Abstract
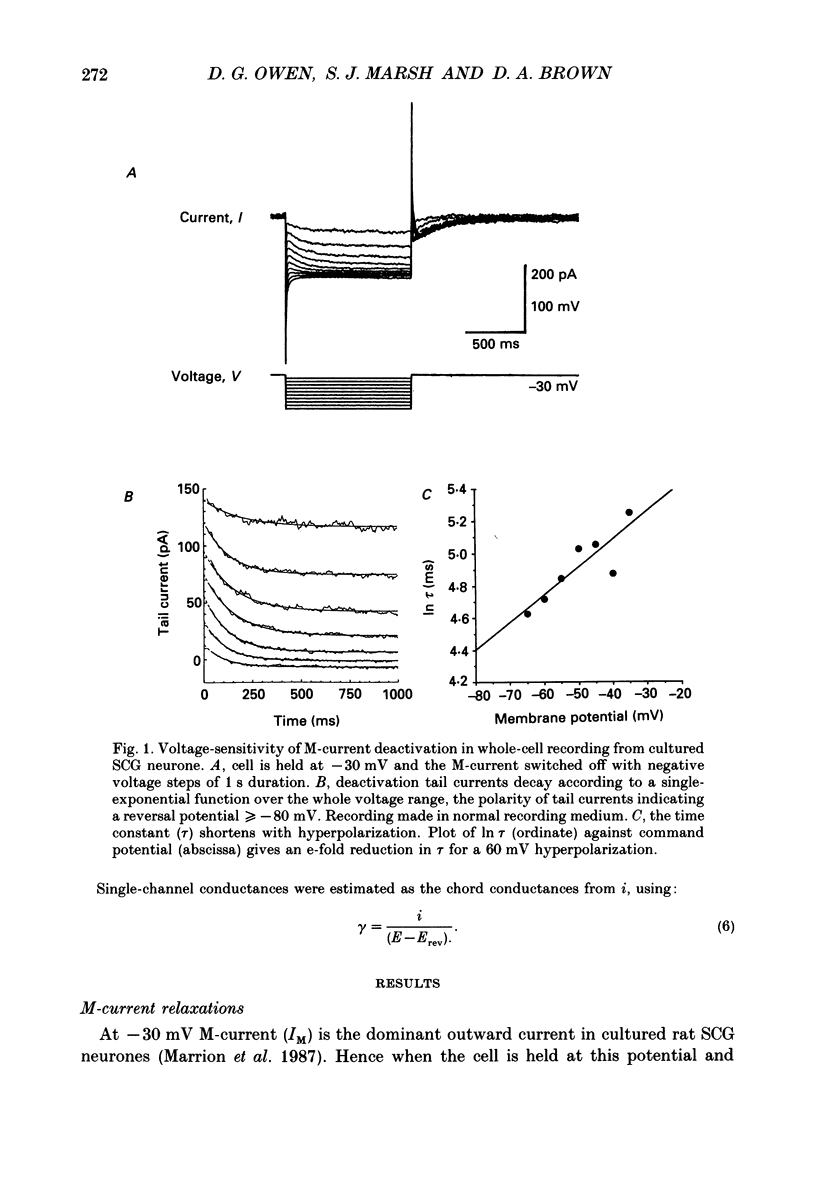
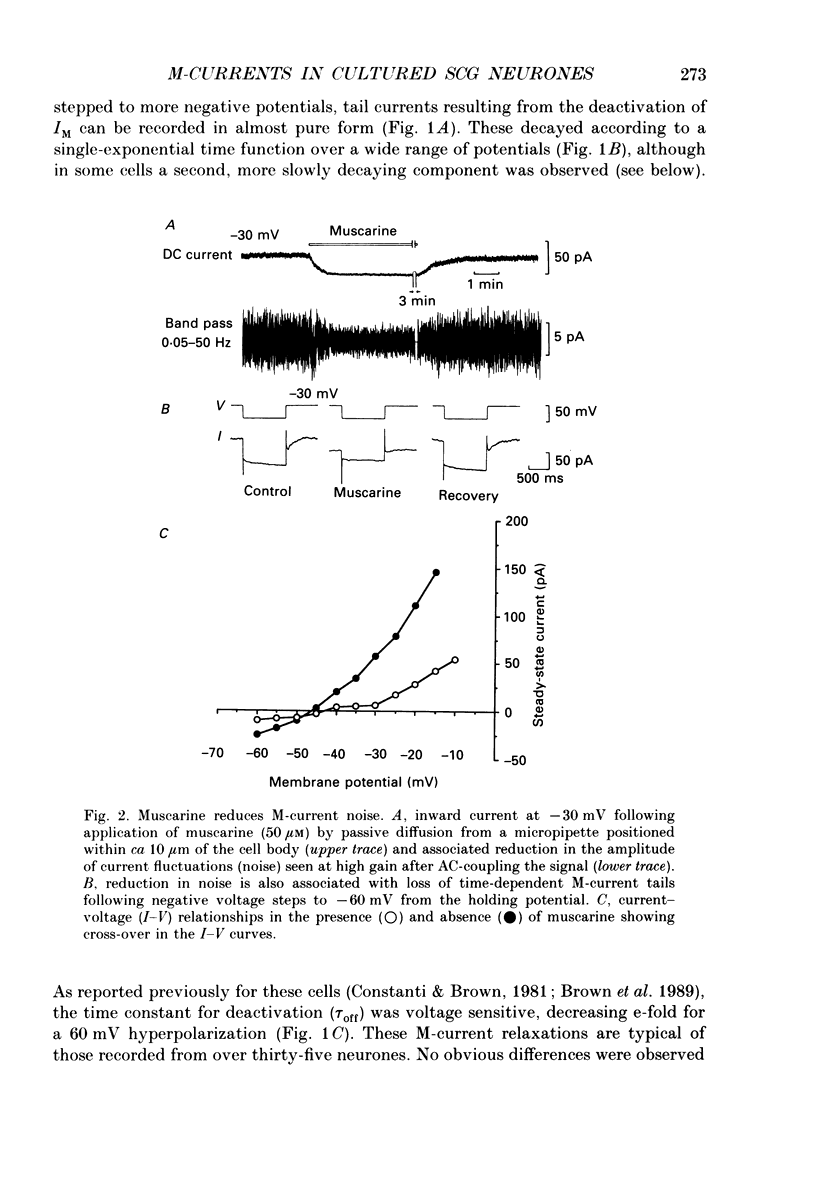
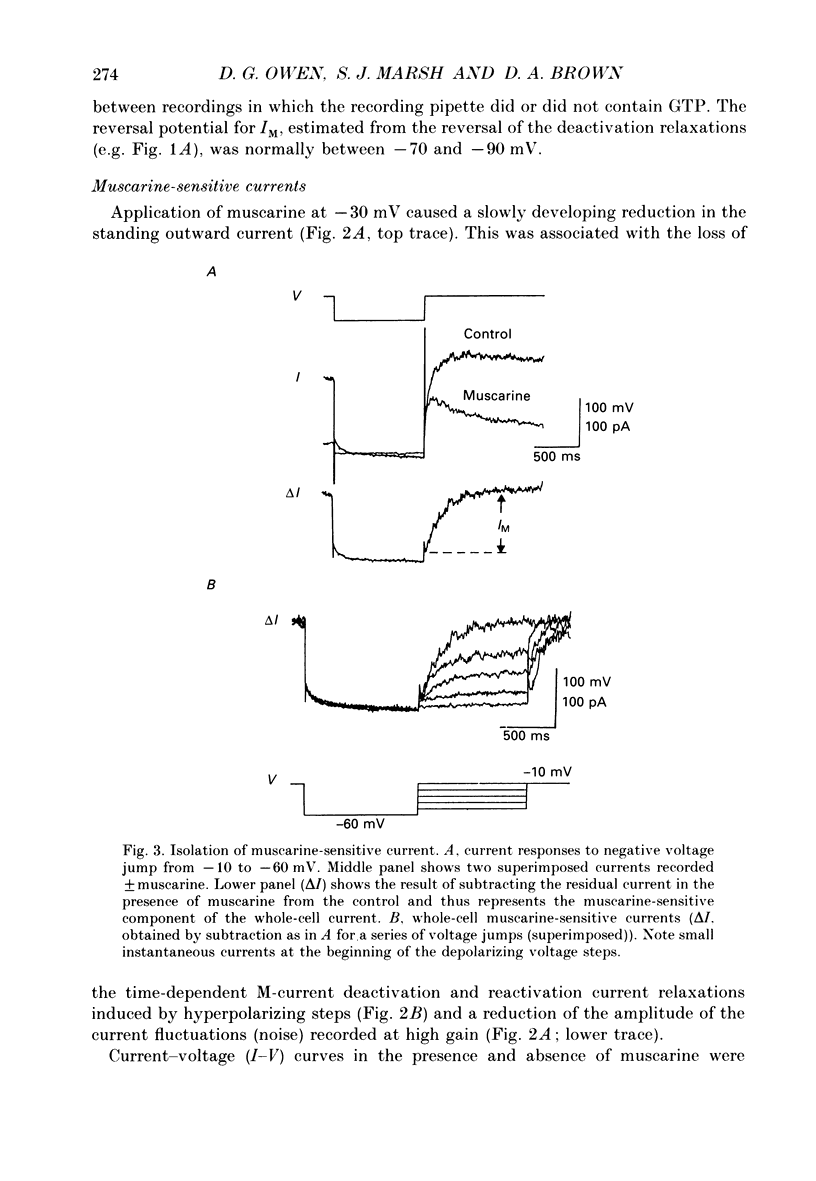
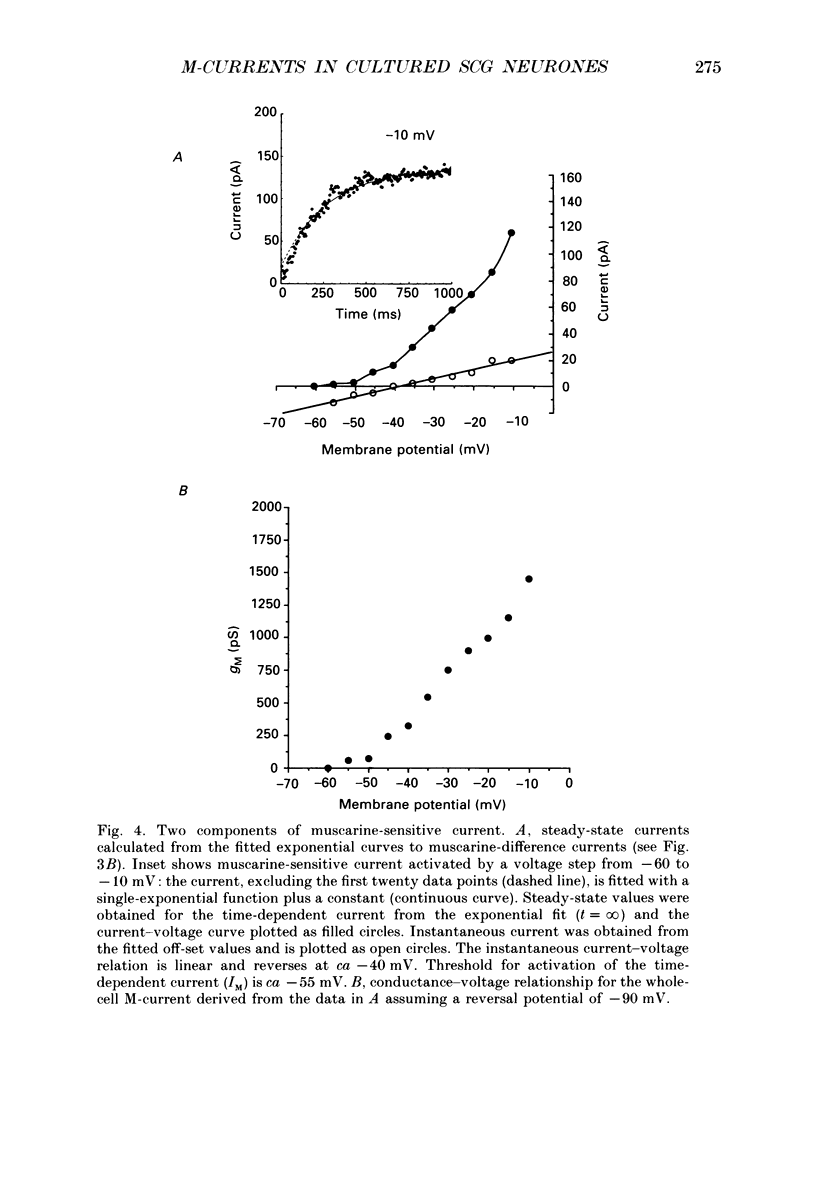
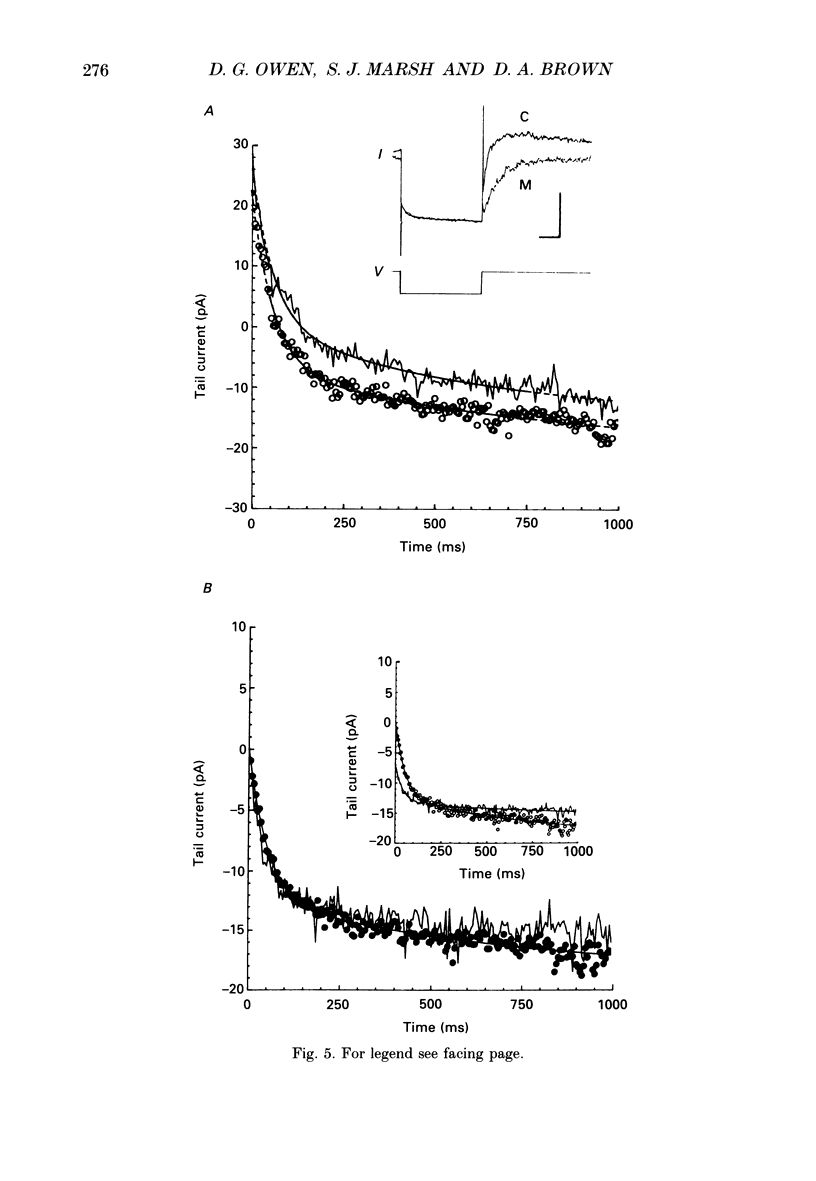
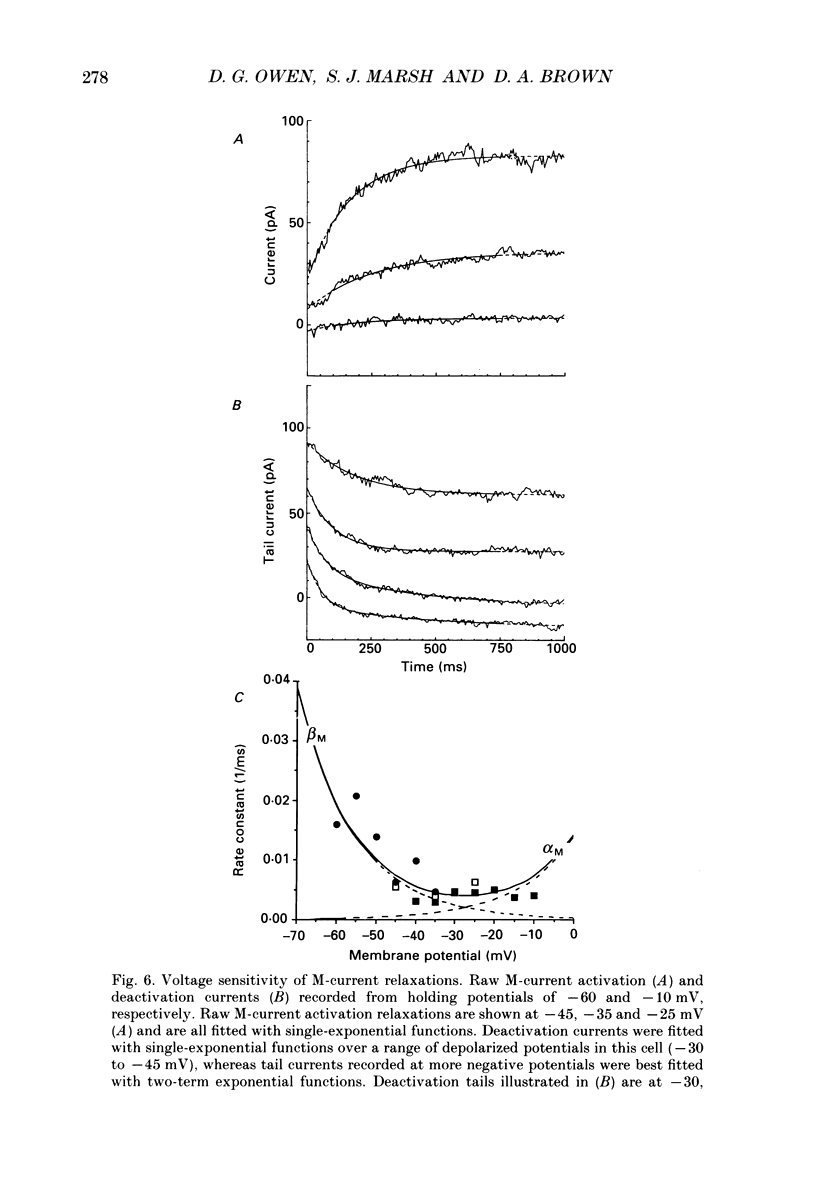
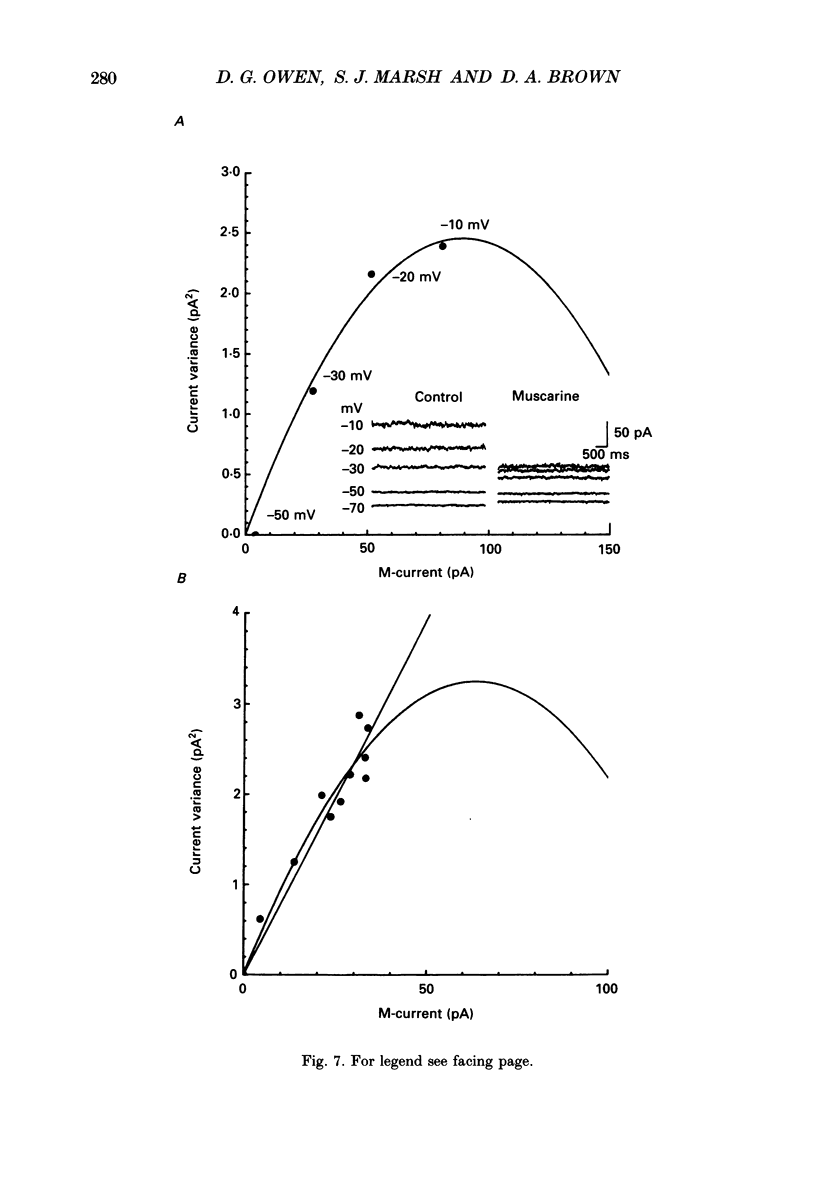
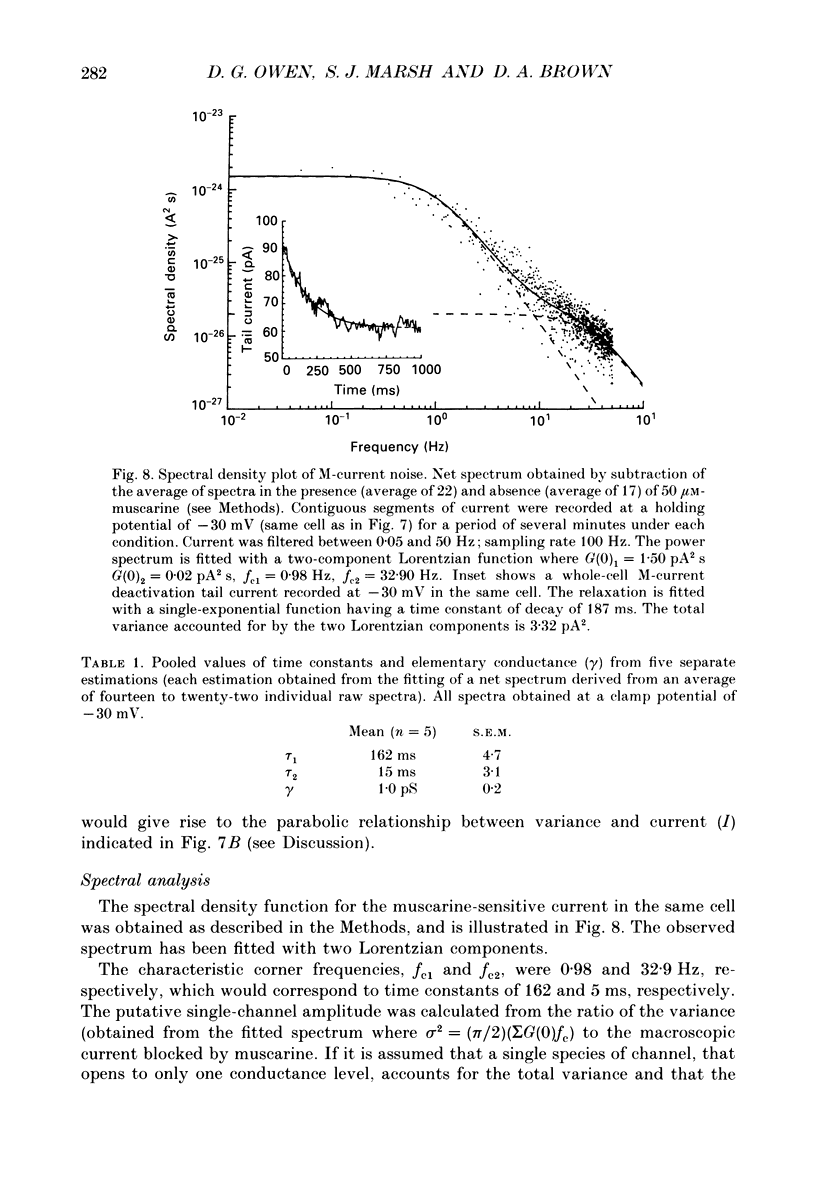
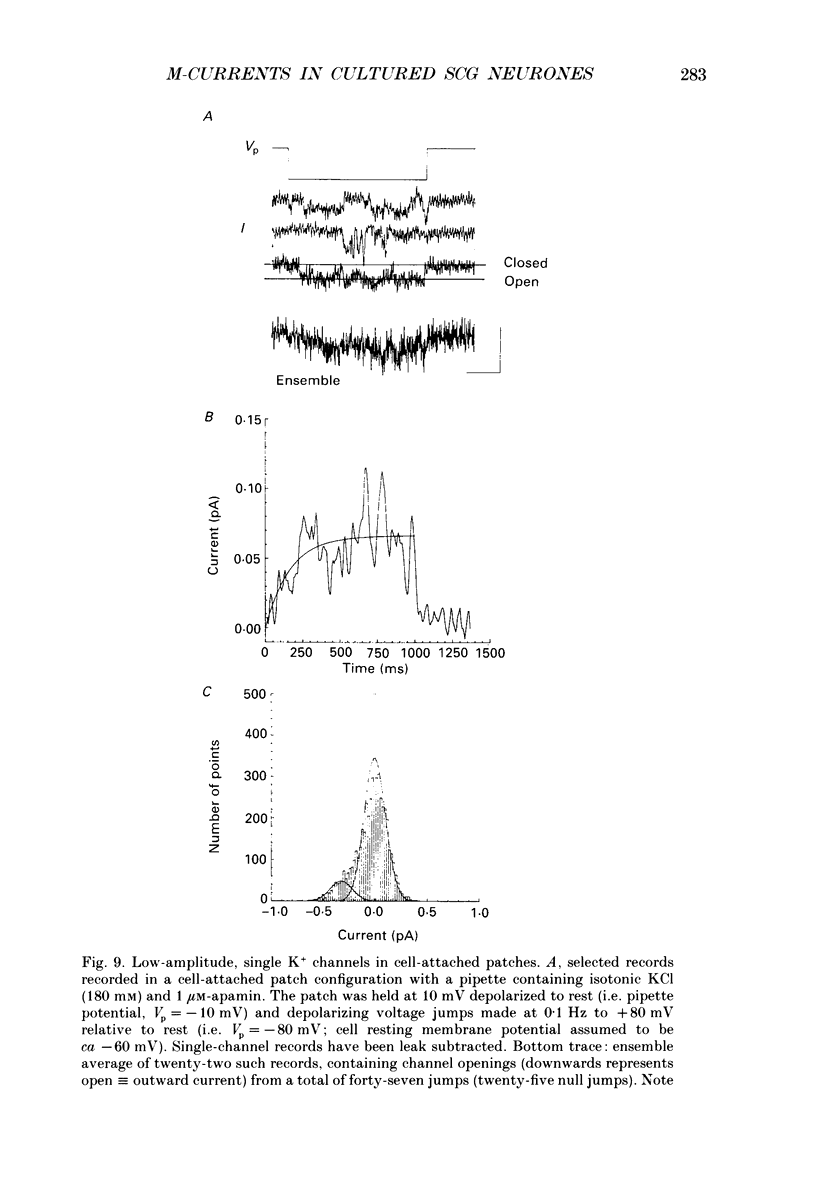
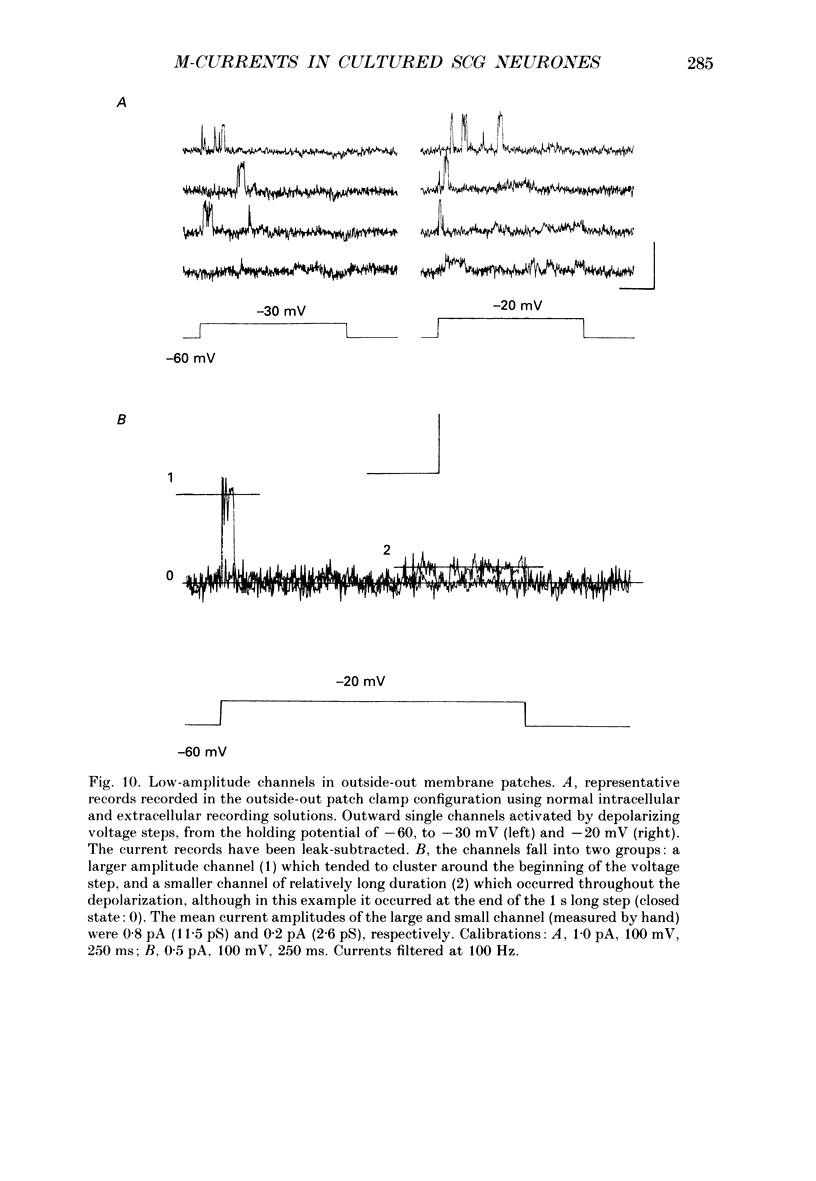
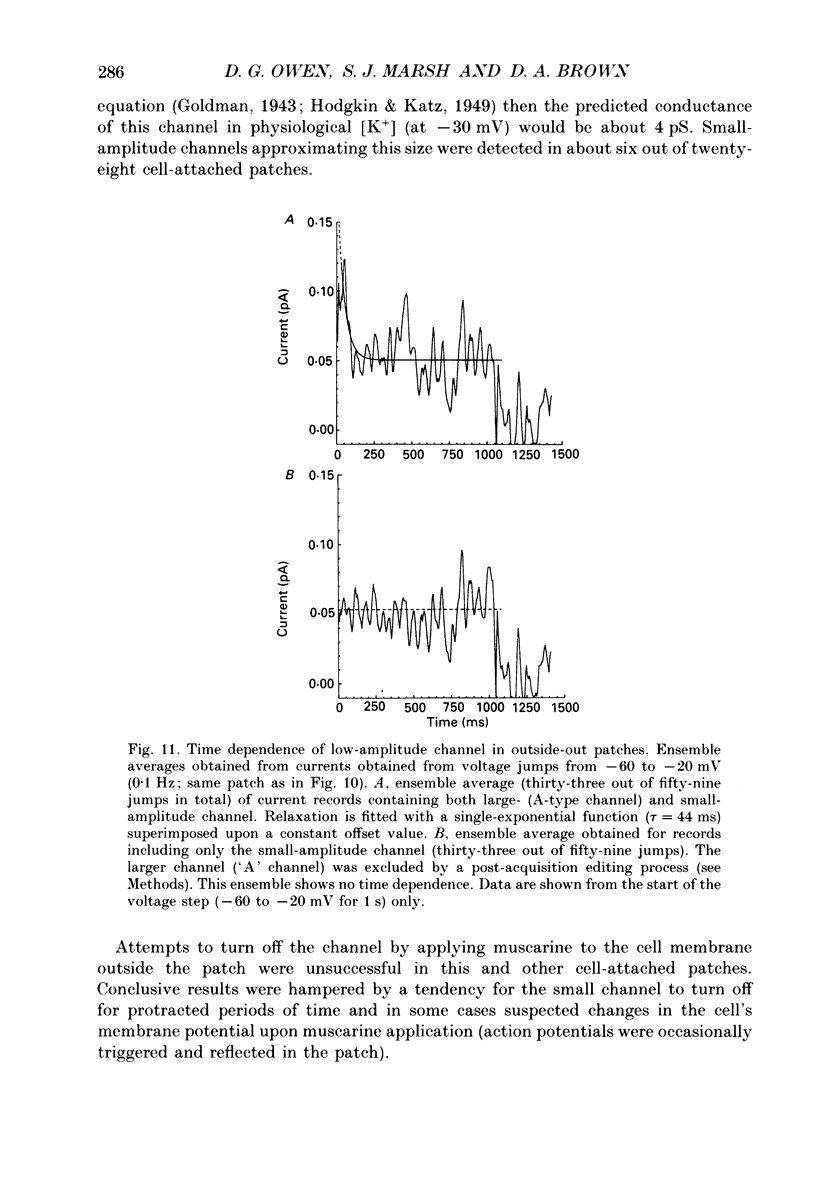
