Abstract
We previously linked Laing-type early-onset autosomal dominant distal myopathy (MPD1) to a 22-cM region of chromosome 14. One candidate gene in the region, MYH7, which is mutated in cardiomyopathy and myosin storage myopathy, codes for the myosin heavy chain of type I skeletal muscle fibers and cardiac ventricles. We have identified five novel heterozygous mutations—Arg1500Pro, Lys1617del, Ala1663Pro, Leu1706Pro, and Lys1729del in exons 32, 34, 35, and 36 of MYH7—in six families with early-onset distal myopathy. All five mutations are predicted, by in silico analysis, to locally disrupt the ability of the myosin tail to form the coiled coil, which is its normal structure. These findings demonstrate that heterozygous mutations toward the 3′ end of MYH7 cause Laing-type early-onset distal myopathy. MYH7 is the fourth distal-myopathy gene to have been identified.
The distal myopathies form a group of disorders that is heterogeneous both clinically and genetically. They are characterized by weakness starting in the anterior or posterior compartment of either the distal upper or the distal lower limb and show either autosomal dominant or autosomal recessive inheritance. (For recent reviews, see Udd and Griggs [2001] and Udd et al. [2002]). To date, the genes for three distal myopathies have been identified: dysferlin, in Miyoshi myopathy (Bashir et al. 1998; Liu et al. 1998); titin, in tibial muscular dystrophy (Hackman et al. 2002); and UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE), in Nonaka myopathy (Kayashima et al. 2002; Nishino et al. 2002). Proteins mutated in other muscle diseases may also cause distal phenotypes (Sjoberg et al. 1999; Tateyama et al. 2002). Other distal myopathies have been linked to loci, but the genes have not been identified. These include Welander distal myopathy (Ahlberg et al. 1999); Miyoshi myopathy, for which a second locus has been linked to chromosome 10 (Linssen et al. 1998); distal myopathy with vocal cord and pharyngeal weakness (Feit et al. 1998); and a unique distal myopathy (MPD3) in a Finnish family (Haravuori et al. 2004). A number of other distal myopathies remain unlinked (Felice et al. 1999; Udd et al. 2002).
Laing-type early-onset distal myopathy (MPD1 [MIM 160500]) is phenotypically different from other dominant distal myopathies in showing onset as early as age 4 years (range 4–25 years). Selective weakness of the anterior tibial muscles is followed by weakness of the finger extensors and of selected proximal muscle groups, such as the hip abductors and rotators, the shoulder abductors, and the sternocleidomastoids in some patients (Laing et al. 1995; Voit et al. 2001; Mastaglia et al. 2002; Hedera et al. 2003). We linked Laing-type distal myopathy to chromosome 14, in 1995 (Laing et al. 1995). Smaller European families with similar phenotypes showed evidence of linkage to the same locus (Voit et al. 2001), and a large U.S. family showed linkage to the locus (Hedera et al. 2003). A recombination in an unaffected individual in the U.S. family made it possible to reduce the linkage region to a still-large 22 cM (Hedera et al. 2003). A positional candidate gene approach (Collins 1995) was therefore the only possible course for identifying the disease gene. Within the linkage region is the gene, MYH7, for the slow skeletal muscle fiber myosin heavy chain, which is also expressed in cardiac muscle (Lompre et al. 1984). Mutations in MYH7 cause dilated and hypertrophic cardiomyopathy (Geisterfer-Lowrance et al. 1990; Seidman and Seidman 2001; Richard et al. 2003) and the skeletal muscle disease myosin storage myopathy (hyaline body myopathy) (Tajsharghi et al. 2003; Bohlega et al. 2004). We investigated MYH7 as a candidate gene for Laing-type distal myopathy in seven separate families.
