Abstract
Hereditary nonpolyposis colorectal cancer (HNPCC) is caused by inherited mutations in DNA mismatch-repair genes, most commonly MLH1 or MSH2. The role MSH6 plays in inherited cancer susceptibility is less well defined. The aim of this study was to investigate the penetrance and expressivity of MSH6 mutations in kindreds ascertained through endometrial cancer probands unselected for family history. Detailed pedigrees were constructed for six MSH6 mutation carriers. All reported cancers and precancers were confirmed, and tissues were obtained when available. Tumors were analyzed for microsatellite instability (MSI) and for expression of MSH2, MLH1, and MSH6. MSH6 mutation status was determined for 59 family members. Of these 59 individuals, 19 (32%) had confirmed cancers and precancers. There was an excess of mutation carriers among the 19 affected family members (11 [58%] of 19) compared with those among the 40 unaffecteds (8 [20%] of 40, P=.0065, odds ratio = 5.5, 95% CI = 1.66–18.19). In four of the seven tumors analyzed from mutation carriers other than the probands, MSI and/or MMR protein expression was consistent with the involvement of MSH6. Overall estimated penetrance of the MHS6 mutations was 57.7%. Of the tumors in mutation carriers, 78% were part of the extended HNPCC spectrum. This study demonstrates that MSH6 germline mutations are, indeed, associated with increased cancer risk and that the penetrance of mutations may be higher than appreciated elsewhere. A combination of MSI and immunohistochemistry analyses may be helpful in screening for MSH6 mutation carriers.
Hereditary nonpolyposis colorectal cancer (HNPCC) is an autosomal dominant cancer-susceptibility syndrome characterized by early onset cancers of the colorectum, endometrium, small bowel, ureter, and renal pelvis (Amsterdam criteria II; see Vasen et al. [1999]). Additional extracolonic lesions associated with the syndrome include cancers of the stomach, ovary, brain, and hepatobiliary tract and benign sebaceous adenomas (Vasen et al. 1999). HNPCC is caused by inherited mutations in DNA mismatch-repair genes, most commonly involving MLH1 (MIM 120436) or MSH2 (MIM 120435) (Fishel et al. 1993; Bronner et al. 1994). Defective DNA mismatch repair in HNPCC-associated tumors results in the microsatellite instability (MSI) tumor phenotype (Aaltonen et al. 1993). MSI is found in >90% of colorectal tumors and ∼75% of endometrial tumors associated with HNPCC (Risinger et al. 1993; Fujiwara et al. 1998).
Germline mutations in MSH6 (MIM 600678) have been reported elsewhere as an uncommon cause of HNPCC (Miyaki et al. 1997; Wijnen et al. 1999; Wu et al. 1999). In fact, most MSH6 mutations have been found in kindreds with suspected HNPCC that do not fulfill the classic diagnostic criteria (Kolodner et al. 1999; Shin et al. 1999; Wijnen et al. 1999; Wagner et al. 2001). MSH6 kindreds are often characterized by multiple endometrial cancers, low penetrance of colorectal cancer, and older age at cancer diagnosis. An additional distinguishing feature of MSH6-associated HNPCC is the observation that many cancers arising in mutation carriers exhibit an MSI-low (MSI-L) or MS-stable (MSS) tumor phenotype (Wijnen et al. 1999; Wu et al. 1999; Berends et al. 2002). Functional redundancy in the DNA mismatch-repair system could explain the less extensive MSI observed in tumors in MSH6 germline mutation carriers compared with the MSI of MSH2- or MLH1-associated cancers (Acharya et al. 1996; Marsischky et al. 1996).
The exact role of MSH6 germline mutations in inherited cancer susceptibility is uncertain at this time. Whether inherited defects in MSH6 cause HNPCC, when some MSH6-associated tumors do not appear to be deficient in DNA mismatch repair, is a subject of debate. One possible explanation is that MSH6 defects may affect pathways preceding mismatch-repair function (Kariola et al. 2002). Alternatively, some of the MSH6 mutations found in kindreds with atypical HNPCC may not be the cause of their cancer susceptibility but, rather, an incidental finding. Lastly, it is also possible that MSH6 mutations seemingly associated with MSS tumors cause low-level MSI that is not detected by the NCI consensus panel (Boland et al. 1998) used by most investigators.
We investigated the penetrance and expressivity of presumed pathogenic MSH6 germline mutations in seven kindreds identified through endometrial cancer probands that were not ascertained on the basis of family or medical history (Goodfellow et al. 2003). The loss-of-function mutations in these kindreds are as follows (kindred identification numbers are in boldface): 1401 L634Z; 1335 R911Z; 1497 R911Z; 1389 frameshift, insert TA at codon 1066; 1064 alanine insertion, insert CTG at codons 1163/1164; 1319 frameshift, del CAAG at 1320/1321, and 1524 frameshift, insert TCAAAAGGGACATAGAAAA at 1320. All of the probands’ cancers had high-level microsatellite instability (MSI-H). Detailed family histories were obtained for six of the seven probands. Case 1497 declined to participate in the study, and we had only the reported cancer status for her parents. Information regarding cancers or precancers was obtained for 278 relatives of the 6 other endometrial cancer probands (mean 46 per family, a range of 27–71) over four generations. Reported cancers, possible precancers (including colonic polyps and endometrial hyperplasia), and any tumors of uncertain malignant potential, as well as ages at diagnosis, were verified by a review of medical records, pathology reports, and/or death certificates when available. Among 53 relatives (including 1 parent of case 1497) reported as having cancers or precancers, 29 cases (55%) were confirmed via medical record review, yielding detailed information on 32 (54%) of 59 reported lesions.
