Abstract
1. We have investigated the endothelial actomyosin system with particular emphasis on its possible role in actively opening a paracellular route for permeability. 2. Actin and myosin comprised 16% of total endothelial protein with a molar actin/myosin ratio of 16.2 which is close to the actin/myosin ratio of muscle (studies on freshly isolated pig pulmonary arterial endothelial cells, PAEC). 3. By immunocytochemistry at the light and electron microscope levels the bulk of actin and myosin was colocalized in close vicinity to the intercellular clefts of both micro- and macrovascular endothelial cells in situ and in vitro. 4. Calcium-ionophore-induced rise in permeability of human umbilical venous endothelial cells (HUVEC) and PAEC monolayers grown on filters in a two-chamber permeability system was caused by opening of intercellular gaps. Gap formation depended on the rise in intracellular Ca2+ and could be blocked by the calmodulin-binding drugs trifluperazine (TFP) and W7. 5. In skinned monolayers of cultured PAEC and in isolated sheets of HUVEC gap formation was shown to require ATP and occurred only when free myosin binding sites were available on endothelial actin filaments (experiments with myosin subfragment 1 modified by N-ethylmaleimide, S1-NEM). 6. These experiments suggest that actin and myosin in endothelial cells play a central role in regulating the width of the intercellular clefts, thereby controlling the paracellular pathway of vascular permeability.
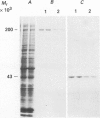
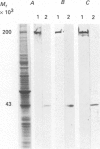
Full text
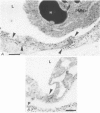
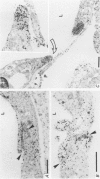
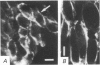
PDF









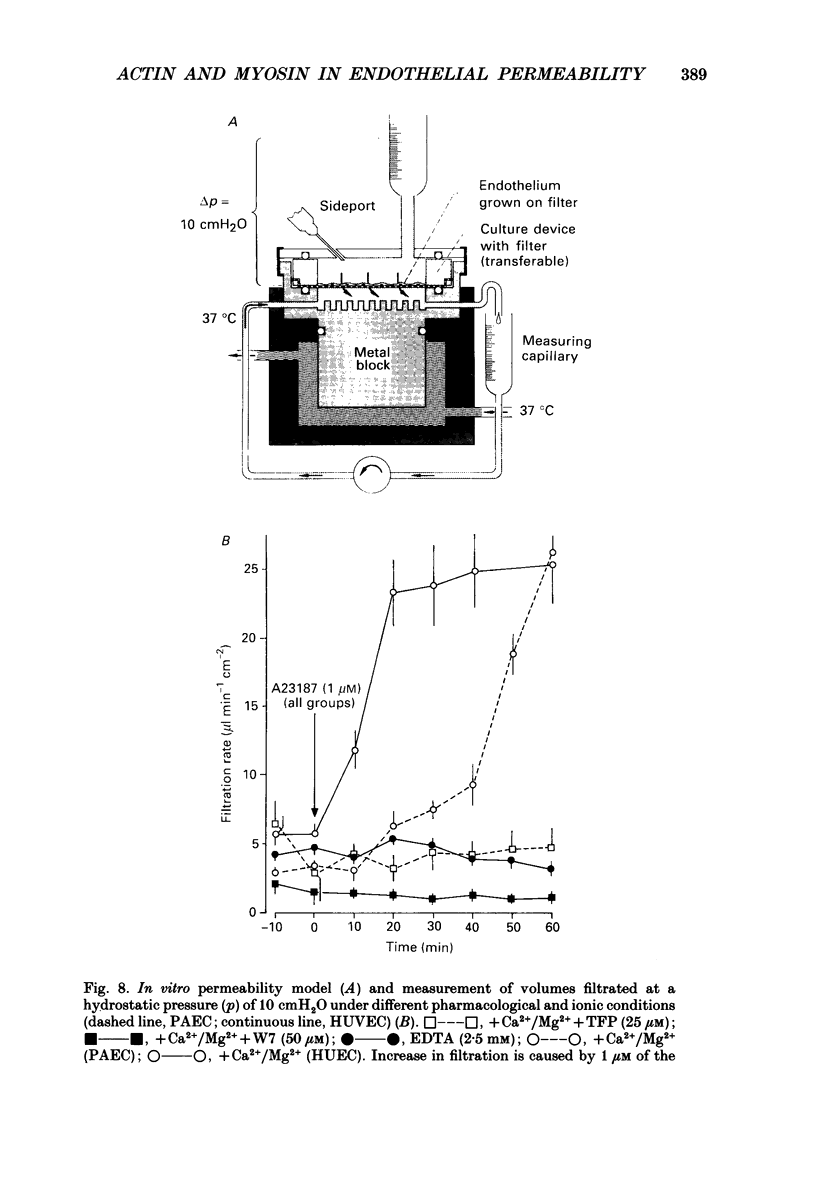











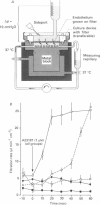
Images in this article
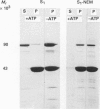
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfors K. E., Rutili G., Svensjö E. Microvascular transport of macromolecules in normal and inflammatory conditions. Acta Physiol Scand Suppl. 1979;463:93–103. [PubMed] [Google Scholar]
- Becker C. G., Nachman R. L. Contractile proteins of endothelial cells, platelets and smooth muscle. Am J Pathol. 1973 Apr;71(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruns R. R., Palade G. E. Studies on blood capillaries. II. Transport of ferritin molecules across the wall of muscle capillaries. J Cell Biol. 1968 May;37(2):277–299. doi: 10.1083/jcb.37.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M. The three-dimensional organization of tight junctions in a capillary endothelium revealed by serial-section electron microscopy. J Ultrastruct Res. 1984 Jul;88(1):1–17. doi: 10.1016/s0022-5320(84)90177-1. [DOI] [PubMed] [Google Scholar]
- Burgess D. R. Reactivation of intestinal epithelial cell brush border motility: ATP-dependent contraction via a terminal web contractile ring. J Cell Biol. 1982 Dec;95(3):853–863. doi: 10.1083/jcb.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z. A permeabilized cell model for studying cytokinesis using mammalian tissue culture cells. J Cell Biol. 1980 Nov;87(2 Pt 1):326–335. doi: 10.1083/jcb.87.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z. Preparation of N-ethylmaleimide-modified heavy meromyosin and its use as a functional probe of actomyosin-based motility. Methods Enzymol. 1986;134:473–477. doi: 10.1016/0076-6879(86)34113-2. [DOI] [PubMed] [Google Scholar]
