Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice. We first reported an S140G mutation of KCNQ1, an α subunit of potassium channels, in one Chinese kindred with AF. However, the molecular defects and cellular mechanisms in most patients with AF remain to be identified. We evaluated 28 unrelated Chinese kindreds with AF and sequenced eight genes of potassium channels (KCNQ1, HERG, KCNE1, KCNE2, KCNE3, KCNE4, KCNE5, and KCNJ2). An arginine-to-cysteine mutation at position 27 (R27C) of KCNE2, the β subunit of the KCNQ1-KCNE2 channel responsible for a background potassium current, was found in 2 of the 28 probands. The mutation was present in all affected members in the two kindreds and was absent in 462 healthy unrelated Chinese subjects. Similar to KCNQ1 S140G, the mutation had a gain-of-function effect on the KCNQ1-KCNE2 channel; unlike long QT syndrome–associated KCNE2 mutations, it did not alter HERG-KCNE2 current. The mutation did not alter the functions of the HCN channel family either. Thus, KCNE2 R27C is a gain-of-function mutation associated with the initiation and/or maintenance of AF.
Atrial fibrillation (AF [MIM 607554]) is the most common cardiac arrhythmia. Its prevalence is 0.4% in the general population, increasing to ∼6% in those >65 years of age. It is estimated that 2.2 million Americans have this rhythm disorder. AF causes a 2-fold increase in mortality and a 2–6-fold increase in risk for stroke. In the United States alone, AF is implicated in ∼75,000 strokes each year. Despite its high prevalence and severe complications, a definitive treatment approach has not been established. AF remains a challenging hurdle in endeavors to cure supraventricular arrhythmias (Ryder and Benjamin 1999; Chugh et al. 2001; Nattel 2002).
AF is characterized by rapid and irregular atrial activation. Since Mackenzie (1904) first described an arrhythmia, now known as AF, important advances in our understanding of the pathophysiology of AF have been made. AF may be due to multiple wavelet re-entry or focal activation mainly from pulmonary vein foci. Whatever the initiating mechanism for AF is, multiple wavelet re-entry has been widely accepted to be the maintaining mechanism of AF. A shortening of the atrial effective refractory period (ERP) has been considered a major substrate for multiple wavelet re-entry for several decades (Nattel 1999, 2002; Nattel et al. 2000). Atrial electrical remodeling also plays a role in the maintenance of AF (Nattel 2002). Nevertheless, the molecular basis of AF remains largely unknown.
We have identified the first molecular genetic defect for familial AF in a Chinese kindred. By linkage analysis, the locus was mapped to chromosome 11p15.5. Candidate gene sequencing revealed an S-to-G mutation at position 140 of KCNQ1, an α subunit of potassium channels. Functional analysis of the mutation revealed a gain-of-function effect on both KCNQ1-KCNE1 and KCNQ1-KCNE2 channels. The mutation was not found in unrelated patients with familial AF (Chen et al. 2003). Other loci on chromosome 10q22-24 (Brugada et al. 1997) and chromosome 6q14-16 (Ellinor et al. 2003) have also been identified as being linked to AF in some families but not in others (Darbar et al. 2003). Therefore, familial AF is genetically heterogeneous.
Long QT syndrome (LQTS [MIM 607542]) is another genetically heterogenous rhythm disorder that is characterized by prolongation of QT duration, ventricular tachyarrhythmia, and sudden cardiac death. It is mainly due to mutations in potassium and sodium channels (Chiang and Roden 2000; Tristani-Firouzi et al. 2002; Mohler et al. 2003). Changes in the properties of these channels have been observed not only in LQTS but also in AF (Van Wagoner and Nerbonne 2000; Nattel 2002). Thus, we hypothesized that familial AF also results, at least in part, from mutations in these channels. In view of this, we sequenced eight potassium-channel genes in 28 unrelated Chinese kindreds with AF and analyzed the function of the mutation. KCNE2, a β subunit of potassium channels (Tinel et al. 2000), was implicated in AF for the first time.
A total of 28 kindreds with familial AF were identified among the Chinese Han population and participated in the study. All of the affected subjects met diagnostic criteria: electrocardiographic evidence of AF and exclusion of organic heart diseases and other causative factors, such as hypertension, coronary artery disease, valvular heart disease, congenital heart disease, dilated or hypertrophic cardiomyopathy, congestive heart failure, pericarditis, diabetes, chronic obstructive pulmonary disease, and hyperthyroidism. The study was approved by the ethical committee at Tongji University School of Medicine (Shanghai). Written informed consent was obtained from the adult subjects and the parents of the minors. Minors also agreed to participate. All subjects were evaluated by a thorough medical history, physical examination, electrocardiography, and echocardiography.
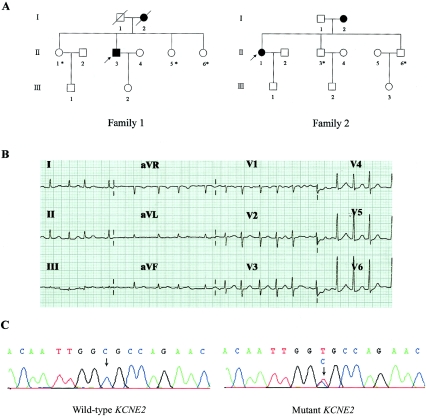
We evaluated the probands in the 28 kindreds with familial AF. The etiology of the AF was not evident in any of the probands. We sequenced the eight potassium ion–channel genes (KCNQ1 [GenBank accession number AJ006345], HERG [NT_007914], KCNE1 [AP001720], KCNE2 [NM_005136], KCNE3 [XM_208561], KCNE4 [NT_005403], KCNE5 [NM_012282], and KCNJ2 [NT_035430]) after PCR amplification of genomic DNA. The same missense mutation in KCNE2 was found in two of the probands. The proband in family 1 presented with paroxysmal AF with rapid ventricular response as frequently as once a week (table 1 and fig. 1A), and the proband in family 2 also presented with paroxysmal AF with rapid ventricular response once or twice a month (table 1 and fig. 1A). The two probands were both heterozygous for a C-to-T transition at position 79 from the translation initiation codon (79C→T) of KCNE2. The 79C→T transition led to the substitution of an arginine for a cysteine at position 27 (R27C) of KCNE2 (fig. 1C).