MSH6 mutation status was determined for 59 relatives (21% of those reported). The PCR primers and methods used to determine mutation status are included in table A1 (online only). The mean age of these 59 relatives was 51.3 years. Of the 59 family members, 19 (32%) had confirmed cancers or precancers (affected). The remaining 40 relatives we analyzed were unaffected by any confirmed cancers or precancers. There was a statistically significant excess of MSH6 mutation carriers among the affected family members. As shown in table 1, 11 (58%) of 19 affected relatives were mutation carriers, whereas 8 (20%) of 40 unaffected relatives had an MSH6 mutation (P=.0065, odds ratio = 5.5, 95% CI = 1.66–18.19, two-tailed Fisher’s exact test).
Table 1.
Details of Family Size, Number of Individuals with Tumors, and MSH6 Mutation Status for Seven Kindreds of MSH6 Germline Mutation Carriers
|
No. of Relatives with Known Mutation Status |
||||
| Kindreds | No. of Reported Relatives | No. of Relatives withCancers and PrecancersReported/Confirmeda | Affected Carriers/Noncarriersa,b | Unaffected Carriers/Noncarriersa |
| ID: | ||||
| 1524 | 71 | 12/12 | 3/2 | 5/4 |
| 1064 | 57 | 7/4 | 1/2 | 0/7 |
| 1497 | 2 | 1/0 | 0/0 | 0/0 |
| 1319 | 27 | 8/0 | 0/0 | 0/2 |
| 1335 | 27 | 9/4 | 2/1 | 2/3 |
| 1401 | 58 | 9/8 | 5/2 | 1/11 |
| 1389 | 38 | 7/1 | 0/1 | 0/5 |
| All | 280 | 53/29 | 11/8 | 8/32c |
Mean age of relatives with cancers and precancers reported/confirmed is 58.4/63.8 years, of affected carriers/noncarriers is 65.6/64.9 years, and of unaffected carriers/noncarriers is 42.8/47.0 years.
Affected includes only confirmed cancers and precancers.
Of the affected relatives, 11 of 19 were mutation carriers, whereas 8 of 40 unaffected relatives had an MSH6 mutation (P=.0065, Fisher’s exact test).
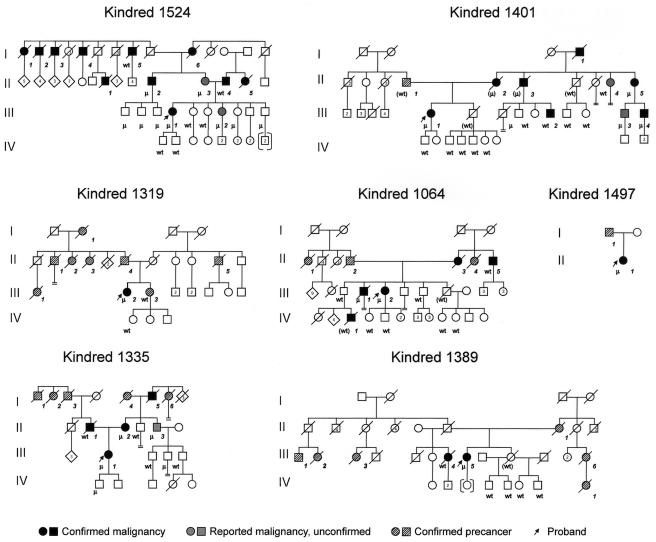
Overall, the affected family members were older than the unaffected ones. The mean age at diagnosis of the 19 affected family members was 64.7 years, whereas the unaffected family members had a mean age of 46.1 years (P=.0018, Student’s t-test). A similar age difference was found between the mean ages of the 11 affected mutation-carriers (65.6 years) and the 8 unaffected mutation-carriers (42.8 years). Table 1 summarizes the information on confirmed cancers and mutation status for each of the seven kindreds. The pedigrees for the seven kindreds, including mutation status and age at onset of tumors, are presented in figure 1.
Figure 1.