- Citi S., Kendrick-Jones J. Regulation in vitro of brush border myosin by light chain phosphorylation. J Mol Biol. 1986 Apr 5;188(3):369–382. doi: 10.1016/0022-2836(86)90161-0. [DOI] [PubMed] [Google Scholar]
- Clough G., Michel C. C. The ultrastructure of frog microvessels following perfusion with the ionophore A23187. Q J Exp Physiol. 1988 Jan;73(1):123–125. doi: 10.1113/expphysiol.1988.sp003109. [DOI] [PubMed] [Google Scholar]
- Crone C. Modulation of solute permeability in microvascular endothelium. Fed Proc. 1986 Feb;45(2):77–83. [PubMed] [Google Scholar]
- Crone C. The Malpighi lecture. From 'Porositates carnis' to cellular microcirculation. Int J Microcirc Clin Exp. 1987;6(2):101–122. [PubMed] [Google Scholar]
- Drenckhahn D., Dermietzel R. Organization of the actin filament cytoskeleton in the intestinal brush border: a quantitative and qualitative immunoelectron microscope study. J Cell Biol. 1988 Sep;107(3):1037–1048. doi: 10.1083/jcb.107.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Gröschel-Stewart U., Kendrick-Jones J., Scholey J. M. Antibody to thymus myosin: its immunological characterization and use for immunocytochemical localization of myosin in vertebrate nonmuscle cells. Eur J Cell Biol. 1983 Mar;30(1):100–111. [PubMed] [Google Scholar]
- Drenckhahn D., Merte C. Restriction of the human kidney band 3-like anion exchanger to specialized subdomains of the basolateral plasma membrane of intercalated cells. Eur J Cell Biol. 1987 Dec;45(1):107–115. [PubMed] [Google Scholar]
- Drenckhahn D., Pollard T. D. Elongation of actin filaments is a diffusion-limited reaction at the barbed end and is accelerated by inert macromolecules. J Biol Chem. 1986 Sep 25;261(27):12754–12758. [PubMed] [Google Scholar]
- Drenckhahn D., Wagner J. Stress fibers in the splenic sinus endothelium in situ: molecular structure, relationship to the extracellular matrix, and contractility. J Cell Biol. 1986 May;102(5):1738–1747. doi: 10.1083/jcb.102.5.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Fox J., Galey F., Wayland H. Action of histamine on the mesenteric microvasculature. Microvasc Res. 1980 Jan;19(1):108–126. doi: 10.1016/0026-2862(80)90087-4. [DOI] [PubMed] [Google Scholar]
- Franke R. P., Gräfe M., Schnittler H., Seiffge D., Mittermayer C., Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984 Feb 16;307(5952):648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Gabbiani F., Lombardi D., Schwartz S. M. Organization of actin cytoskeleton in normal and regenerating arterial endothelial cells. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2361–2364. doi: 10.1073/pnas.80.8.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z., Ghitescu L., Simionescu M. Fatty acids binding to albumin increases its uptake and transcytosis by the lung capillary endothelium. Eur J Cell Biol. 1988 Dec;47(2):358–365. [PubMed] [Google Scholar]
