Abstract
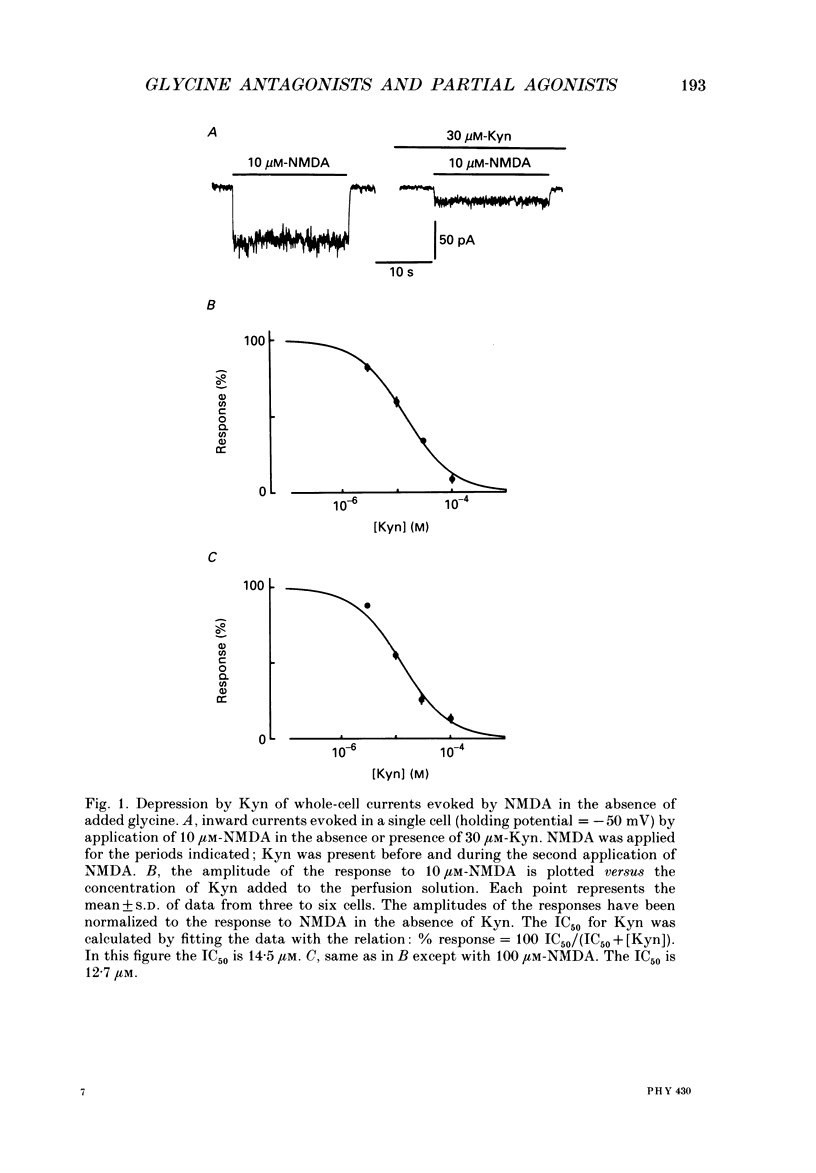
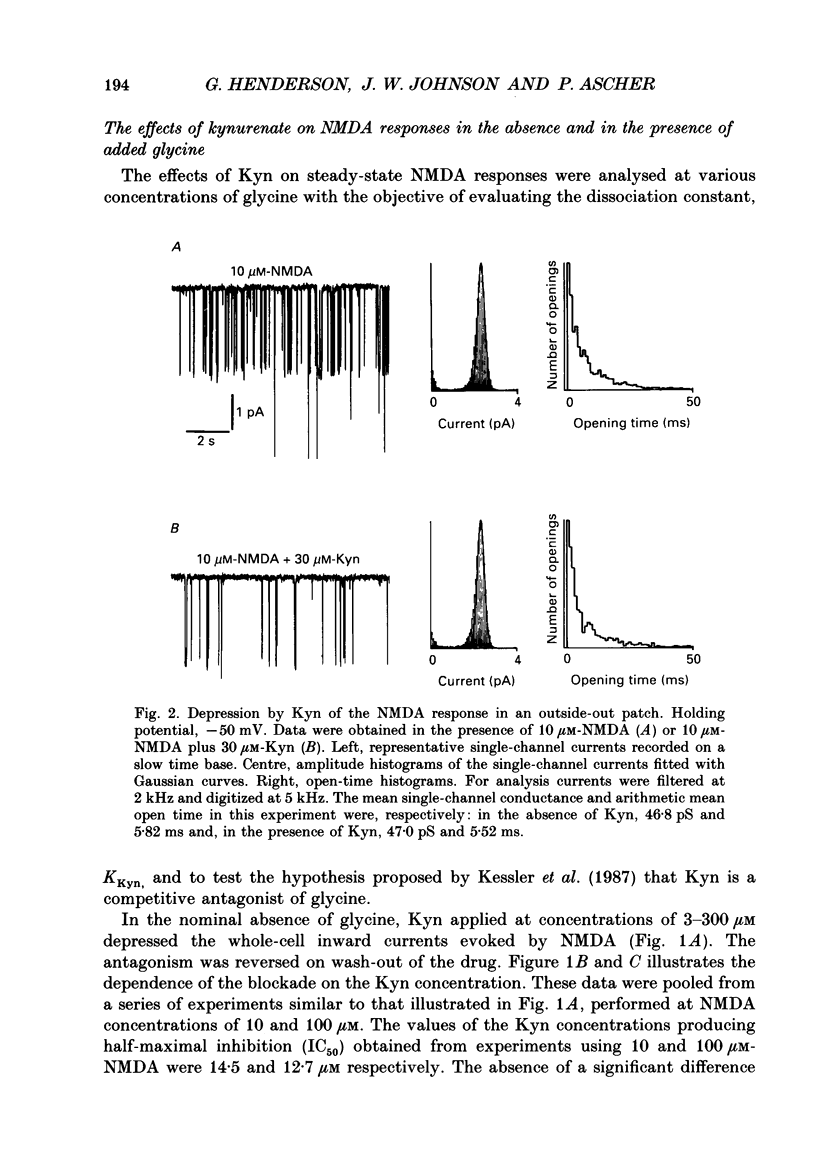
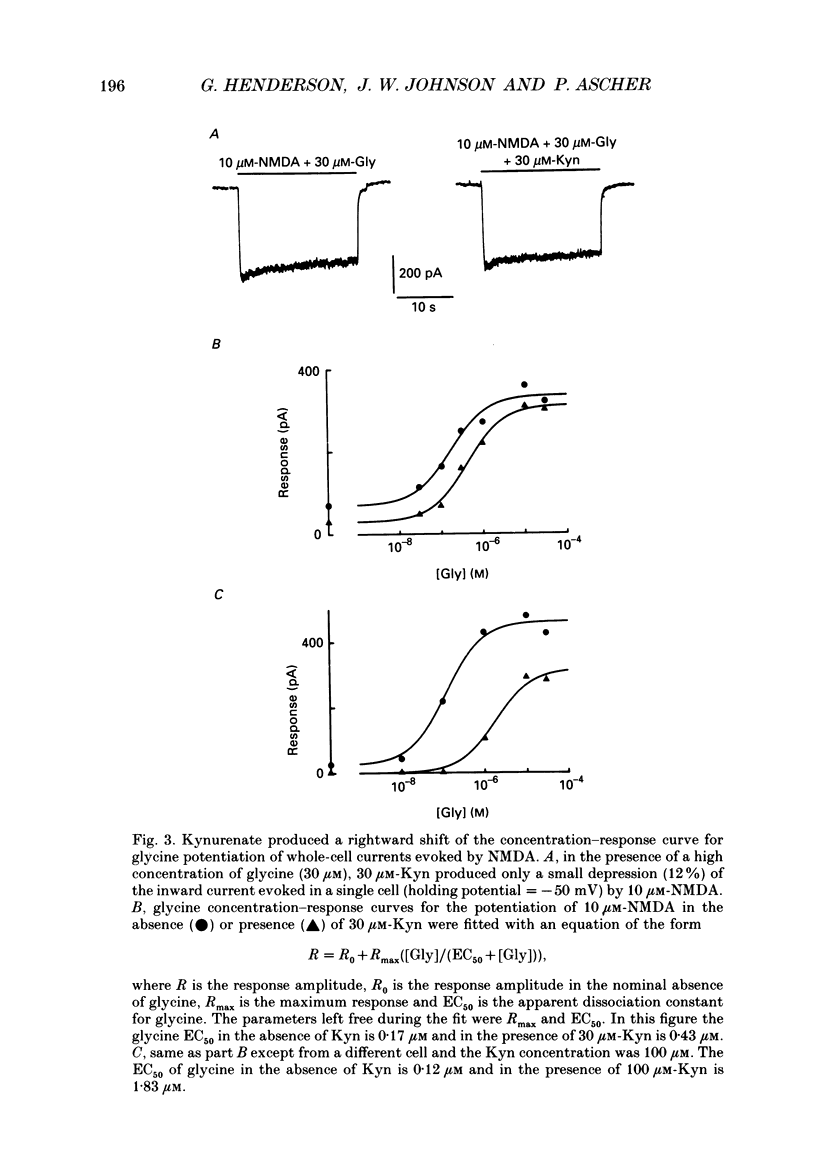
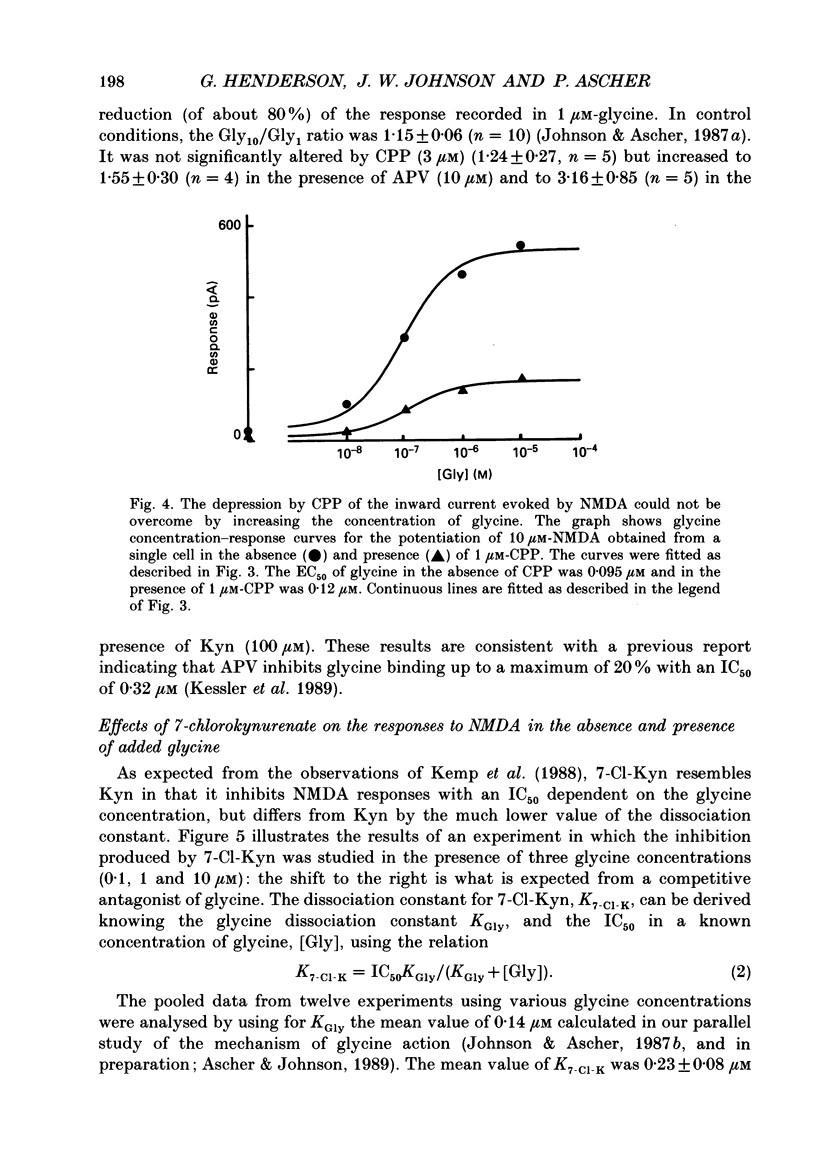
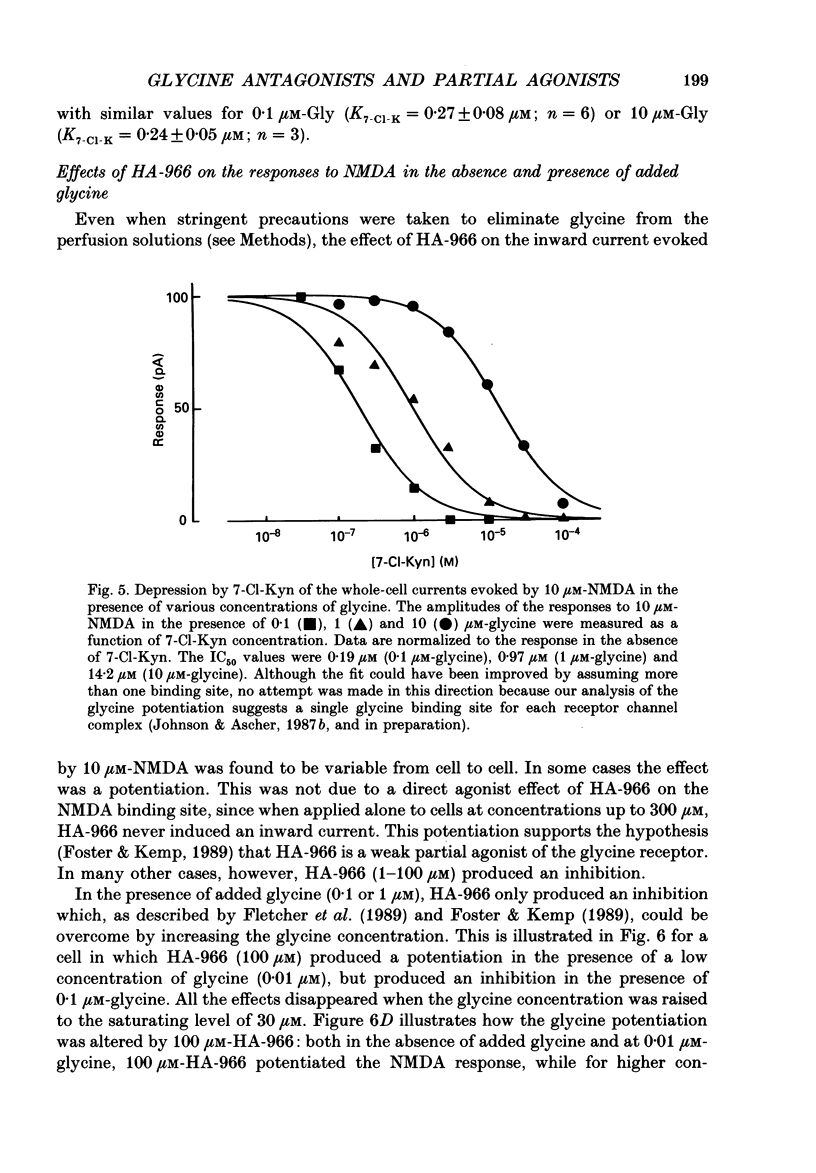
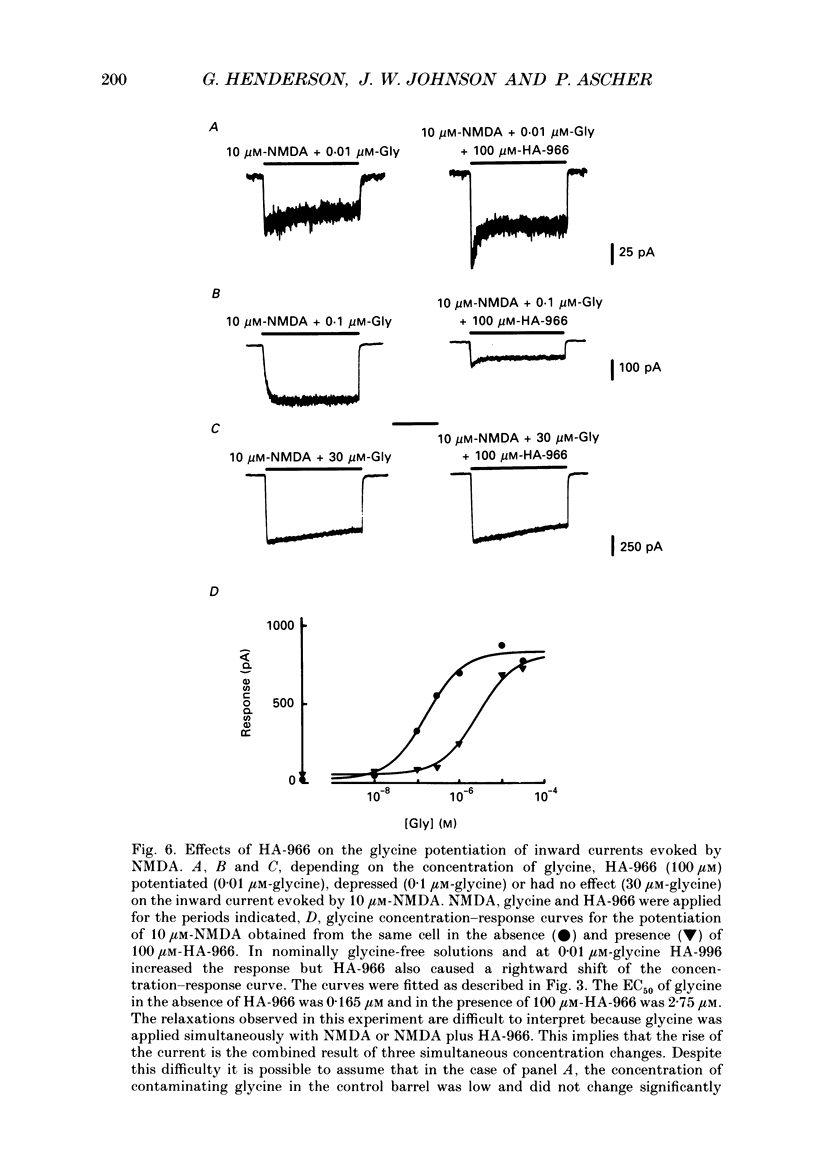
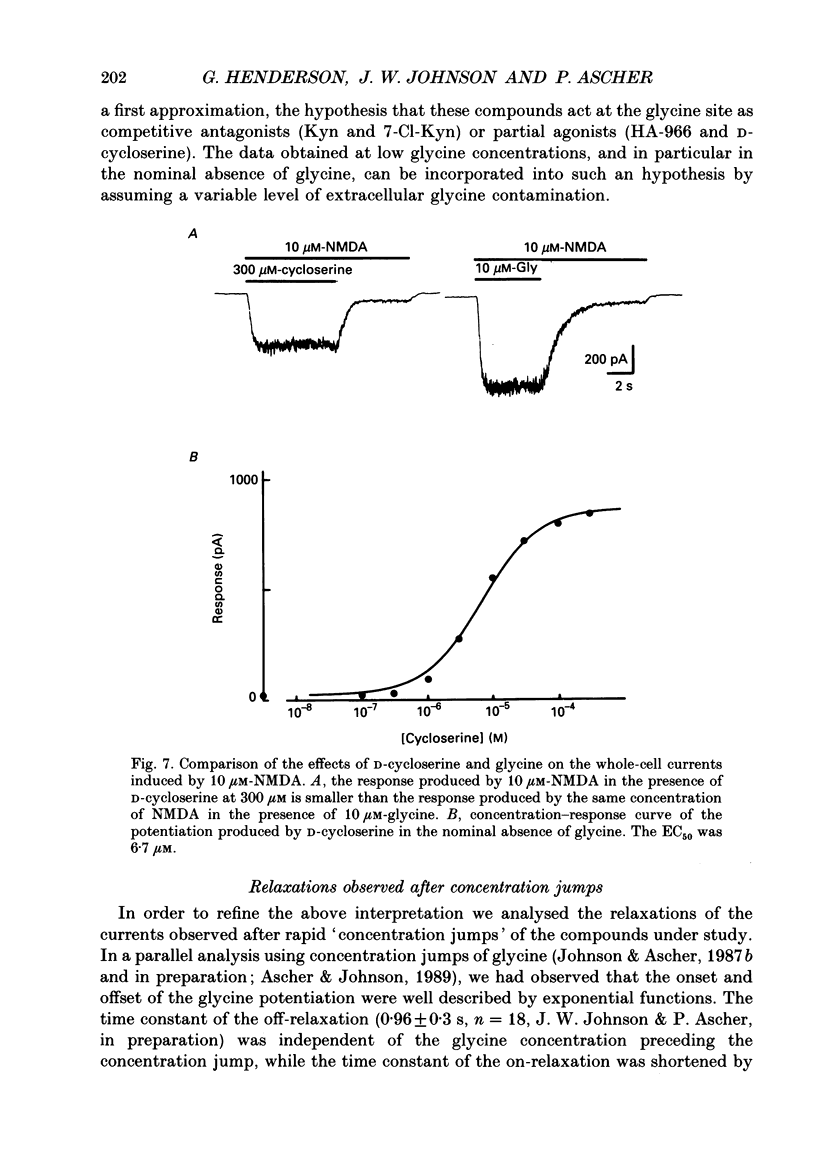
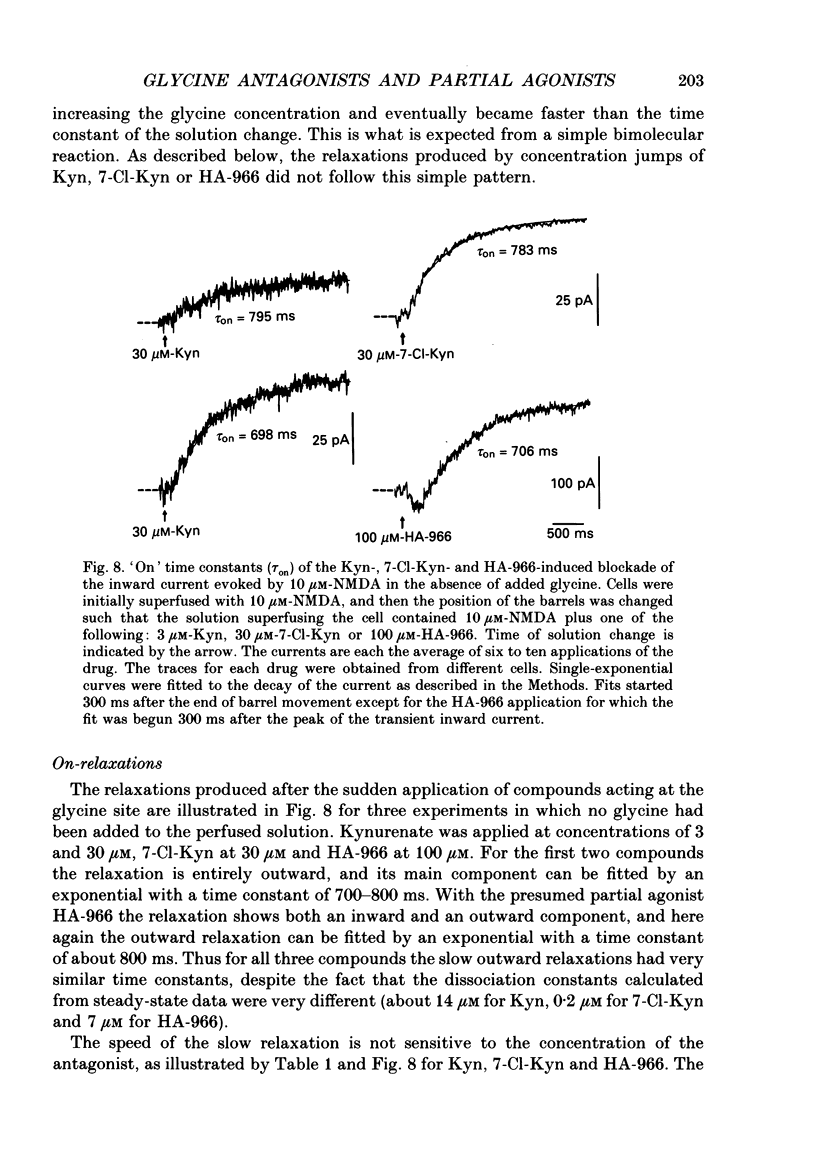
1. Kynurenate (Kyn), 7-chlorokynurenate (7-Cl-Kyn), 3-amino-1-hydroxypyrrolid-2-one (HA-966) and D-cycloserine are known to bind to the glycine site that modulates the N-methyl-D-aspartate (NMDA) response of vertebrate central neurones. The effects of these compounds were investigated with patch-clamp and fast-perfusion techniques on mouse cortical neurones in primary culture in an effort to establish whether they act as antagonists, partial agonists and/or inverse agonists of glycine. A fast drug application method allowed the study of both steady-state and transient responses. 2. The analysis of steady-state responses indicates that the main effects of Kyn and 7-Cl-Kyn are those expected from competitive antagonists of glycine, with a dissociation constant of 15 microM for Kyn, and of 0.3 microM for 