MSH6 kindreds. Kindred 1524, I-1 Hodgkin lymphoma, age 58; I-2 Hodgkin lymphoma, age 44; I-3 lung cancer, age 36; I-4 laryngeal cancer, age 61; I-5 colon cancer, age 92; I-6 endometrial cancer, age 65; II-1 lung cancer, age 69; II-2 prostate cancer, age 73; II-3 colonic tubular adenoma, age 69; II-4 lung cancer, age 74; II-5 lung cancer (small cell), age 71, and lung cancer (oat cell), age 78; III-1 endometrial cancer, age 46; III-2 atypical pituitary adenoma, age 38. Kindred 1401, I-1 colon cancer, age 80; II-1 pancreatic cancer, age 73; II-2 cholangiocarcinoma, age 86; II-3 glioblastoma, age 80s; II-4 uterine cancer, age 40s (unconfirmed), and adenomatous colon polyps, age 86; II-5 renal cell cancer, age 50s (unconfirmed), and breast cancer, age 97; III-1 endometrial cancer, age 57; III-2 basal cell cancer, age 56; III-3 adenomatous colon polyps, age 53; III-4 prostate cancer, age 62, and adenomatous colon polyps, age 66. Kindred 1319, I-1 skin cancer, age 60s; II-1 cancer, unknown primary, age 40s; II-2 cancer, unknown primary, age 61; II-3 cancer, unknown primary, age 40; II-4 cancer, unknown primary, age 60; II-5 multiple myeloma, age 70s; III-1 endometrial cancer, age 40s; III-2 endometrial cancer, age 74; III-3 skin cancer, age 50s. Kindred 1064, II-1 breast cancer, age late 20s; II-2 brain cancer, age 60s; II-3 oat cell lung cancer, age 66; II-4 ovarian cancer, late 30s, and colon cancer, age 40s; II-5 rectal cancer, age 65; III-1 tongue squamous cell carcinoma, age 63; III-2 endometrial cancer, age 53, and rectal cancer, age 61; IV-1 medulloblastoma, age 3. Kindred 1497, I-1 esophageal cancer, age unknown; II-1 endometrial cancer, age 52, and ovarian cancer, age 52. Kindred 1335, I-1 lung cancer, age unknown; I-2 liver cancer, age unknown; I-3 prostate cancer, age 40s; I-4 breast cancer, age 60; I-5 prostate cancer, age 60; I-6 endometrial cancer, age 50s; II-1 pancreatic cancer, age 71; II-2 endometrial cancer, age 35, and colon cancer, age 67; II-3 adenomatous colon polyps, age 65; III-1 endometrial cancer, age 44. Kindred 1389, II-1 endometrial cancer, age 30; III-1 cancer, unknown primary, age unknown; III-2 lung cancer, age 60s; III-3 breast cancer, age 70s; III-4 bladder cancer, age 72; III-5 endometrial cancer, age 61; III-6 cancer, unknown primary, age 76; IV-1 cancer, unknown primary, age unknown.
In an effort to further determine whether inherited MSH6 mutations confer an increased risk for cancers in relatives, we compared the rates of confirmed cancers in the first-degree relatives of our 6 MSH6 probands and 40 MSS endometrial cancer probands from the same patient series (described in Whelan et al. [2002]). We reasoned that this MSS cohort is representative of sporadic endometrial cancer families. As the risk of developing cancer increases with age, only first-degree relatives >60 years old and free from cancer or individuals affected with cancer at any age were considered informative and were included in the analysis. A significantly higher number of cancers were found in first-degree relatives of the 6 MSH6 probands than in those of the 40 MSS probands. Among 16 informative first-degree relatives in the MSH6 cohort, 7 cancers were confirmed, versus 17 confirmed cancers in 150 informative first-degree relatives in the MSS cohort (44% vs. 11.3%, P=.0027, two-tailed Fisher’s exact test).
We assessed tumors for MSI and mismatch-repair protein expression by immunohistochemistry. Archival tissue specimens were available for seven cancers/precancers from seven MSH6 mutation carriers. Results of the combined MSI analysis and immunohistochemistry for MSH6 are presented in table 2. For three of the seven mutation carriers (1064:III-1 with a squamous cell carcinoma [SCC] of the tongue, 1524:II-2 with prostate cancer, and 1335:II-3 with a colonic adenoma), the molecular analyses did not suggest an association between MSH6 germline mutation and the development of their tumors. The specimens showed no evidence of MSI at any marker and stained positively for all three mismatch-repair proteins. Because the MSI phenotype is variable in MSH6 mutation carriers, it may be unreliable to use solely MSI to make a connection between mutation and disease, but, if the expression data is considered as well, it seems unlikely that these three tumors have a defect in mismatch repair.
Table 2.