- Hidaka H., Asano M., Tanaka T. Activity-structure relationship of calmodulin antagonists, Naphthalenesulfonamide derivatives. Mol Pharmacol. 1981 Nov;20(3):571–578. [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Keller T. C., 3rd, Mooseker M. S. Ca++-calmodulin-dependent phosphorylation of myosin, and its role in brush border contraction in vitro. J Cell Biol. 1982 Dec;95(3):943–959. doi: 10.1083/jcb.95.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beux Y. J., Willemot J. Actin-like filaments in the endothelial cells of adult rat brain capillaries. Exp Neurol. 1978 Feb;58(3):446–454. doi: 10.1016/0014-4886(78)90100-0. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L. Loosening tight junctions. Lessons from the intestine. J Clin Invest. 1989 Apr;83(4):1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan W. G., Joyner W. L. The effect of altering the external calcium concentration and a calcium channel blocker, verapamil, on microvascular leaky sites and dextran clearance in the hamster cheek pouch. Microvasc Res. 1984 Sep;28(2):159–179. doi: 10.1016/0026-2862(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Meza I., Ibarra G., Sabanero M., Martínez-Palomo A., Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol. 1980 Dec;87(3 Pt 1):746–754. doi: 10.1083/jcb.87.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C. Capillary permeability and how it may change. J Physiol. 1988 Oct;404:1–29. doi: 10.1113/jphysiol.1988.sp017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C., Phillips M. E. The effects of ionophore A23187 on permeability of the frog mesentery microvasculature. Q J Exp Physiol. 1989 Jan;74(1):7–18. doi: 10.1113/expphysiol.1989.sp003241. [DOI] [PubMed] [Google Scholar]
- Michel C. C. The Malpighi lecture. Vascular permeability--the consequence of Malpighi's hypothesis. Int J Microcirc Clin Exp. 1985;4(3):265–284. [PubMed] [Google Scholar]
- Moeremans M., Daneels G., Van Dijck A., Langanger G., De Mey J. Sensitive visualization of antigen-antibody reactions in dot and blot immune overlay assays with immunogold and immunogold/silver staining. J Immunol Methods. 1984 Nov 30;74(2):353–360. doi: 10.1016/0022-1759(84)90303-x. [DOI] [PubMed] [Google Scholar]
- Nagy Z., Peters H., Hüttner I. Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab Invest. 1984 Mar;50(3):313–322. [PubMed] [Google Scholar]
- Nicolaysen G. Increase in capillary filtration rate resulting from reduction in the intravascular calcium ion-concentration. Acta Physiol Scand. 1971 Apr;81(4):517–527. doi: 10.1111/j.1748-1716.1971.tb04929.x. [DOI] [PubMed] [Google Scholar]
- Northover A. M., Northover B. J. Changes of vascular endothelial cell shape and of membrane potential in response to the ionophore A23187. Int J Microcirc Clin Exp. 1987;6(2):137–148. [PubMed] [Google Scholar]
- Olesen S. P. An electrophysiological study of microvascular permeability and its modulation by chemical mediators. Acta Physiol Scand Suppl. 1989;579:1–28. [PubMed] [Google Scholar]