We identified heterozygous mutations in the light meromyosin (LMM) region of the MYH7 tail in six of the seven families (table 1). We identified the mutation in family 1 by sequencing the entire coding region of MYH7 from cDNA. The other mutations were identified from genomic DNA by amplification and sequencing of all 40 MYH7 exons. The mutations identified in families 1–4 were not seen in 400 control chromosomes, and the mutations in families 5 and 6 were not seen in 200 control chromosomes. The mutations identified in the families showing dominant inheritance (families 1, 2, 3, and 5) segregated with the disease, resulting in a combined LOD score of 8.7 at a recombination fraction of 0.
Table 1.
Families and Heterozygous Mutations Identified in the Present Study
Families 4 and 6 consist of previously unpublished isolated patients from the Netherlands and Western Australia who received diagnoses (from M.d.V. and P.L.) of Laing myopathy based on the published descriptions of the disease (Laing et al. 1995; Voit et al. 2001; Mastaglia et al. 2002). The Dutch patient is a 32-year-old woman who has had bilateral foot drop since the age of 5 years and slowly progressive proximal weakness since the age of 6 years. She also has severe weakness and atrophy of the sternocleidomastoid muscles and a rigid neck. The Western Australian patient, from family 6, was noted at birth to have mild talipes equinovarus, but this corrected itself. Early motor milestones were appropriate in this patient, but, by the time she was 4 years old, she was noted to have weakness of ankle dorsiflexion that was more severe on the right side than on the left. Since then, she has had slowly progressive weakness of her legs and hands. Her arms started to become weak at 11 years of age. Intellectually, she is an excellent student. On examination, there was mild facial weakness, but she had full external ocular movements and no ptosis. The patient had a Trendelenburg gait, foot drop, mild scapular winging, and flexion contractures of the fingers. There was generalized limb weakness, in the legs more than in the arms. There was marked weakness of the sternocleidomastoid muscles bilaterally. Other axial muscles were strong. Deep tendon reflexes were present but markedly reduced. Results of cardiac examination, creatine kinase tests, and nerve conduction studies were normal.
The c.R1500P and c.L1706P mutations identified in the isolated patients in families 4 and 6 were not present in either parent, whereas paternity/maternity was consistent in analysis of multiple microsatellites. The mutations are thus de novo mutations arising in the pedigrees at the same point as the disease.
Family 7 is an Italian family with early-onset distal myopathy (Scoppetta et al. 1995). Sequencing all 40 MYH7 exons did not result in identification of a mutation, despite the family showing evidence of linkage to the MYH7 region (Voit et al. 2001).
Family 2 from Germany and family 3 from Austria have the same c.K1617del mutation. It is possible, therefore, that the two families share a founder mutation. However, haplotype analysis (table 2) gives no indication that the families share a disease haplotype, suggesting that the same mutation has probably arisen independently in the two families.
Table 2.
Haplotype Analysis of Families 2 and 3
|
Allele in |
||||
| Marker | Position ondeCODE Map(cM) | Location in theHuman Genome Draft Sequencea(bp) | Family2 | Family3 |
| D14S1003 | 13.4 | 20106473–20106637 | 167 | 165 |
| D14S283 | 14.7 | 20677673–20677803 | 136 | 145 |
| D14S990 | 15.4 | 21576515–21576761 | 147 | 155 |
| MYH7 | … | 21872077–21894978 | … | … |
| D14S581 | 18.5 | 22288637–22288827 | 192 | 196 |
| D14S972 | 18.5 | 22337862–22338070 | 206 | 204 |
| D14S264 | 19.6 | 23270071–23270286 | 226 | 228 |
From the University of California Santa Cruz Genome Bioinformatics Web site.