Results of MSI Analysis and IHC of MMR Proteins on Available Tumors of MSH6 Mutation Carriers[Note]
|
MSI Markers |
IHC |
||||||||||||
| Kindred ID | Relationship to Proband | Tumor Typea | Age atDiagnosis | D17S250 | D2S123 | D5S346 | BAT25 | BAT26 | BAT40 | MSIStatus | MSH6 | MSH2 | MLH1 |
| 1064:III-1 | Brother | SCC tongue | 63 | NI | NL | NL | NI | NI | NI | MSS | + | + | + |
| 1524:II-3 | Mother | Colonic adenoma | 69 | MSI | NL | NL | NI | NI | NI | MSI-L | Focal weak | + | + |
| 1524:II-2 | Maternal uncle | Prostate ca | 73 | NL | NL | NL | NI | NI | NL | MSS | + | + | + |
| 1524:III-2 | Sister | Pituitary adenoma | 38 | LOH | LOH | NL | MSI | NI | NI | MSI-L | − | + | + |
| 1335:II-2 | Mother | Colon ca | 67 | MSI | MSI | MSI | MSI | MSI | MSI | MSI-H | − | + | + |
| 1335:II-3 | Maternal uncle | Colonic adenoma | 65 | NL | NL | NI | ND | NI | NI | MSS | + | + | + |
| 1401:III-4 | Maternal cousin | Prostate ca | 62 | ND | ND | ND | ND | ND | ND | ND | − | ND | + |
Note.— MSI analysis was performed using five consensus markers (Boland et al. 1998). Since MSH6 mutations tend to have lower levels of MSI and MSI is more frequently found in mononucleotide repeats, an additional mononucleotide marker (BAT40) was typed. Samples were classified as MSI-H if instability was detected at two or more consensus markers, as MSI-L if instability was confined to one consensus marker, and as MSS if none of the consensus markers revealed instability. IHC for MLH1, MSH2, and MSH6 was performed with the use of 5-μm thick paraffin sections mounted on charged slides, as described elsewhere (Buttin et al. 2004). Sections of normal appendix served as external positive controls. Nuclear staining was read as positive (+), and absence of nuclear staining of any of these was read as negative (−). Abbreviations in the table are as follows: ca = carcinoma, LOH = loss of heterozygosity, NL = no loss, NI = not informative, and ND = no data.
Includes only confirmed cancers and precancers (see “Subjects and Methods”).
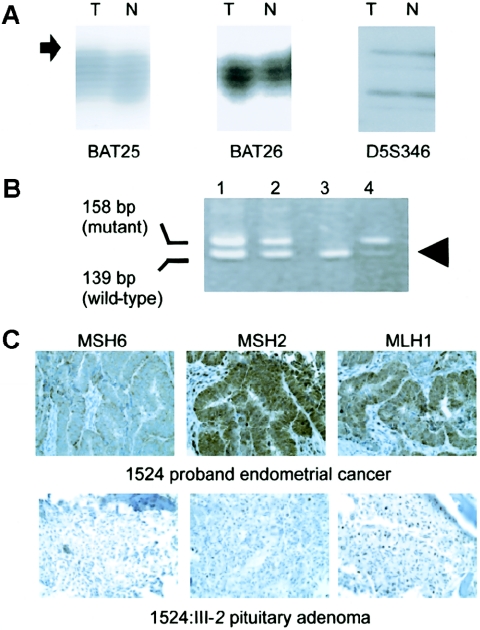
For two of the seven mutation carriers (1524:III-2 with a pituitary adenoma and 1335:II-2 with colon cancer) the molecular analysis of the tumors suggested a causative role for MSH6. The pituitary adenoma had MSI at BAT25 (fig. 2A) as well as loss of the wild-type copy of MSH6 (fig. 2B), whereas the colon cancer had MSI at all markers (data not shown). MSH6 staining was absent for both the pituitary adenoma and colon carcinoma (fig. 2C and additional data, not shown). For the remaining two mutation carriers (1524:II-3 with a colonic adenoma and 1401:III-4 with prostate cancer), evidence for the involvement of MSH6 was inconclusive. The adenoma from 1524:II-3 showed focally positive staining for MSH6 but had MSI at D17S250 (data not shown). The prostate carcinoma from 1401:III-4 had absent MSH6 staining (data not shown); however, there was insufficient tissue to perform MSI analysis on this tumor.
Figure 2.
Molecular analysis of an atypical pituitary adenoma that arose in an MSH6 mutation carrier. A, MSI analysis. Instability at the BAT25 marker is seen as a subtle increase in the size of the PCR products in the tumor DNA (T), compared with those in the normal DNA (N), and as a novel insertion (arrow). The tumor and matched normal specimens show identical patterns with the BAT26 and D5S346 markers. B, Mutation analysis revealing loss of the wild-type allele in the tumor (arrowhead). C, Immunohistochemistry revealing loss of expression of MSH6 in the proband’s endometrial cancer (upper panel) and in the atypical pituitary adenoma (lower panel). Note the positive staining with MLH1 and MSH2 in both tumors.
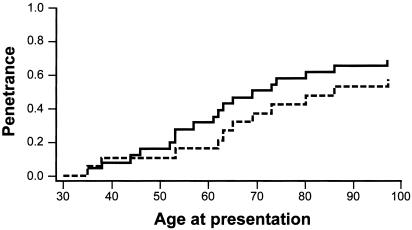
The overall penetrance of MSH6 germline mutations was 69.2% (18 affected individuals among 26 mutation carriers, including the probands), on the assumption that all cancers/precancers were associated with MSH6 mutation. Figure 3 illustrates the fraction of penetrant family members plotted against age in years. The estimated penetrance decreases to 57.7% if we exclude the three affected mutation-carriers for whom no evidence for MSH6 involvement in their tumors was found.
Figure 3.