7-Cl-Kyn. Concentration jumps indicate that at all concentrations of glycine, and in particular in the absence of added glycine, the blockade by Kyn and 7-Cl-Kyn develops at a rate which is close to the rate of dissociation of glycine from its binding site and is independent of antagonist concentration. 3. The main effects of D-cycloserine and of HA-966 are those of partial agonists of high and low efficacy, respectively. In the absence of added glycine, D-cycloserine always produced a potentiation, while HA-966 produced either a potentiation or an inhibition. This can be explained by assuming the presence of a variable level of contaminating glycine. With both D-cycloserine and HA-966, concentration jumps produced biphasic relaxations in which the onset rate of the slow component was, here again, close to the rate of dissociation of glycine from its binding site. 4. These results can be interpreted by assuming that (1) Kyn and 7-Cl-Kyn are competitive antagonists of glycine, (2) HA-966 and D-cycloserine are partial agonists, (3) in the absence of added glycine some glycine is present in the extracellular solution and (4) the response in the total absence of glycine is very small or negligible.
Full text
PDF







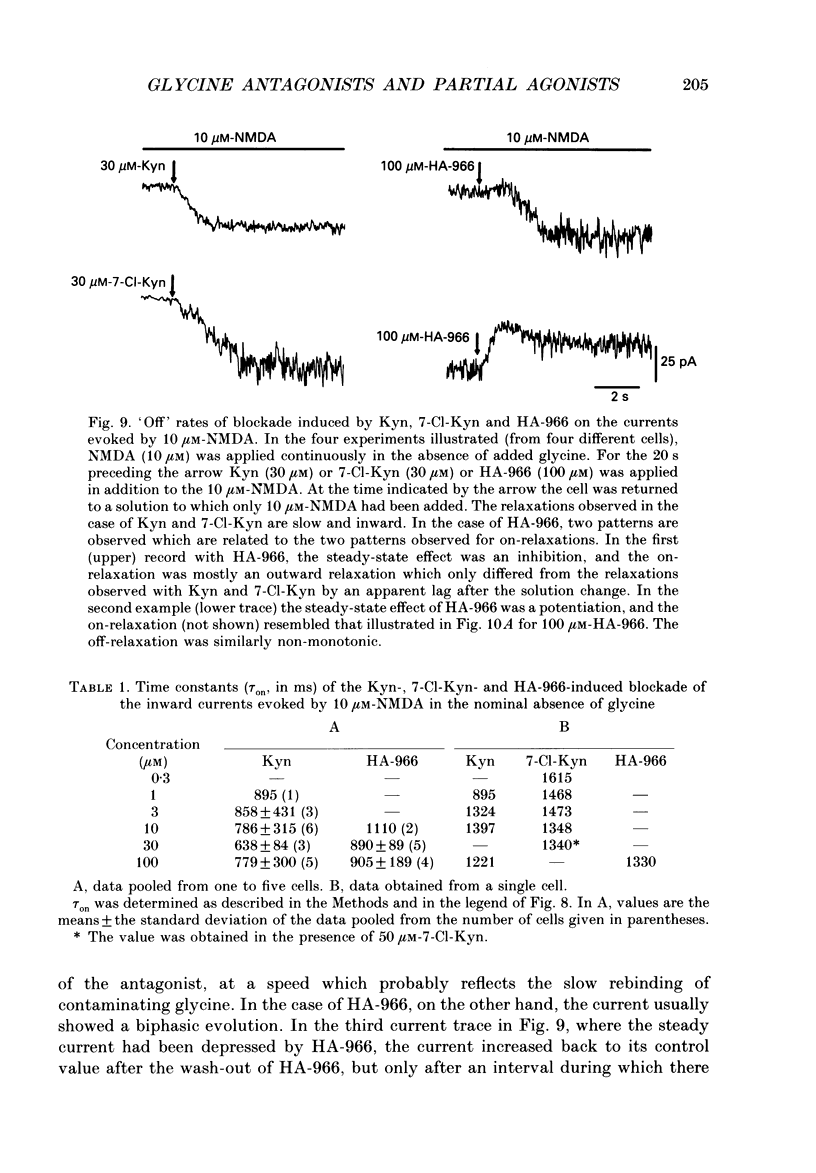
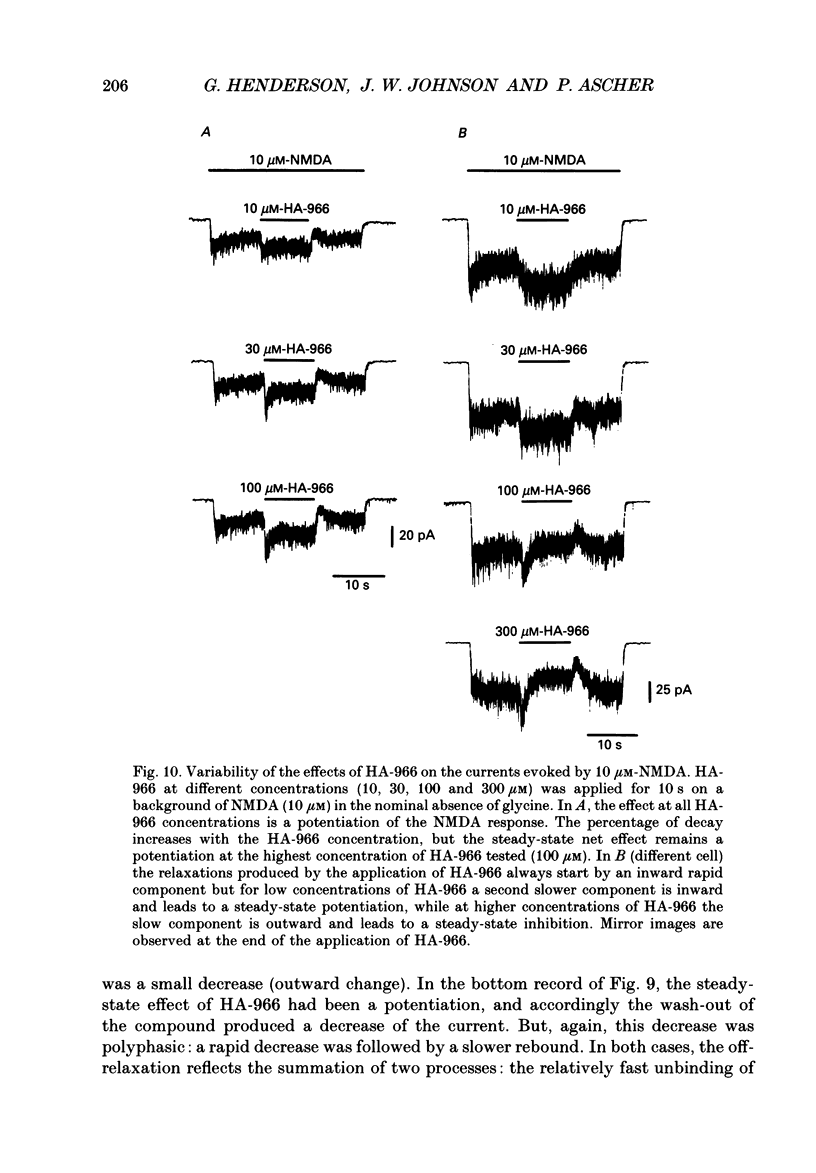






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M., Clements J., Vyklický L., Jr, Mayer M. L. A kinetic analysis of the modulation of N-methyl-D-aspartic acid receptors by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:333–357. doi: 10.1113/jphysiol.1990.sp018215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch P. J., Grossman C. J., Hayes A. G. Kynurenate and FG9041 have both competitive and non-competitive antagonist actions at excitatory amino acid receptors. Eur J Pharmacol. 1988 Jul 7;151(2):313–315. doi: 10.1016/0014-2999(88)90814-x. [DOI] [PubMed] [Google Scholar]
- Birch P. J., Grossman C. J., Hayes A. G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Sep 1;154(1):85–87. doi: 10.1016/0014-2999(88)90367-6. [DOI] [PubMed] [Google Scholar]
- Danysz W., Fadda E., Wroblewski J. T., Costa E. Different modes of action of 3-amino-1-hydroxy-2-pyrrolidone (HA-966) and 7-chlorokynurenic acid in the modulation of N-methyl-D-aspartate-sensitive glutamate receptors. Mol Pharmacol. 1989 Dec;36(6):912–916. [PubMed] [Google Scholar]