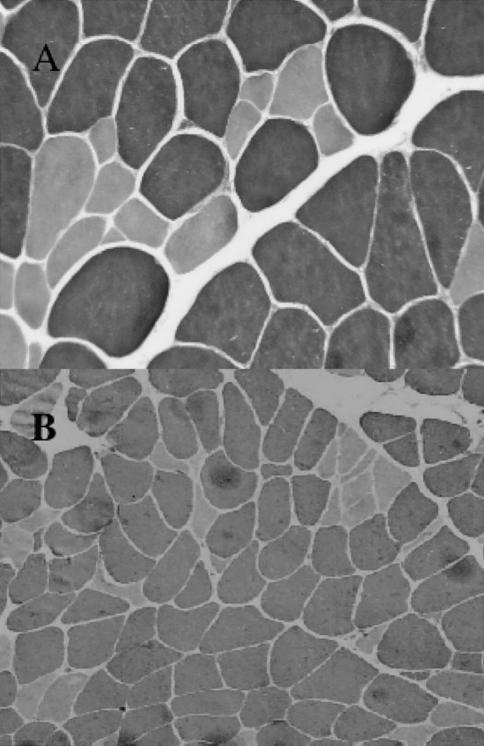
MYH7 codes for the isoform of myosin present in slow (type I) skeletal muscle fibers. Consistent with this, the commonest findings on muscle biopsy, present in four families, were atrophy and grouping of type I muscle fibers (fig. 1A). In some patients, the numbers of type I fibers were depleted. However, there was considerable variability in the severity of pathological changes in affected individuals. Nonspecific myopathic changes, including excessive variation in fiber size, central nucleation, fiber splitting, “moth-eaten” fibers, and ring fibers, were present to variable degrees in different families. In the biopsy specimens from older patients, there were secondary effects in type II fibers (fig. 1B). In the biopsy specimen from one patient (family 4), muscle fiber vacuolation and necrosis were present in both fiber types. The muscle pathology is, thus, pleomorphic.
Figure 1.
Sections stained for myosin ATPase (pH 9.4) from vastus lateralis biopsies in the probands from family 6 (age 12 years) (A) and family 1 (age 48 years) (B). In the section shown in panel A, there is selective atrophy of the type I fibers (lightly stained). In the section shown in panel B, there is atrophy and angulation, mainly of type I fibers, but type II fibers are also occasionally atrophic. Magnifications: A, ×400; B, ×160.
We have therefore identified five mutations in exons 32, 34, 35, and 36 of MYH7 in six families with Laing distal myopathy. The pathophysiology of the mutations is unclear. Three of the mutations introduce proline, whereas the other two are deletions of single amino acids. The myosin tail forms the coiled coil that is the basis of the myosin dimerization and the structure of the thick filament (McLachlan and Karn 1982; Craig 1994). Prolines are incompatible with coiled coils (O’Neil and DeGrado 1990) (there are normally no prolines in the myosin rod [McLachlan and Karn 1982]), whereas deletion of a single amino acid disrupts the heptad repeat essential to the formation of a coiled coil (O’Neil and DeGrado 1990). The effects of the five Laing myopathy mutations and of the LMM hypertrophic cardiomyopathy mutations (Blair et al. 2002; Richard et al. 2003) on the coiled coil structure of myosin were analyzed using the COILS program (Lupas et al. 1991). The probability of coiled coil formation was predicted for each mutation and compared with the predicted probability for the normal protein sequence. Probabilities were calculated using the MTK (myosin, tropomyosin, and keratin) matrix with amino acid window lengths of 14, 21, and 28. All five Laing myopathy mutations significantly decreased the probability of coiled coil formation over segments of the myosin tail (fig. 2A). However, the LMM hypertrophic cardiomyopathy mutations had no significant effect on the probability of coiled coil formation (fig. 2B). The Laing myopathy mutations should therefore more severely affect the ability of the MYH7 LMM to make a coiled coil than the mutations associated with cardiomyopathy (Blair et al. 2002; Richard et al. 2003). Nevertheless, patients with Laing myopathy usually do not demonstrate cardiomyopathy (Laing et al. 1995; Voit et al. 2001; Mastaglia et al. 2002; Hedera et al. 2003). This is a situation similar to the myosin storage (hyaline body) myopathy caused by mutations in exons 37 and 39 of MYH7, in which cardiac involvement is minimal (Tajsharghi et al. 2003; Bohlega et al. 2004).
Figure 2.