Penetrance of MSH6 mutations in 26 mutation carriers. The seven endometrial cancer probands are included in one plot (solid line) and are excluded in the other plot (dashed line).
The different types of confirmed cancers and precancers found in MSH6 mutation carriers and noncarriers, excluding the probands, are presented in table 3. Overall, 9 (69%) of 13 cancers/precancers in mutation carriers were HNPCC-associated lesions in accordance with the ICG Research Criteria (Vasen et al. 1999), compared with 4 (50%) of 8 cancers/precancers in the nonmutation carrier group. As described elsewhere (Buttin et al. 2004), two of the seven probands, 1064 and 1497, had second HNPCC-associated cancers: rectal and ovarian carcinomas, respectively.
Table 3.
Summary of Cancers and Precancers Found in MSH6 Mutation Carriers Versus Noncarriers
| Precancer/Cancera,b | No. in MSH6 Mutation Carriers | No. inNoncarriers |
| Colonic adenoma | 4 | 1 |
| Colon ca | 1 | 1 |
| Endometrial ca | 1 | 0 |
| Rectal ca | 0 | 1 |
| Hepatobiliary ca | 1 | 0 |
| Bladder ca | 0 | 1 |
| Brain ca | 2 | 1 |
| Pancreatic ca | 0 | 1 |
| Lung ca | 0 | 1 |
| Skin ca | 1 | 1 |
| Prostate ca | 2 | 0 |
| Breast ca | 1 | 0 |
| All HNPCC-associated | 9 (of 13) | 4 (of 8) |
ca = carcinoma.
Tumors in italics are associated with HNPCC in accordance with the ICG definition of “Lynch Syndrome” (Vasen et al. 1999).
Although the link between MSH2, MLH1, and HNPCC has been firmly established, the exact role of MSH6 in inherited cancer susceptibility continues to evolve. MSH6 germline mutations are a cause of HNPCC, although most families with mutations do not conform to the Amsterdam II criteria. Screening for MSH6 mutation is recommended for kindreds with suspected inherited cancer susceptibility suggestive of HNPCC who test negative for MSH2 and MLH1 germline mutations (Lynch and de la Chapelle 2003). It should be emphasized that mutations in MSH6 are more frequent in kindreds that fail to meet HNPCC diagnostic criteria. The true incidence of MSH6 germline mutations in HNPCC is yet to be determined.
Efforts to define the role of MSH6 in HNPCC, and in cancer susceptibility in general, have been hampered by the fact that MSH6 mutations may be associated with tumors that lack features usually associated with defective DNA mismatch repair and the fact that kindreds segregating MSH6 mutations may be lacking the clinical hallmarks of HNPCC. Hence, screening on the basis of either tumor MSI status or cancer family history may fail to identify a subset of patients with MSH6 germline defects. There is a clear need to further characterize the natural history of MSH6 germline mutations, to better define MSH6-associated HNPCC and, ultimately, to devise appropriate identification, clinical screening, and treatment strategies.
We evaluated seven kindreds in an effort to clarify the role of MSH6 germline mutations in inherited cancer susceptibility. Unlike prior studies, we investigated families of MSH6 germline mutation carriers that were identified independent of family history or age at diagnosis. The MSH6 germline mutations were detected in women with MSI-H endometrial cancers. Given the reported association between MSH6 germline mutations and MSI-L or MSS tumors (Wu et al. 1999; Wagner et al. 2001; Berends et al. 2002), our results may suggest that the mutations are different from those described elsewhere. It is possible that kindreds ascertained through probands with MSI-L or MSS tumors have different penetrance and expressivity from that of the kindreds we studied. Regardless, the family histories of our MSH6 germline mutation carriers underscore the uncertainty as to who should be screened for MSH6 germline mutations. We note a heterogeneous spectrum of cancers and precancers (colonic adenomas). Two kindreds (1335 and 1401) could be classified as atypical HNPCC families (because of two, as opposed to three, first-degree relatives with endometrial/colon cancer in kindred 1335 and because of older age at diagnosis in kindred 1401). However, the other four kindreds may not have been identified as families with increased risk for inherited cancer susceptibility on the basis of family history alone.
The families we studied have many features in common with MSH6 kindreds that have been described elsewhere. However, we detected only 1 case of colon carcinoma among 13 different types of cancers and precancers found in 11 affected mutation-carriers (excluding the probands). This finding confirms the suggestion that MSH6 kindreds may have a lower penetrance of colorectal cancer (Wijnen et al. 1999) in comparison with that of MSH2 and MLH1 HNPCC kindreds. We did find an increased number of colonic adenomas in the mutation carriers compared with the number found in noncarriers (four vs. one), possibly suggesting increased potential for carcinogenesis with very slow progression. We found one case of endometrial cancer among the five female affected mutation-carriers with a mean age of 65 years, in addition to the six known endometrial cancers in the probands. However, many of the female family members in the older generations had hysterectomies at an early age for benign reasons. Other authors have reported a predominance of endometrial cancers (52%–73%) in female MSH6 germline mutation carriers (Wijnen et al. 1999; Wagner et al. 2001; Berends et al. 2002). Including our six endometrial cancer probands, the incidence of endometrial cancer in female mutation carriers in this study (7 [64%] of 11) confirms this suggestion.
