–350. doi: 10.1016/S0006-3495(87)83222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


