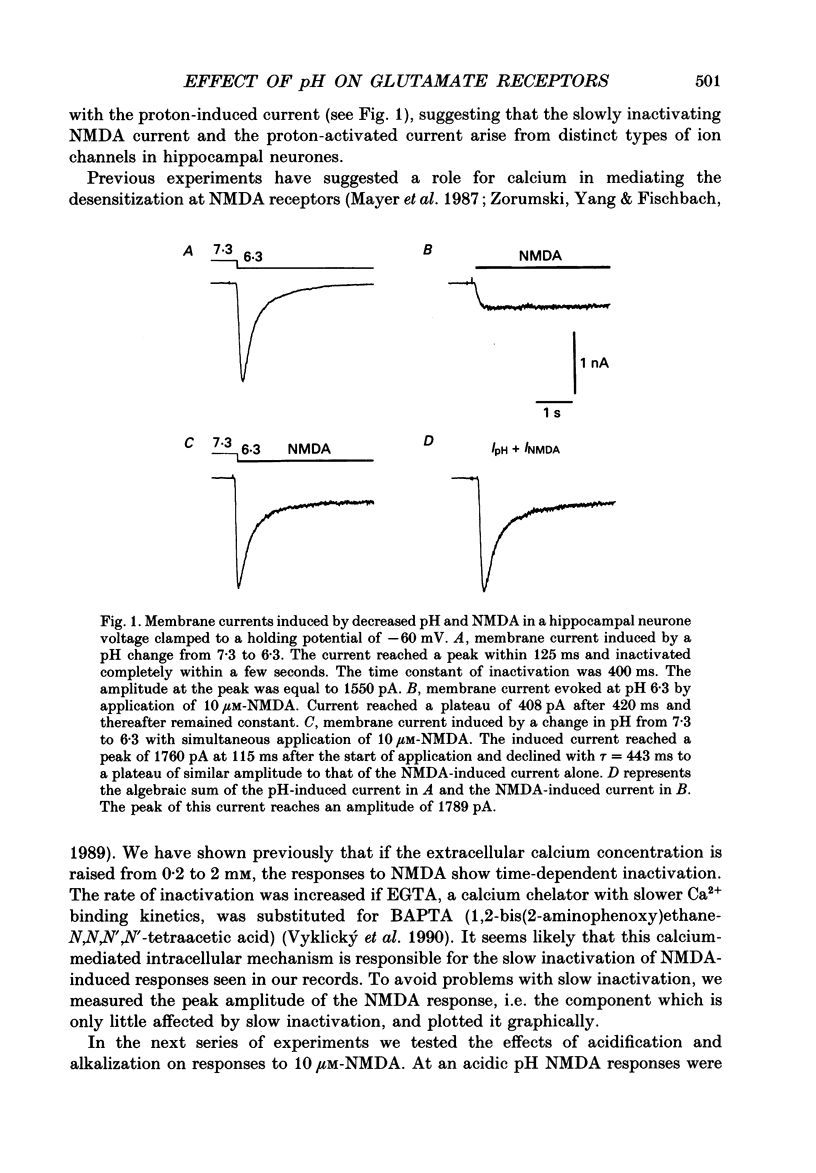
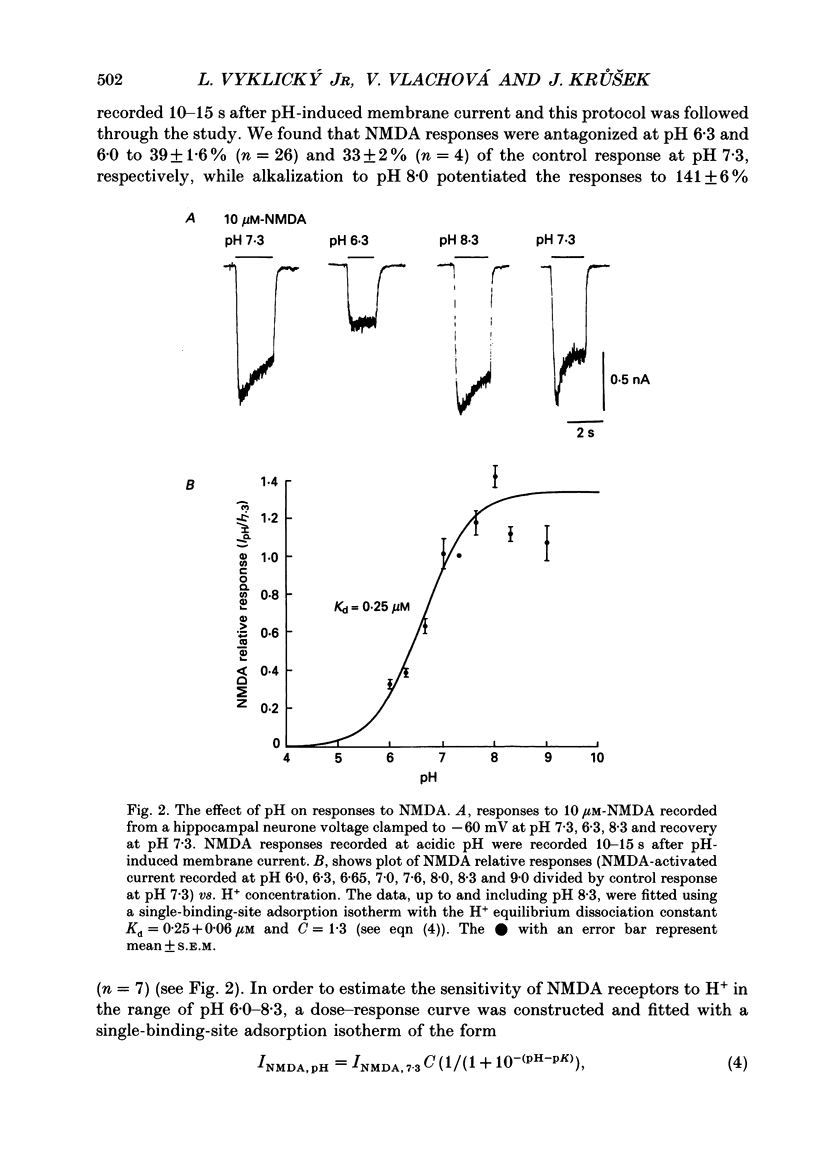
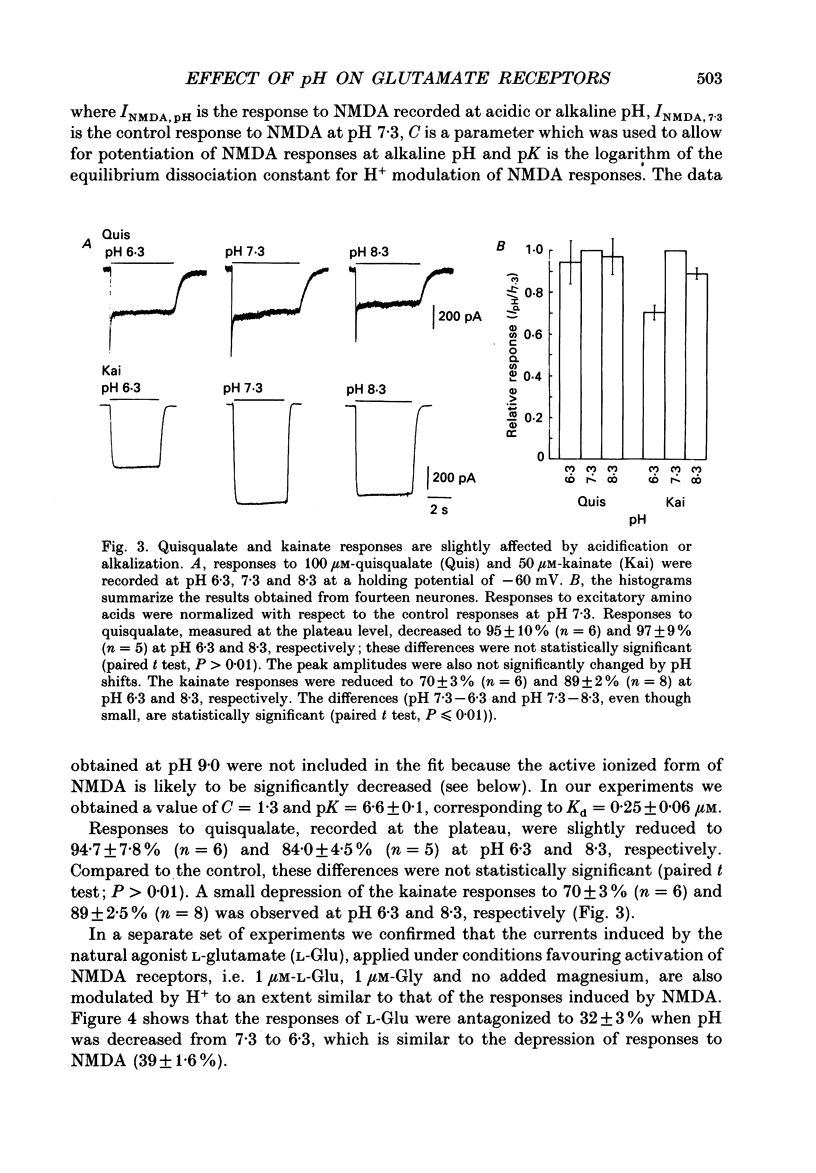
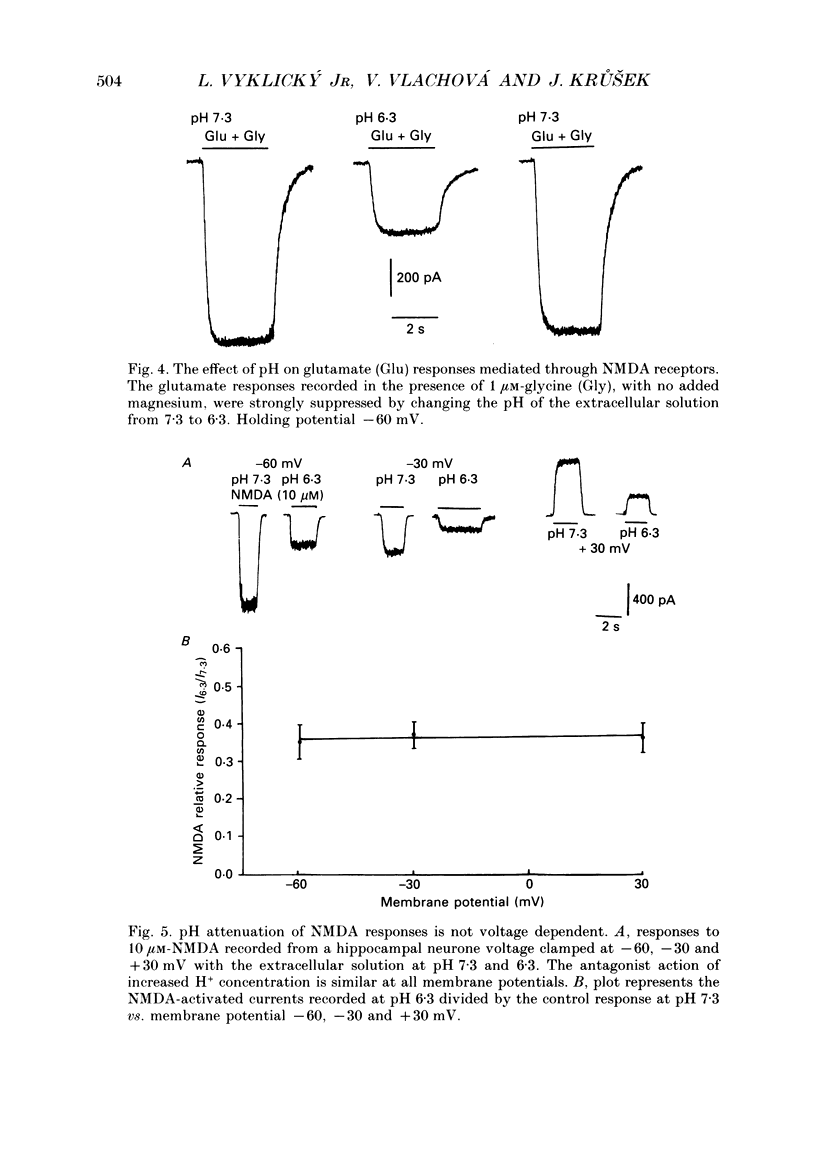
Abstract
1. The whole-cell and outside-out configurations of the patch-clamp technique were used to record responses to excitatory amino acids in mouse hippocampal neurones in cell culture at different pH. The amino acids kainate, quisqualate, N-methyl-D-aspartate (NMDA) and L-glutamate were applied by a rapid perfusion system. 2. In the whole-cell recording mode the responses to NMDA or to low concentrations of glutamate, recorded in the absence of Mg2+ and with glycine in the extracellular superfusion solution, were antagonized by acidic pH and potentiated by an alkaline extracellular solution. Decrease in pH from 7.3 to 6.0 reduced NMDA responses to 33 +/- 2% and an increase in pH from 7.3 to 8.0 potentiated it to 141 +/- 6%. The responses to quisqualate and kainate were only slightly changed by altering the pH from 7.3 to 6.3 or 8.3. 3. The equilibrium dissociation constant (Kd) for H+ antagonism of responses to NMDA, estimated from the fit of a single-binding-site adsorption isotherm, was calculated to be 0.25 +/- 0.06 microM, corresponding to pH 6.6 +/- 0.1. The H+ attenuation of NMDA current was voltage independent at membrane potentials -60 to +30 mV. 4. H+ antagonism of responses to NMDA was reduced when the NMDA concentration was lowered. In the pH range 6.3-8.3 the H(+)-induced reduction did not vary with the concentration of glycine or Mg2+. The sensitivity of NMDA current to Zn2+ was unchanged in the pH range 6.3 +/- 8.0. These results suggest that H+ ions do not directly interfere with the binding of NMDA to its agonist recognition site or with the binding of glycine, Mg2+ and Zn2+ to the specific allosteric sites on the NMDA receptor-channel complex. 5. In outside-out patches held at -60 mV, unitary NMDA-activated currents were recorded at pH 7.3 and 6.3. The mean NMDA single-channel conductance (gamma) obtained for the largest and most