Although the comparison was limited by small numbers, there was no significant difference between the types of cancers found in mutation carriers and those found in nonmutation carriers (table 3), with the exception of an excess of colonic adenomas in the mutation carriers. This clinical heterogeneity (variable expressivity) underlines the limitations of screening patients for a family history suggestive of HNPCC or atypical HNPCC to detect those with germline MSH6 defects.
Our data demonstrate that MSH6 germline mutations are, indeed, associated with increased cancer risk. Alhough the mouse studies by Edelmann et al. (1997) suggest that MSH6 mutation is associated with highly penetrant, late-onset disease, studies of humans, to date, could not truly address onset and penetrance, as the majority of kindreds evaluated were chosen on the basis of increased familial risk. Screening patients for inherited cancer susceptibility associated with MSH6 mutation perhaps should include an awareness of the overall increased numbers of relatives with any cancer at any age, rather than a specific focus on HNPCC-type disease. The exact tumor spectrum associated with MSH6 defects will require further investigation.
Lastly, the results of our molecular correlative studies suggest the utility of immunohistochemistry in the assessment of the role of MSH6 in tumorigenesis. We demonstrated a very consistent pattern of absent staining for MSH6 in all endometrial tumors of our seven probands, whereas both MSH2 and MLH1 staining remained intact. These findings confirm a report by de Leeuw et al. (2000), in which the authors described consistent isolated absence of MSH6 staining in tumors from MSH6 germline mutation carriers, consistent absence of both MSH2 and MSH6 staining in MSH2 mutation carriers, and inconsistent results in tumors from MLH1 mutation carriers. De Leeuw et al.'s study (2000) showed more variability with regard to MSI than did ours. As noted, all of our index endometrial cancers were MSI-H, and the mutations we evaluated are all presumed loss-of-function defects. However, some of the other tumors found in MSH6 mutation carriers with absent or questionable MSH6 staining were MSI-L (the colonic adenoma in 1524:II-3 had MSI at D17S250, and the pituitary adenoma in 1524:III-2 had MSI at BAT25), and none were MSS (table 2). The combination of MSI and immunohistochemistry (IHC) analysis was able to demonstrate that three tumors arising in MSH6 mutation carriers, (a SCC of the tongue in 1064:III-1, a prostate carcinoma in 1524:II-2, and a colonic adenoma in 1335:II-3) were unlikely to be associated with loss of MSH6 function.
Our data provide only an estimate of the penetrance of MSH6 germline mutations because we were unable to obtain tissue on all confirmed cancers in mutation carriers. Therefore, it is possible that we may have overestimated the penetrance of the mutations. However, our conservative estimate of 57.7% total penetrance (excluding the three affected mutation-carriers without any evidence of MSH6 involvement in their tumors) is still higher than elsewhere appreciated. As expected, the mean age of cancer diagnosis among affected mutation-carriers (65.6 years) is significantly higher than the mean age reported for HNPCC patients with MLH1 or MSH2 mutations (41–48 years, depending on cancer type; see Wagner et al. [2001]).
In summary, our study represents an unbiased analysis of the natural history, including penetrance and expressivity, of MSH6 germline mutations. We demonstrate an increased cancer risk associated with these mutations, although the exact tumor spectrum remains to be elucidated. Our data confirm other reports that some MSH6 kindreds fall under the extended or atypical HNPCC spectrum and also show that other kindreds are characterized by tumors only recently implicated in HNPCC, such as prostate cancer (Soravia et al. 2003), or not previously associated with HNPCC, such as an atypical pituitary adenoma. The overall penetrance of MSH6 germline mutations may be as high as 58%, and, as noted by other investigators, the age at onset of cancers is later than is seen in kindreds segregating MSH2 and MLH1 mutations. Neither tumor MSI status, young age at cancer diagnosis, nor a family history suggestive of atypical or classic HNPCC will identify all MSH6 mutation carriers. A combination of MSI and IHC analyses for mismatch-repair–protein expression in tumor tissue may be helpful in screening for possible mutation carriers.
Acknowledgments
We thank Jennifer Ivanovich for her assistance in collecting family histories, Rhonda Walters for her help with immunohistochemistry, Arie Perry for assistance with procurement of archived pathology specimens, and Ken Walls for his help with SSCV analysis. We also thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis for the use of the Hereditary Cancer Core. The Siteman Cancer Center is supported in part by National Cancer Institute Cancer Center Support Grant P30 CA91842. Our study is supported in part by grant RO1-CA71754 from the National Cancer Institute.
Appendix A
Table A1.