1. Whole-cell recordings of M-currents and single-channel recordings have been made in cultured rat sympathetic ganglion (SCG) neurones using the patch clamp technique. 2. Muscarine caused a reduction in macroscopic M-current relaxations, induced by voltage steps, and a concomitant reduction in whole-cell current noise. Power spectra of the muscarine-sensitive component of current noise were fitted with two Lorentzian components corresponding, on average, to 162 and 15 ms. The longer time constant was very similar to that of deactivation tail currents measured at the same potential. 3. The single-channel conductance at -30 mV was estimated from power density spectra and whole-cell current-variance relationships to be 1-2 pS. 4. Putative single M-channels, activated by depolarization, were identified in cell-attached and outside-out patches from cultured SCG neurones. In particular, the ensemble average of a small amplitude channel (estimated to be ca4 pS in physiological [K+]) in a cell-attached patch, exhibited a similar time dependence to whole-cell M-current.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardetti F., Kandel E. R., Siegelbaum S. A. Neuronal inhibition by the peptide FMRFamide involves opening of S K+ channels. Nature. 1987 Jan 8;325(7000):153–156. doi: 10.1038/325153a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Marrion N. V., Smart T. G. On the transduction mechanism for muscarine-induced inhibition of M-current in cultured rat sympathetic neurones. J Physiol. 