COILS analysis of the effect of the four distal-myopathy mutations (A) and seven hypertrophic cardiomyopathy mutations (B) in the LMM region of MYH7 on the probability of the mutant myosin tails forming a coiled coil. All four distal-myopathy mutations have a significant effect on the probability of the myosin tail forming a coiled coil, whereas the hypertrophic cardiomyopathy mutations have no significant effect on the ability of the myosin tail to form a coiled coil.
It has been argued that MYH7 mutations produce either dilated or hypertrophic cardiomyopathy through effects on different myosin protein interactions (Seidman and Seidman 2001; Blair et al. 2002). Two of the MYH7 Laing myopathy mutations, c.K1617del and c.K1729del, are caused by loss of one of three consecutive lysines, presumably by mechanisms similar to those that change microsatellite allele size (Xu et al. 2000). Deletion of a single amino acid residue—one (c.E930del) in a series of three glutamate residues—in the S2 region of slow myosin causes cardiomyopathy (Richard et al. 2003). Skeletal muscle disease was not described in these patients (Richard et al. 2003). Thus, single amino acid deletions in the MYH7 rod do not always cause distal myopathy, suggesting that MYH7 mutations have to lie within a restricted region to produce the Laing myopathy phenotype and probably interrupt a specific myosin function.
The mutations causing Laing myopathy may disrupt the binding of myosin to titin, myomesin, or M-protein. The titin binding site (Houmeida et al. 1995) corresponds to residues 1815–1831 in slow skeletal myosin (Jaenicke et al. 1990). Mutations in the M-band region of titin cause tibial muscular dystrophy (Hackman et al. 2002), which shows a pattern of muscle involvement similar to Laing myopathy, though it has a later onset (Mastaglia et al. 2002). The similar distribution of affected muscles might suggest linked pathophysiology. The M-line proteins M-protein and myomesin have been shown to bind to the equivalent of residues 1503–1671 in slow skeletal myosin (Obermann et al. 1997, 1998). Residues 1503–1671 encompass two of the Laing myopathy mutations, and the other three are close (fig. 2). It may be that the MYH7 mutations that cause Laing myopathy disrupt the binding of myosin to titin, myomesin, or M-protein, whereas the mutations in MYH7 that cause cardiomyopathy (Blair et al. 2002; Richard et al. 2003) or myosin storage myopathy (Tajsharghi et al. 2003; Bohlega et al. 2004) do not. Linked pathology in Laing myopathy and tibial muscular dystrophy perhaps results from interaction of mutated myosin and titin with myomesin, M-protein, or another component of the sarcomere.
Rimmed vacuoles and filamentous inclusions occur in many distal myopathies, including Laing myopathy (Voit et al. 2001), leading to the proposition they are inclusion-body myopathies (Askanas 1997). Therefore, it is interesting that mutation of fast myosin IIa causes autosomal dominant hereditary inclusion-body myopathy, IBM3 (Martinsson et al. 2000).
We found MYH7 mutations in six of the seven families investigated. We did not find a mutation in the seventh family, despite this family showing evidence of linkage to MYH7. This may reflect the already identified genetic heterogeneity in early-onset distal myopathy (von Deimling et al. 2001) or that the techniques used to identify the MYH7 mutations failed to identify the mutation in the seventh family. MYH7 is the fourth distal-myopathy gene identified after dysferlin (Bashir et al. 1998; Liu et al. 1998), GNE (Kayashima et al. 2002), and titin (Hackman et al. 2002; Van den Bergh et al. 2003) and encodes the second sarcomeric protein shown to be involved.
Acknowledgments
N.G.L. was supported by Australian National Health and Medical Research Council Fellowship Grant 139170 and Project Grant 254544, as well as by the West Australian Medical and Health Research Infrastructure Fund; T.V. was supported by the Alfried-Krupp-von-Bohlen-und-Halbach Stiftung; J.K.F. is supported by a Veterans Affairs Merit Review Award, the National Institutes of Health (National Institute of Neurological Disorders and Stroke grants R01NS33645, R01NS36177, and R01NS38713), and the Spastic Paraplegia Foundation; and P.H. is supported by National Institutes of Health grant K08NS42743 and by the U.S. Muscular Dystrophy Association. We thank Koert P. Dingemans for performing the ultrastructural studies in family 5. This article is dedicated to the memory of Robin William Laing (March 25, 1951–June 10, 2002).