Primers and PCR Conditions for Heteroduplex Analysis of MSH6 Germline Mutations and for SSCV Analysis of MSH6 Polymorphisms[Note]
| Sequence Variant | MSH6 Exon | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Annealing Temperature (°C) | Size (bp) | Restriction |
| 1401 mutation | 4 | agattcttctggccatactcg | gctcctgatcaataaggcattt | 60 | 458 | None |
| 1389 mutation | 5 | ctgataaaacccccaaacgatg | agtcttcgtaatgcaaggatgg | 60 | 186 | None |
| 1064 mutation | 6 | gaccttttcctccctcattcac | tgaatgagaacttaagtgggaaacaa | 61 | 177 | None |
| 1319 mutation | 9 | tctgttgctagcacatgtatcg | cccttccccttttactgtttct | 60 | 315 | None |
| 1524 mutation | 9 | ttcattaagggagcttgtcct | accgaaataatcgtagtgactga | 60 | 139 | None |
| Polymorphism | 1A | ggagctccgtccgacagaacg | cctccgttgaggttcttc | 55 | 271 | None |
| Polymorphism | 2 | actgcctttaaggaaacttgacc | cacacacatggcagtagtgactc | 60 | 284 | None |
| Polymorphism | 3 | ggattacagtcgtgagcctctg | taatacaccctccccctttctttt | 60 | 302 | None |
| Polymorphism | 4A | ggttttccaaattttgatttgttt | aatgctagttgcttgtttggtg | 55 | 410 | HinfI |
| Polymorphism | 5 | ctgataaaacccccaaacgatg | ctgtgtttggaaaatgatcacc | 60 | 403 | Sau3AI |
| Polymorphism | 7B | gtgaaactgccagcatactcat | tgagtgcgtgctctaaaaacat | 55 | 184 | None |
Note.— Mutation analysis was performed on blood and/or archival tissue specimens of all available family members. The single-base substitution mutation in kindred 1335 was demonstrated by direct sequencing. The remaining mutations associated with insertions or deletions were demonstrated by heteroduplex analysis, resolving PCR products on nondenaturing 10% acrylamide gels. PCR primers and annealing temperatures are listed. For deceased relatives for whom no tissue specimens could be obtained, MSH6 mutation status was deduced by reconstruction of haplotypes for the MSH6 region on chromosome 2p. DNA from the surviving spouse and children of the deceased was genotyped for six intragenic MSH6 polymorphic markers (exons 1, 2, 3, 4, 5, and 7) as well as three polymorphic markers in introns 2 and 7 of MSH2. The intragenic MSH6 variants were detected by single strand conformational variant (SSCV) analysis. The MSH2 intragenic markers were as described elsewhere by Desai et al. (2000) for a TTTA repeat in intron 2. TAAA and TTTG repeats in intron 7 were also evaluated.
Electronic-Database Information
URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
References
- Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Powell SM, Jen J, Hamilton SR, Petersen GM, Kinzler KW, Vogelstein B, de la Chapelle A (1993) Clues to the pathogenesis of familial colorectal cancer. Science 260:812–816 [DOI] [PubMed] [Google Scholar]
- Acharya S, Wilson T, Gradia S, Kane MF, Guerrette S, Marsischky GT, Kolodner R, Fishel R (1996) hMSH2 forms specific mispair-binding complexes with hMSH3 and hMSH6. Proc Natl Acad Sci USA 93:13629–13634 10.1073/pnas.93.24.13629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berends MJ, Wu Y, Sijmons RH, Mensink RG, van der Sluis T, Hordijk-Hos JM, de Vries EG, Hollema H, Karrenbeld A, Buys CH, van der Zee AG, Hofstra RM, Kleibeuker JH (2002) Molecular and clinical characteristics of MSH6 variants: an analysis of 25 index carriers of a germline variant. Am J Hum Genet 70:26–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S (1998) A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 58:5248–5257 [PubMed] [Google Scholar]
- Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, Kane M, Earabino C, Lipford J, Lindblom A, Tannergård P, Bollag RJ, Godwin AR, Ward DC, Nordenskjld M, Fishel R, Kolodner R, Liskay RM (1994) Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 368:258–261 10.1038/368258a0 [DOI] [PubMed] [Google Scholar]
- Buttin BM, Powell MA, Mutch DG, Rader JS, Herzog TJ, Gibb RK, Huettner P, Bocker-Edmonston T, Goodfellow PJ (2004) Increased risk for HNPCC-associated synchronous and metachronous malignancies in patients with MSI-positive endometrial carcinoma lacking MLH1 promoter methylation. Clin Cancer Res 10:481–490 [DOI] [PubMed] [Google Scholar]
- de Leeuw WJ, Dierssen J, Vasen HF, Wijnen JT, Kenter GG, Meijers-Heijboer H, Brocker-Vriends A, Stormorken A, Moller P, Menko F, Cornelisse CJ, Morreau H (2000) Prediction of a mismatch repair gene defect by microsatellite instability and immunohistochemical analysis in endometrial tumours from HNPCC patients. J Pathol 192:328–335 [DOI] [PubMed] [Google Scholar]