frequent openings were: gamma 7.3 = 52.5 +/- 0.8 pS and gamma 6.3 = 51.8 +/- 0.9 pS. The duration of the mean channel open time, tau o, decreased from 4.75 +/- 0.25 ms in the control at pH 7.3 to 3.59 +/- 0.21 ms at pH 6.3. The mean burst duration, tau b, was reduced from 8.51 +/- 0.78 ms at control pH 7.3 to 5.1 +/- 0.34 ms at pH 6.3. The frequency of NMDA channel bursts was reduced by 31%.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



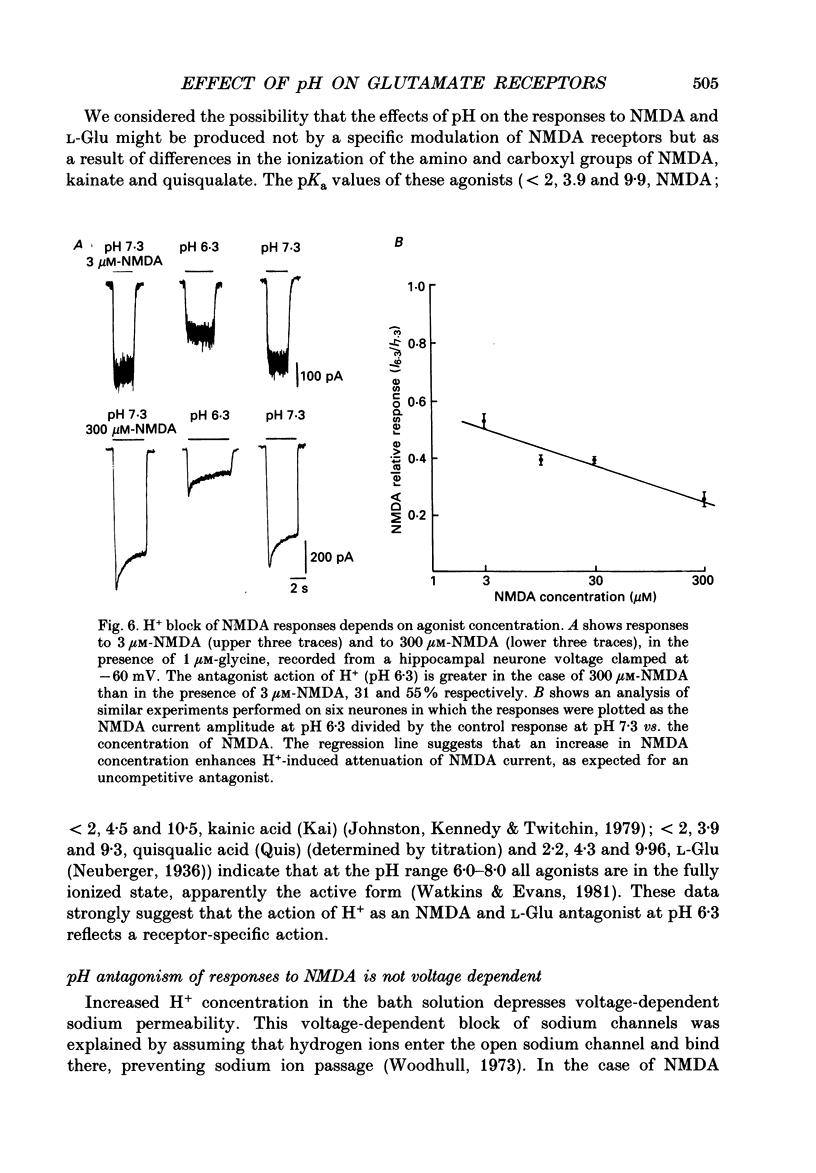
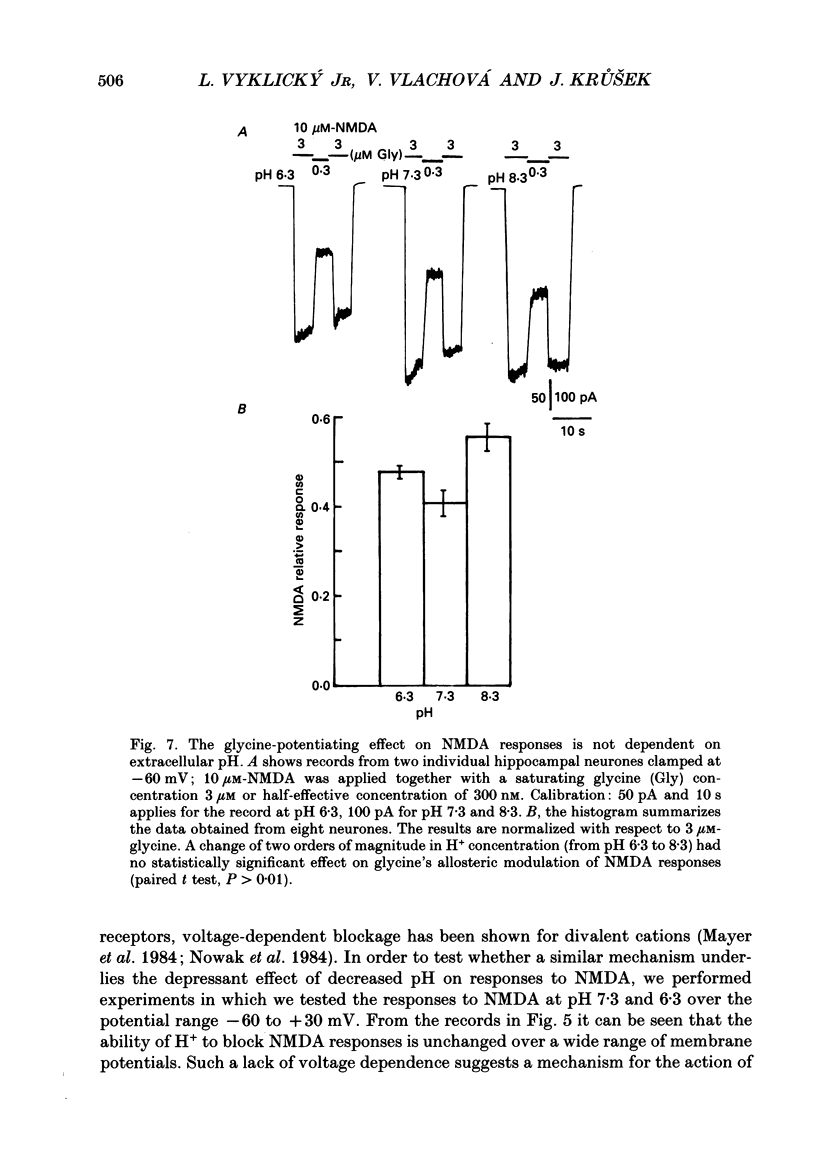

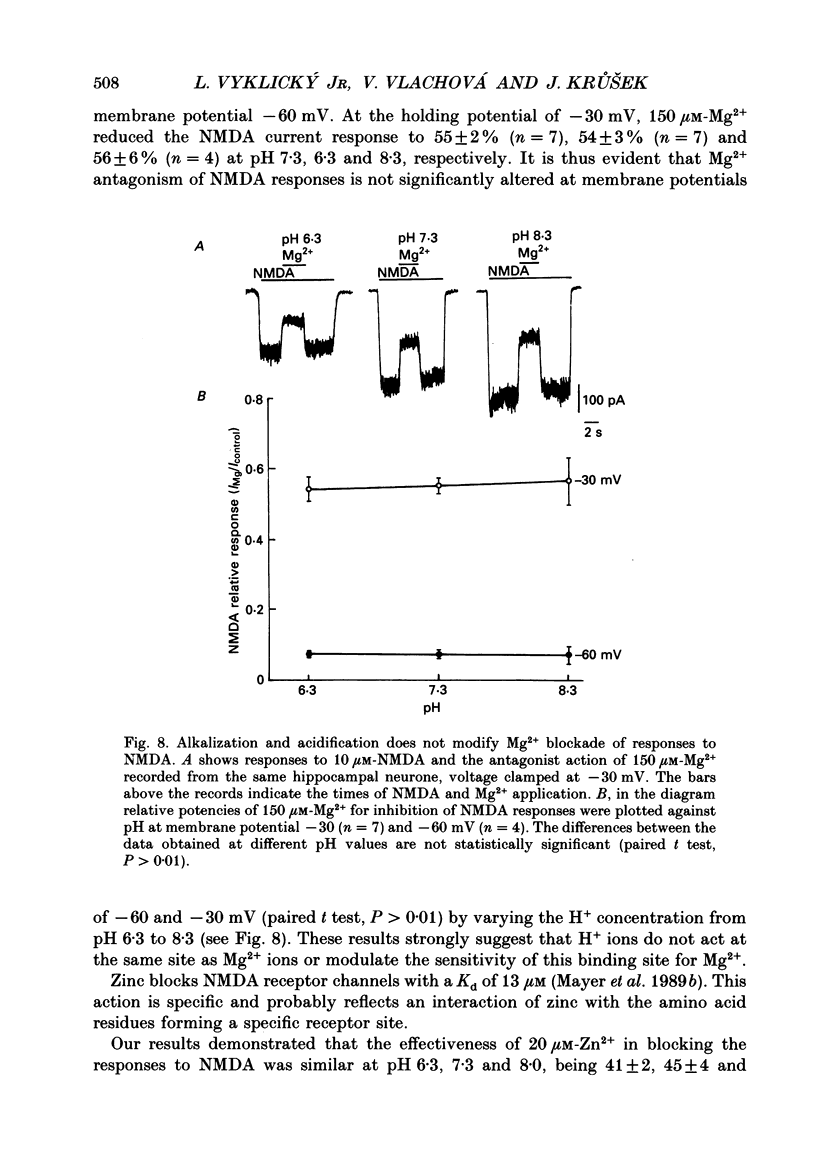
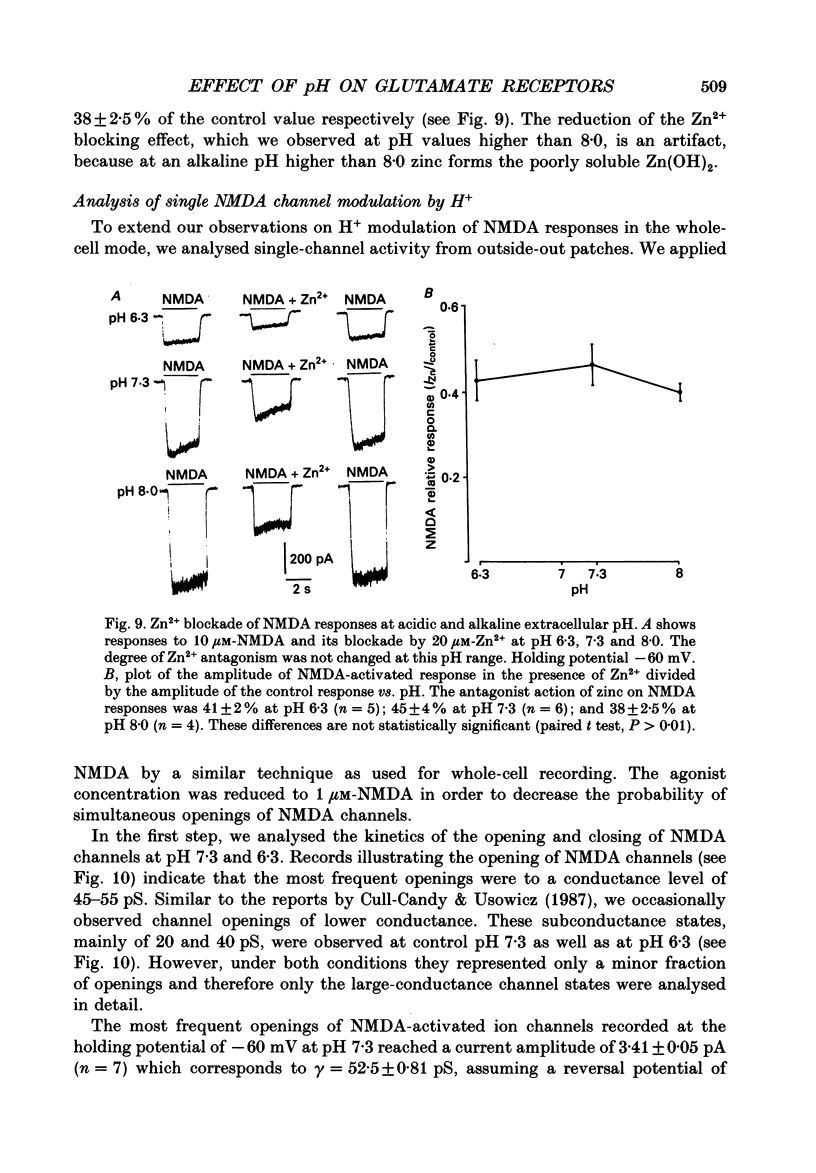






Selected References
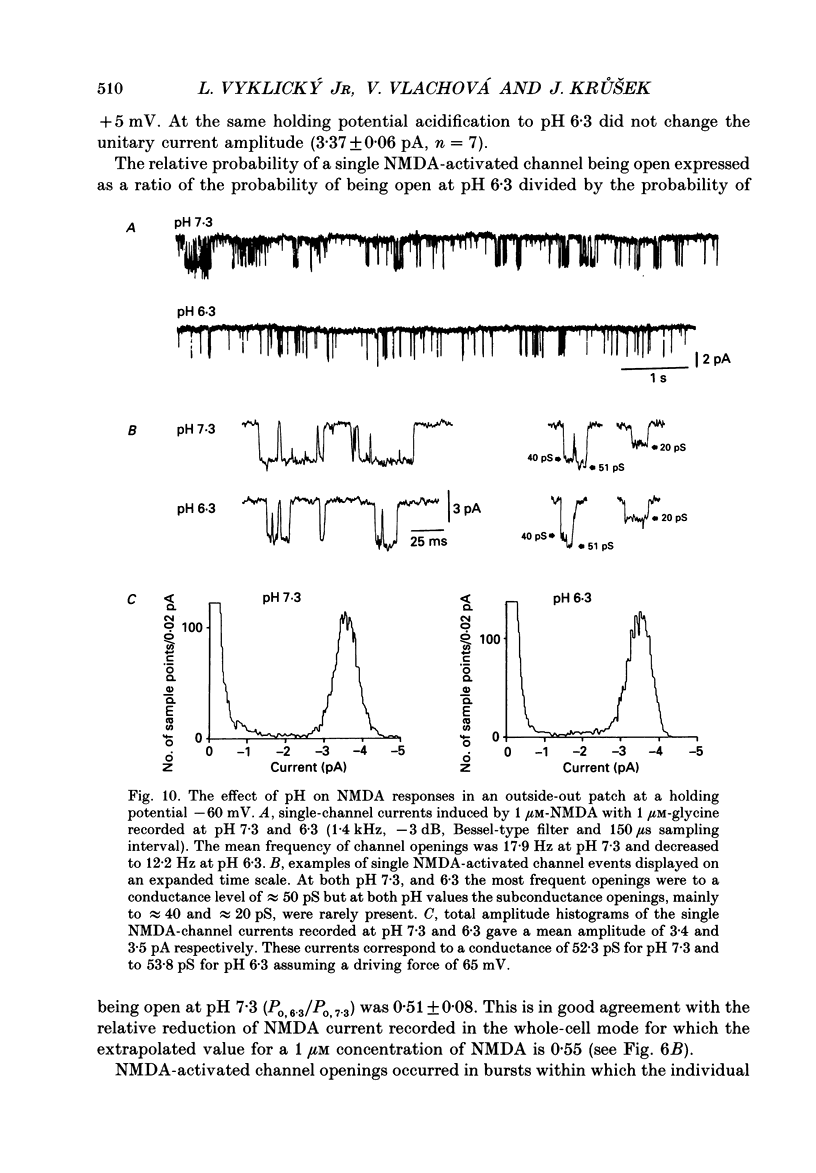
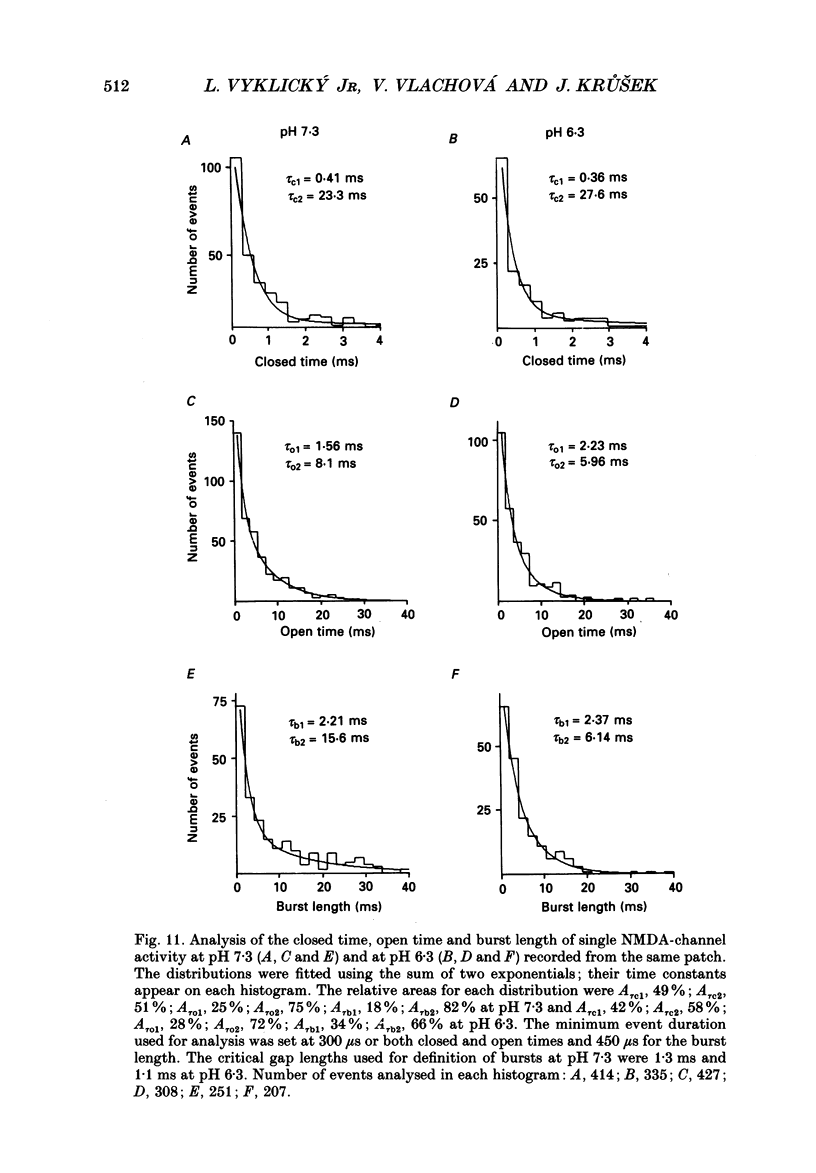
These references are in PubMed. This may not be the complete list of references from this article.