Electronic-Database Information
The URLs for data presented herein are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MPD1) [PubMed]
- University of California Santa Cruz Genome Bioinformatics, http://genome.cse.ucsc.edu/ (for the working draft sequence of the human genome)
References
- Ahlberg G, von Tell D, Borg K, Edstrom L, Anvret M (1999) Genetic linkage of Welander distal myopathy to chromosome 2p13. Ann Neurol 46:399–404 [DOI] [PubMed] [Google Scholar]
- Askanas V (1997) New developments in hereditary inclusion body myopathies. Ann Neurol 41:421–422 [DOI] [PubMed] [Google Scholar]
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno M, Moreira E, Zatz M, Beckmann J, Bushby K (1998) A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet 20:37–42 10.1038/1689 [DOI] [PubMed] [Google Scholar]
- Blair E, Redwood C, de Jesus Oliveira M, Moolman-Smook JC, Brink P, Corfield VA, Ostman-Smith I, Watkins H (2002) Mutations of the light meromyosin domain of the β-myosin heavy chain rod in hypertrophic cardiomyopathy. Circ Res 90:263–269 10.1161/hh0302.104532 [DOI] [PubMed] [Google Scholar]
- Bohlega S, Abu-Amero SN, Wakil SM, Carroll P, Al-Amr R, Lach B, Al-Sayed Y, Cupler EJ, Meyer BF (2004) Mutation of the slow myosin heavy chain rod domain underlies hyaline body myopathy. Neurology 62:1518–1521 [DOI] [PubMed] [Google Scholar]
- Collins FS (1995) Positional cloning moves from perditional to traditional. Nat Genet 9:347–350 10.1038/ng0495-347 [DOI] [PubMed] [Google Scholar]
- Craig R (1994) The structure of the contractile filaments. In: Engel AG, Franzini-Armstrong C (eds) Myology. McGraw-Hill, New York, pp 134–175 [Google Scholar]
- Feit H, Silbergleit A, Schneider LB, Gutierrez JA, Fitoussi RP, Reyes C, Rouleau GA, Brais B, Jackson CE, Beckmann JS, Seboun E (1998) Vocal cord and pharyngeal weakness with autosomal dominant distal myopathy: clinical description and gene localization to 5q31. Am J Hum Genet 63:1732–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice KJ, Meredith C, Binz N, Butler A, Jacob R, Akkari P, Hallmayer J, Laing N (1999) Autosomal dominant distal myopathy not linked to the known distal myopathy loci. Neuromuscul Disord 9:59–65 10.1016/S0960-8966(98)00099-6 [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance AAT, Kass S, Tanigawa G, Vosberg H-P, McKenna W, Seidman CE, Seidman JG (1990) A molecular basis for familial hypertrophic cardiomyopathy: a β cardiac myosin heavy chain gene missense mutation. Cell 62:999–1006 10.1016/0092-8674(90)90274-I [DOI] [PubMed] [Google Scholar]
- Hackman P, Vihola A, Haravuori H, Marchand S, Sarparanta J, De Seze J, Labeit S, Witt C, Peltonen L, Richard I, Udd B (2002) Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Genet 71:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haravuori H, Siitonen HA, Mahjneh I, Hackman P, Lahti L, Somer H, Peltonen L, Kestila M, Udd B (2004) Linkage to two separate loci in a family with a novel distal myopathy phenotype (MPD3). Neuromuscul Disord 14:183–187 10.1016/j.nmd.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Hedera P, Petty EM, Bui MR, Blaivas M, Fink JK (2003) The second kindred with autosomal dominant distal myopathy linked to chromosome 14q: genetic and clinical analysis. Arch Neurol 60:1321–1325 10.1001/archneur.60.9.1321 [DOI] [PubMed] [Google Scholar]