- Olesen S. P., Crone C. Substances that rapidly augment ionic conductance of endothelium in cerebral venules. Acta Physiol Scand. 1986 Jun;127(2):233–241. doi: 10.1111/j.1748-1716.1986.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Olesen S. P. Regulation of ion permeability in frog brain venules. Significance of calcium, cyclic nucleotides and protein kinase C. J Physiol. 1987 Jun;387:59–68. doi: 10.1113/jphysiol.1987.sp016562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. Cytoplasmic contractile proteins. J Cell Biol. 1981 Dec;91(3 Pt 2):156s–165s. doi: 10.1083/jcb.91.3.156s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. Myosin purification and characterization. Methods Cell Biol. 1982;24:333–371. doi: 10.1016/s0091-679x(08)60665-2. [DOI] [PubMed] [Google Scholar]
- Rogers K. A., Kalnins V. I. A method for examining the endothelial cytoskeleton in situ using immunofluorescence. J Histochem Cytochem. 1983 Nov;31(11):1317–1320. doi: 10.1177/31.11.6352798. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Smith R. C., Drenckhahn D., Groschel-Stewart U., Kendrick-Jones J. Thymus myosin. Isolation and characterization of myosin from calf thymus and thymic lymphocytes, and studies on the effect of phosphorylation of its Mr = 20,000 light chain. J Biol Chem. 1982 Jul 10;257(13):7737–7745. [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Sullivan J. M., Peach M. J. Role of endothelial cell cytoskeleton in control of endothelial permeability. Circ Res. 1982 Nov;51(5):657–661. doi: 10.1161/01.res.51.5.657. [DOI] [PubMed] [Google Scholar]
- Sheterline P. Trifluoperazine can distinguish between myosin light chain kinase-linked and troponin C-linked control of actomyosin interaction by Ca++. Biochem Biophys Res Commun. 1980 Mar 13;93(1):194–200. doi: 10.1016/s0006-291x(80)80265-8. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975 Dec;67(3):863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobieszek A. Calmodulin antagonist action in smooth-muscle myosin phosphorylation. Different mechanisms for trifluoperazine and calmidazolium inhibition. Biochem J. 1989 Aug 15;262(1):215–223. doi: 10.1042/bj2620215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B. R., Anderson J. M., Bullivant S. The epithelial tight junction: structure, function and preliminary biochemical characterization. Mol Cell Biochem. 1988 Oct;83(2):129–145. doi: 10.1007/BF00226141. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Fuchs T., Seeger W., Wilke A., Drenckhahn D. Role of Ca2+ and Mg2+ for endothelial permeability of water and albumin in vitro. Lab Invest. 1989 Aug;61(2):183–191. [PubMed] [Google Scholar]
- Suttorp N., Hessz T., Seeger W., Wilke A., Koob R., Lutz F., Drenckhahn D. Bacterial exotoxins and endothelial permeability for water and albumin in vitro. Am J Physiol. 1988 Sep;255(3 Pt 1):C368–C376. doi: 10.1152/ajpcell.1988.255.3.C368. [DOI] [PubMed] [Google Scholar]
- White G. E., Gimbrone M. A., Jr, Fujiwara K. Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J Cell Biol. 1983 Aug;97(2):416–424. doi: 10.1083/jcb.97.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissig S. L. Identification of the small pore in muscle capillaries. Acta Physiol Scand Suppl. 1979;463:33–44. [PubMed] [Google Scholar]
- Wong A. J., Pollard T. D., Herman I. M. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983 Feb 18;219(4586):867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]