- Anis N. A., Berry S. C., Burton N. R., Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983 Jun;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Ascher P., Bregestovski P., Nowak L. N-methyl-D-aspartate-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988 May;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987 Feb 5;325(6104):525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. doi: 10.1016/0165-0173(84)90020-1. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Lux H. D. Similarity and mutual exclusion of NMDA- and proton-activated transient Na+-currents in rat tectal neurons. Neurosci Lett. 1988 Jun 29;89(2):198–203. doi: 10.1016/0304-3940(88)90381-3. [DOI] [PubMed] [Google Scholar]
- Guthrie P. B., Brenneman D. E., Neale E. A. Morphological and biochemical differences expressed in separate dissociated cell cultures of dorsal and ventral halves of the mouse spinal cord. Brain Res. 1987 Sep 15;420(2):313–323. doi: 10.1016/0006-8993(87)91252-2. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris R. J., Richards P. G., Symon L., Habib A. H., Rosenstein J. pH, K+, and PO2 of the extracellular space during ischaemia of primate cerebral cortex. J Cereb Blood Flow Metab. 1987 Oct;7(5):599–604. doi: 10.1038/jcbfm.1987.111. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Colquhoun D., Cull-Candy S. G. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988 May 23;233(1273):407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Kennedy S. M., Twitchin B. Action of the neurotoxin kainic acid on high affinity uptake of L-glutamic acid in rat brain slices. J Neurochem. 1979 Jan;32(1):121–127. doi: 10.1111/j.1471-4159.1979.tb04518.x. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Foster A. C., Leeson P. D., Priestley T., Tridgett R., Iversen L. L., Woodruff G. N. 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6547–6550. doi: 10.1073/pnas.85.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig R. P., Ferreira-Filho C. R., Nicholson C. Alkaline and acid transients in cerebellar microenvironment. J Neurophysiol. 1983 Mar;49(3):831–850. doi: 10.1152/jn.1983.49.3.831. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., MacDermott A. B., Westbrook G. L., Smith S. J., Barker J. L. Agonist- and voltage-gated calcium entry in cultured mouse spinal cord neurons under voltage clamp measured using arsenazo III. J Neurosci. 1987 Oct;7(10):3230–3244. doi: 10.1523/JNEUROSCI.07-10-03230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Westbrook G. L. Modulation of excitatory amino acid receptors by group IIB metal cations in cultured mouse hippocampal neurones. J Physiol. 1989 Aug;415:329–350. doi: 10.1113/jphysiol.1989.sp017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Vyklický L., Jr Sites of antagonist action on N-methyl-D-aspartic acid receptors studied using fluctuation analysis and a rapid perfusion technique. J Neurophysiol. 1988 Aug;60(2):645–663. doi: 10.1152/jn.1988.60.2.645. [DOI] [PubMed] [Google Scholar]
- Mody I., Salter M. W., MacDonald J. F. Requirement of NMDA receptor/channels for intracellular high-energy phosphates and the extent of intraneuronal calcium buffering in cultured mouse hippocampal neurons. Neurosci Lett. 1988 Oct 31;93(1):73–78. doi: 10.1016/0304-3940(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mutch W. A., Hansen A. J. Extracellular pH changes during spreading depression and cerebral ischemia: mechanisms of brain pH regulation. J Cereb Blood Flow Metab. 1984 Mar;4(1):17–27. doi: 10.1038/jcbfm.1984.3. [DOI] [PubMed] [Google Scholar]
- Neuberger A. Dissociation constants and structures of glutamic acid and its esters. Biochem J. 1936 Nov;30(11):2085–2094. doi: 10.1042/bj0302085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Koh J., Choi D. W. Zinc selectively blocks the action of N-methyl-D-aspartate on cortical neurons. Science. 1987 May 1;236(4801):589–593. doi: 10.1126/science.2883728. [DOI] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Direct measurement of proton transfer rates to a group controlling the dihydropyridine-sensitive Ca2+ channel. Nature. 1987 Sep 17;329(6136):243–246. doi: 10.1038/329243a0. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P., Hahn S. Ketamine-xylazine anaesthesia blocks consolidation of ocular dominance changes in kitten visual cortex. Nature. 1987 Mar 12;326(6109):183–185. doi: 10.1038/326183a0. [DOI] [PubMed] [Google Scholar]
- Sernagor E., Kuhn D., Vyklicky L., Jr, Mayer M. L. Open channel block of NMDA receptor responses evoked by tricyclic antidepressants. Neuron. 1989 Mar;2(3):1221–1227. doi: 10.1016/0896-6273(89)90306-1. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab. 1989 Apr;9(2):127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., von Hanwehr R., Nergelius G., Nevander G., Ingvar M. Extra- and intracellular pH in the brain during seizures and in the recovery period following the arrest of seizure activity. J Cereb Blood Flow Metab. 1985 Mar;5(1):47–57. doi: 10.1038/jcbfm.1985.7. [DOI] [PubMed] [Google Scholar]
- Urbanics R., Leniger-Follert E., Lübbers D. W. Time course of changes of extracellular H+ and K+ activities during and after direct electrical stimulation of the brain cortex. Pflugers Arch. 1978 Dec 15;378(1):47–53. doi: 10.1007/BF00581957. [DOI] [PubMed] [Google Scholar]
- Vyklický L., Jr, Benveniste M., Mayer M. L. Modulation of N-methyl-D-aspartic acid receptor desensitization by glycine in mouse cultured hippocampal neurones. J Physiol. 1990 Sep;428:313–331. doi: 10.1113/jphysiol.1990.sp018214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Westbrook G. L., Mayer M. L. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987 Aug 13;328(6131):640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C. F., Yang J., Fischbach G. D. Calcium-dependent, slow desensitization distinguishes different types of glutamate receptors. Cell Mol Neurobiol. 1989 Mar;9(1):95–104. doi: 10.1007/BF00711446. [DOI] [PubMed] [Google Scholar]
- von Hanwehr R., Smith M. L., Siesjö B. K. Extra- and intracellular pH during near-complete forebrain ischemia in the rat. J Neurochem. 1986 Feb;46(2):331–339. doi: 10.1111/j.1471-4159.1986.tb12973.x. [DOI] [PubMed] [Google Scholar]