- Houmeida A, Holt J, Tskhovrebova L, Trinick J (1995) Studies of the interaction between titin and myosin. J Cell Biol 131:1471–1481 10.1083/jcb.131.6.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke T, Diederich KW, Haas W, Schleich J, Lichter P, Pfordt M, Bach A, Vosberg H-P (1990) The complete sequence of the human β-myosin heavy chain gene and a comparative analysis of its product. Genomics 8:194–206 [DOI] [PubMed] [Google Scholar]
- Kayashima T, Matsuo H, Satoh A, Ohta T, Yoshiura K, Matsumoto N, Nakane Y, Niikawa N, Kishino T (2002) Nonaka myopathy is caused by mutations in the UDP-N-acetylglucosamine-2-epimerase/N-acetylmannosamine kinase gene (GNE). J Hum Genet 47:77–79 10.1007/s100380200004 [DOI] [PubMed] [Google Scholar]
- Laing NG, Laing BA, Meredith C, Wilton SD, Robbins P, Honeyman K, Dorosz S, Kozman H, Mastaglia FL, Kakulas BA (1995) Autosomal dominant distal myopathy: linkage to chromosome 14. Am J Hum Genet 56:422–427 [PMC free article] [PubMed] [Google Scholar]
- Linssen WHJP, Devisser M, Notermans NC, Vreyling JP, Vandoorn PA, Wokke JHJ, Baas F, Bolhuis PA (1998) Genetic heterogeneity in Miyoshi-type distal muscular dystrophy. Neuromuscul Disord 8:317–320 10.1016/S0960-8966(98)00020-0 [DOI] [PubMed] [Google Scholar]
- Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea J, Hentati F, Benhamida M, Bohlega S, Culper E, Amato A, Bossie K, Oeltjen T, Bejaoui K, Mckenna-Yasek D, Hosler BA, Schurr E, Arahata K, Dejong P, Brown R (1998) Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet 20:31–36 10.1038/1682 [DOI] [PubMed] [Google Scholar]
- Lompre AM, Nadal-Ginard B, Mahdavi V (1984) Expression of the cardiac ventricular α- and β-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem 259:6437–6446 [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J (1991) Predicting coiled coils from protein sequences. Science 252:1162–1164 [DOI] [PubMed] [Google Scholar]
- Martinsson T, Oldfors A, Darin N, Berg K, Tajsharghi H, Kyllerman M, Wahlstrom J (2000) Autosomal dominant myopathy: Missense mutation (Glu-706 →Lys) in the myosin heavy chain IIa gene. Proc Natl Acad Sci USA 97:14614–14619 10.1073/pnas.250289597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastaglia FL, Phillips BA, Cala LA, Meredith C, Egli S, Akkari PA, Laing NG (2002) Early onset chromosome 14-linked distal myopathy (Laing). Neuromuscul Disord 12:350–357 10.1016/S0960-8966(01)00287-5 [DOI] [PubMed] [Google Scholar]
- McLachlan AD, Karn J (1982) Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature 299:226–231 [DOI] [PubMed] [Google Scholar]
- Nishino I, Noguchi S, Murayama K, Driss A, Sugie K, Oya Y, Nagata T, Chida K, Takahashi T, Takusa Y, Ohi T, Nishimiya J, Sunohara N, Ciafaloni E, Kawai M, Aoki M, Nonaka I (2002) Distal myopathy with rimmed vacuoles is allelic to hereditary inclusion body myopathy. Neurology 59:1689–1693 [DOI] [PubMed] [Google Scholar]
- Obermann WMJ, Gautel M, Weber K, Fürst DO (1997) Molecular structure of the sarcomeric M band: mapping of titin and myosin binding domains in myomesin and the identification of a potential regulatory phosphorylation site in myomesin. EMBO J 16:211–220 10.1093/emboj/16.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann WMJ, van der Ven PFM, Steiner F, Weber K, Fürst DO (1998) Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band. Mol Biol Cell 9:829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil KT, DeGrado WF (1990) A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science 250:646–651 [DOI] [PubMed] [Google Scholar]
- Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M (2003) Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107:2227–2232 10.1161/01.CIR.0000066323.15244.54 [DOI] [PubMed] [Google Scholar]
- Scoppetta C, Casali C, La Cesa I, Sermoni A, Mercuri B, Pierelli F, Vaccario ML (1995) Infantile autosomal dominant distal myopathy. Acta Neurol Scand 92:122–126 [DOI] [PubMed] [Google Scholar]
- Seidman JG, Seidman C (2001) The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 104:557–567 10.1016/S0092-8674(01)00242-2 [DOI] [PubMed] [Google Scholar]
- Sjoberg G, Saavedra-Matiz CA, Rosen DR, Wijsman EM, Borg K, Horowitz SH, Sejersen T (1999) A missense mutation in the desmin rod domain is associated with autosomal dominant distal myopathy, and exerts a dominant negative effect on filament formation. Hum Mol Genet 8:2191–2198 10.1093/hmg/8.12.2191 [DOI] [PubMed] [Google Scholar]
- Tajsharghi H, Thornell LE, Lindberg C, Lindvall B, Henriksson KG, Oldfors A (2003) Myosin storage myopathy associated with a heterozygous missense mutation in MYH7. Ann Neurol 54:494–500 10.1002/ana.10693 [DOI] [PubMed] [Google Scholar]
- Tateyama M, Aoki M, Nishino I, Hayashi YK, Sekiguchi S, Shiga Y, Takahashi T, Onodera Y, Haginoya K, Kobayashi K, Iinuma K, Nonaka I, Arahata K, Itoyoma Y (2002) Mutation in the caveolin-3 gene causes a peculiar form of distal myopathy. Neurology 58:323–325 [DOI] [PubMed] [Google Scholar]
- Udd B, Bushby K, Nonaka I, Griggs R (2002) 104th European Neuromuscular Centre (ENMC) International Workshop: Distal Myopathies, 8–10th March 2002 in Naarden, The Netherlands. Neuromuscul Disord 12:897–904 10.1016/S0960-8966(02)00116-5 [DOI] [PubMed] [Google Scholar]
- Udd B, Griggs R (2001) Distal myopathies. Curr Opin Neurol 14:561–566 10.1097/00019052-200110000-00003 [DOI] [PubMed] [Google Scholar]
- Van den Bergh PY, Bouquiaux O, Verellen C, Marchand S, Richard I, Hackman P, Udd B (2003) Tibial muscular dystrophy in a Belgian family. Ann Neurol 54:248–251 10.1002/ana.10647 [DOI] [PubMed] [Google Scholar]
- Voit T, Kutz P, Leube B, Neuen-Jacob E, Schroder JM, Cavallotti D, Vaccario ML, Schaper J, Broich P, Cohn R, Baethmann M, Gohlich-Ratmann G, Scoppetta C, Herrmann R (2001) Autosomal dominant distal myopathy: further evidence of a chromosome 14 locus. Neuromuscul Disord 11:11–19 10.1016/S0960-8966(00)00158-9 [DOI] [PubMed] [Google Scholar]
- von Deimling F, Haravuori H, Bonnemann C, Herrmann R, Brockman K, Osse G, Hanefeld F, Udd B, Voit T (2001) Distal myopathy with childhood onset: a new form with autosomal dominant inheritance. Neuromuscul Disord 11:653–654 [Google Scholar]
- Xu X, Peng M, Fang Z (2000) The direction of microsatellite mutations is dependent upon allele length. Nat Genet 24:396–399 10.1038/74238 [DOI] [PubMed] [Google Scholar]
- Zimprich F, Djamshidian A, Hainfellner JA, Budka H, Zeitlhofer J (2000) An autosomal dominant early adult-onset distal muscular dystrophy. Muscle Nerve 23:1876–1879 [DOI] [PubMed] [Google Scholar]