- Desai DC, Lockman JC, Chadwick RB, Gao X, Percesepe A, Evans DG, Miyaki M, Yuen ST, Radice P, Maher ER, Wright FA, de La Chapelle A (2000) Recurrent germline mutation in MSH2 arises frequently de novo. J Med Genet 37:646–652 10.1136/jmg.37.9.646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann W, Yang K, Umar A, Heyer J, Lau K, Fan K, Liedtke W, Cohen PE, Kane MF, Lipford JR, Yu N, Crouse GF, Pollard JW, Kunkel T, Lipkin M, Kolodner R, Kucherlapati R (1997) Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell 91:467–477 10.1016/S0092-8674(00)80433-X [DOI] [PubMed] [Google Scholar]
- Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R (1993) The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75:1027–1038 10.1016/0092-8674(93)90546-3 [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Stolker JM, Watanabe T, Rashid A, Longo P, Eshleman JR, Booker S, Lynch HT, Jass JR, Green JS, Kim H, Jen J, Vogelstein B, Hamilton SR (1998) Accumulated clonal genetic alterations in familial and sporadic colorectal carcinomas with widespread instability in microsatellite sequences. Am J Pathol 153:1063–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, Look K, Walls KC, Fan MY, Mutch DG (2003) Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci USA 100:5908–5913 10.1073/pnas.1030231100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariola R, Raevaara TE, Lonnqvist KE, Nystrom-Lahti M (2002) Functional analysis of MSH6 mutations linked to kindreds with putative hereditary non-polyposis colorectal cancer syndrome. Hum Mol Genet 11:1303–1310 10.1093/hmg/11.11.1303 [DOI] [PubMed] [Google Scholar]
- Kolodner RD, Tytell JD, Schmeits JL, Kane MF, Gupta RD, Weger J, Wahlberg S, Fox EA, Peel D, Ziogas A, Garber JE, Syngal S, Anton-Culver H, Li FP (1999) Germ-line msh6 mutations in colorectal cancer families. Cancer Res 59:5068–5074 [PubMed] [Google Scholar]
- Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348:919–932 10.1056/NEJMra012242 [DOI] [PubMed] [Google Scholar]
- Marsischky GT, Filosi N, Kane MF, Kolodner R (1996) Redundancy of saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev 10:407–420 [DOI] [PubMed] [Google Scholar]
- Miyaki M, Konishi M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Yasuno M, Igari T, Koike M, Chiba M, Mori T (1997) Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet 17:271–272 [DOI] [PubMed] [Google Scholar]
- Risinger JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J (1993) Genetic instability of microsatellites in endometrial carcinoma. Cancer Res 53:5100–5103 [PubMed] [Google Scholar]
- Shin KH, Ku JL, Park JG (1999) Germline mutations in a polycytosine repeat of the hMSH6 gene in Korean hereditary nonpolyposis colorectal cancer. J Hum Genet 44:18–21 10.1007/s100380050099 [DOI] [PubMed] [Google Scholar]
- Soravia C, van der Klift H, Brundler MA, Blouin JL, Wijnen J, Hutter P, Fodde R, Delozier-Blanchet C (2003) Prostate cancer is part of the hereditary non-polyposis colorectal cancer (HNPCC) tumor spectrum. Am J Med Genet 121A:159–162 [DOI] [PubMed] [Google Scholar]
- Vasen HF, Watson P, Mecklin JP, Lynch HT (1999) New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 116:1453–1456 [DOI] [PubMed] [Google Scholar]
- Wagner A, Hendriks Y, Meijers-Heijboer EJ, de Leeuw WJ, Morreau H, Hofstra R, Tops C, Bik E, Brocker-Vriends AH, van Der Meer C, Lindhout D, Vasen HF, Breuning MH, Cornelisse CJ, van Krimpen C, Niermeijer MF, Zwinderman AH, Wijnen J, Fodde R (2001) Atypical HNPCC owing to MSH6 germline mutations: analysis of a large Dutch pedigree. J Med Genet 38:318–322 10.1136/jmg.38.5.318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan AJ, Babb S, Mutch DG, Rader J, Herzog TJ, Todd C, Ivanovich JL, Goodfellow PJ (2002) MSI in endometrial carcinoma: absence of MLH1 promoter methylation is associated with increased familial risk for cancers. Int J Cancer 99:697–704 10.1002/ijc.10429 [DOI] [PubMed] [Google Scholar]
- Wijnen J, de Leeuw W, Vasen H, van der Klift H, Moller P, Stormorken A, Meijers-Heijboer H, Lindhout D, Menko F, Vossen S, Moslein G, Tops C, Brocker-Vriends A, Wu Y, Hofstra R, Sijmons R, Cornelisse C, Morreau H, Fodde R (1999) Familial endometrial cancer in female carriers of MSH6 germline mutations. Nat Genet 23:142–144 10.1038/13773 [DOI] [PubMed] [Google Scholar]
- Wu Y, Berends MJ, Mensink RG, Kempinga C, Sijmons RH, van Der Zee AG, Hollema H, Kleibeuker JH, Buys CH, Hofstra RM (1999) Association of hereditary nonpolyposis colorectal cancer-related tumors displaying low microsatellite instability with MSH6 germline mutations. Am J Hum Genet 65:1291–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]