1989 Jun;413:469–488. doi: 10.1113/jphysiol.1989.sp017664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Selyanko A. A. Two components of muscarine-sensitive membrane current in rat sympathetic neurones. J Physiol. 1985 Jan;358:335–363. doi: 10.1113/jphysiol.1985.sp015554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capiod T., Ogden D. C. The properties of calcium-activated potassium ion channels in guinea-pig isolated hepatocytes. J Physiol. 1989 Feb;409:285–295. doi: 10.1113/jphysiol.1989.sp017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Brown D. A. M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981 Jul 17;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Pennefather P. Potassium currents evoked by brief depolarizations in bull-frog sympathetic ganglion cells. J Physiol. 1987 Jun;387:519–548. doi: 10.1113/jphysiol.1987.sp016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez H. S., Adams P. R. A G Protein Mediates the Inhibition of the Voltage-Dependent Potassium M Current by Muscarine, LHRH, Substance P and UTP in Bullfrog Sympathetic Neurons. Eur J Neurosci. 1989 Sep;1(5):529–542. doi: 10.1111/j.1460-9568.1989.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Marrion N. V., Smart T. G., Brown D. A. Membrane currents in adult rat superior cervical ganglia in dissociated tissue culture. Neurosci Lett. 1987 Jun 1;77(1):55–60. doi: 10.1016/0304-3940(87)90606-9. [DOI] [PubMed] [Google Scholar]
- Marty A., Tan Y. P., Trautmann A. Three types of calcium-dependent channel in rat lacrimal glands. J Physiol. 1984 Dec;357:293–325. doi: 10.1113/jphysiol.1984.sp015501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A., Fukuda K., Kubo T., Numa S. Intracellular calcium release mediated by two muscarinic receptor subtypes. FEBS Lett. 1988 Nov 21;240(1-2):88–94. doi: 10.1016/0014-5793(88)80345-4. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. Muscarine and t-LHRH suppress M-current by activating an IAP-insensitive G-protein. J Neurosci. 1988 Sep;8(9):3343–3353. doi: 10.1523/JNEUROSCI.08-09-03343.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]