- Drejer J., Sheardown M., Nielsen E. O., Honoré T. Glycine reverses the effect of HA-966 on NMDA responses in cultured rat cortical neurons and in chick retina. Neurosci Lett. 1989 Apr 10;98(3):333–338. doi: 10.1016/0304-3940(89)90424-2. [DOI] [PubMed] [Google Scholar]
- Evans R. H., Francis A. A., Watkins J. C. Mg2+-like selective antagonism of excitatory amino acid-induced responses by alpha, epsilon-diaminopimelic acid, D-alpha-aminoadipate and HA-966 in isolated spinal cord of frog and immature rat. Brain Res. 1978 Jun 16;148(2):536–542. doi: 10.1016/0006-8993(78)90744-8. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Fox A. P., Krasne S. Membrane patches and whole-cell membranes: a comparison of electrical properties in rat clonal pituitary (GH3) cells. J Physiol. 1984 Nov;356:565–585. doi: 10.1113/jphysiol.1984.sp015483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher E. J., Millar J. D., Zeman S., Lodge D. Non-competitive antagonism of N-methyl-d-aspartate by displacement of an endogenous glycine-like substance. Eur J Neurosci. 1989;1(3):196–203. doi: 10.1111/j.1460-9568.1989.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Kemp J. A. HA-966 antagonizes N-methyl-D-aspartate receptors through a selective interaction with the glycine modulatory site. J Neurosci. 1989 Jun;9(6):2191–2196. doi: 10.1523/JNEUROSCI.09-06-02191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hood W. F., Compton R. P., Monahan J. B. D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989 Mar 13;98(1):91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Foster A. C., Leeson P. D., Priestley T., Tridgett R., Iversen L. L., Woodruff G. N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Terramani T., Lynch G., Baudry M. A glycine site associated with N-methyl-D-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989 Apr;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Selectivity of quinoxalines and kynurenines as antagonists of the glycine site on N-methyl-D-aspartate receptors. Mol Pharmacol. 1989 Sep;36(3):430–436. [PubMed] [Google Scholar]
- Kloog Y., Lamdani-Itkin H., Sokolovsky M. The glycine site of the N-methyl-D-aspartate receptor channel: differences between the binding of HA-966 and of 7-chlorokynurenic acid. J Neurochem. 1990 May;54(5):1576–1583. doi: 10.1111/j.1471-4159.1990.tb01207.x. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Nowak L. M. Mechanisms of blockade of excitatory amino acid receptor channels. Trends Pharmacol Sci. 1990 Apr;11(4):167–172. doi: 10.1016/0165-6147(90)90070-O. [DOI] [PubMed] [Google Scholar]
- Marvizón J. C., Lewin A. H., Skolnick P. 1-Aminocyclopropane carboxylic acid: a potent and selective ligand for the glycine modulatory site of the N-methyl-D-aspartate receptor complex. J Neurochem. 1989 Mar;52(3):992–994. doi: 10.1111/j.1471-4159.1989.tb02554.x. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- McBain C. J., Kleckner N. W., Wyrick S., Dingledine R. Structural requirements for activation of the glycine coagonist site of N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Mol Pharmacol. 1989 Oct;36(4):556–565. [PubMed] [Google Scholar]
- Monahan J. B., Corpus V. M., Hood W. F., Thomas J. W., Compton R. P. Characterization of a [3H]glycine recognition site as a modulatory site of the N-methyl-D-aspartate receptor complex. J Neurochem. 1989 Aug;53(2):370–375. doi: 10.1111/j.1471-4159.1989.tb07344.x. [DOI] [PubMed] [Google Scholar]
- Nadler V., Kloog Y., Sokolovsky M. 1-Aminocyclopropane-1-carboxylic acid (ACC) mimics the effects of glycine on the NMDA receptor ion channel. Eur J Pharmacol. 1988 Nov 15;157(1):115–116. doi: 10.1016/0014-2999(88)90478-5. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. [3H]D-2-amino-5-phosphonopentanoate as a ligand for N-methyl-D-aspartate receptors in the mammalian central nervous system. Neuroscience. 1988 Jul;26(1):1–15. doi: 10.1016/0306-4522(88)90123-6. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Stone T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982 Sep 9;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J., Harris K. M., Miller R. J. NMDA receptor antagonists that bind to the strychnine-insensitive glycine site and inhibit NMDA-induced Ca2+ fluxes and [3H]GABA release. Eur J Pharmacol. 1989 Mar 7;172(1):9–17. doi: 10.1016/0922-4106(89)90040-0. [DOI] [PubMed] [Google Scholar]
- STEPHENSON R. P. A modification of receptor theory. Br J Pharmacol Chemother. 1956 Dec;11(4):379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather W., Johnson J. W., Henderson G., Ascher P. Glycine-insensitive desensitization of NMDA responses in cultured mouse embryonic neurons. Neuron. 1990 May;4(5):725–731. doi: 10.1016/0896-6273(90)90198-o. [DOI] [PubMed] [Google Scholar]
- Sigel E., Baur R. Allosteric modulation by benzodiazepine receptor ligands of the GABAA receptor channel expressed in Xenopus oocytes. J Neurosci. 1988 Jan;8(1):289–295. doi: 10.1523/JNEUROSCI.08-01-00289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Donald A. E., Foster A. C., Hutson P. H., Iversen L. L., Iversen S. D., Kemp J. A., Leeson P. D., Marshall G. R., Oles R. J. Enantiomers of HA-966 (3-amino-1-hydroxypyrrolid-2-one) exhibit distinct central nervous system effects: (+)-HA-966 is a selective glycine/N-methyl-D-aspartate receptor antagonist, but (-)-HA-966 is a potent gamma-butyrolactone-like sedative. Proc Natl Acad Sci U S A. 1990 Jan;87(1):347–351. doi: 10.1073/pnas.87.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Trautmann A. A patch-clamp study of the partial agonist actions of tubocurarine on rat myotubes. J Physiol. 1984 Apr;349:353–374. doi: 10.1113/jphysiol.1984.sp015160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]