Table 1.
Clinical Characteristics of Subjects with the KCNE2 R27C Substitution[Note]
| Familyand Member | Sex | Age(years) | Age at AFDiagnosis(years) | RecurrentPalpitation | PrematureAtrial Complexes | AF Classification | MeanVentricular Rate(bpm) | Mean QTca(ms) | Left Atrial Size(mm) | LVEFb(%) |
| Family 1: | ||||||||||
| I-2 | F | 69c | 50 | + | + | Paroxysmal | 83/118d | .37/.43d | 44 | 61 |
| II-1 | F | 52 | NA | + | + | NA | 65 | .38 | 36 | 70 |
| II-3 | M | 50 | 46 | + | + | Paroxysmal | 66/148d | .36/.40d | 35 | 64 |
| II-5 | F | 48 | NA | + | + | NA | 71 | .42 | 37 | 67 |
| II-6 | F | 46 | NA | − | + | NA | 89 | .41 | 34 | 65 |
| Family 2: | ||||||||||
| I-2 | F | 80 | 62 | + | + | Permanent | 91d | .44d | 42 | 60 |
| II-1 | F | 57 | 56 | + | + | Paroxysmal | 78/138d | .43/.42d | 31 | 69 |
| II-3 | M | 55 | NA | + | + | NA | 72 | .36 | 36 | 71 |
| II-6 | M | 51 | NA | + | + | NA | 74 | .42 | 34 | 64 |
Note.— NA = not available or not applicable; + = present; − = absent.
Corrected QT interval.
Left-ventricular ejection fraction.
Age at death.
The value during AF.
Figure 1.
KCNE2 R27C mutation associated with AF. A, Pedigrees of families 1 and 2. Squares indicate male family members; circles, female family members; symbols with a slash, members who had died; blackened symbols, affected members; unblackened symbols, unaffected members; and arrows, probands. Asterisks indicate mutation carriers who had premature atrial complexes. II-1 and II-5 in family 1 and II-3 and II-6 in family 2 had apparent recurrent palpitation. B, Standard 12-lead electrocardiogram of an affected member (II-1 in family 2). C, Sequence analysis of DNA from the affected family members, after PCR amplification revealed a C→T substitution at nucleotide 79 in KCNE2 causing an R27C mutation in families 1 and 2.
We then amplified and sequenced KCNE2 in all members of these two families. The R27C mutation was found in three sisters of the proband in family 1 and in the mother and two younger brothers of the proband in family 2. The mother of the proband in family 1 had died before this study and had a history of paroxysmal AF, and the mother of the proband in family 2 had permanent AF. At the time of the study, neither the sisters of the proband in family 1 nor the brothers of the proband in family 2 had AF during monitoring of 12-lead electrocardiograms. Mutation R27C and AF were absent in the other members of families 1 and 2. Individuals II-1 and II-5 in family 1 and II-3 and II-6 in family 2 had apparent recurrent palpitation (table 1 and fig. 1A).
By 24-h electrocardiographic monitoring of all members of these two families, we found that all patients with paroxysmal AF had frequent premature atrial complexes when in sinus rhythm and that all R27C carriers had 17–120 premature atrial complexes during 24-h recordings. Individual I-2 in family 2 also had frequent premature atrial complexes before the onset of permanent AF (table 1).
To confirm that the R27C substitution in KCNE2 was not a benign polymorphism, we amplified and sequenced the exons flanking the substitution in 462 unrelated healthy Chinese individuals of Han nationality. None of them carried the substitution. In addition, the same substitution was found in two families with AF. Furthermore, the KCNE2 R27C substitution was also absent in a panel of 744 healthy individuals, in a recent study of the frequency and spectrum of cardiac K+ channel variants in white, black, Asian, and Hispanic populations (Ackerman et al. 2003). These results indicated that KCNE2 R27C was a novel mutation.
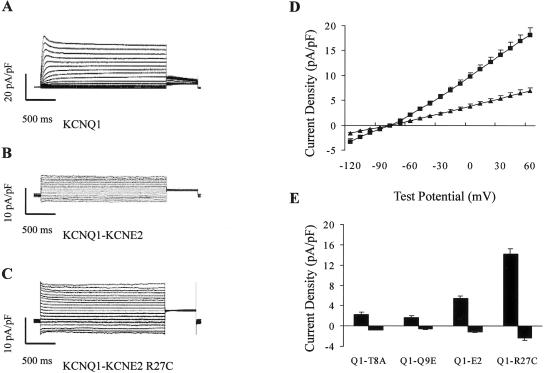
We then analyzed the effect of R27C on potassium channels by mutagenesis, transfection, and whole-cell patch clamping (methods for mutagenesis, transfection, and whole-cell patch clamping are available on request). KCNE2 is the β subunit of the KCNQ1-KCNE2 channel, which produces background potassium current (Tinel et al. 2000). The KCNQ1-KCNE2 current density at all voltage levels was significantly increased when the R27C mutant was coexpressed with KCNQ1 in COS-7 cells at room temperature. Both inward and outward KCNQ1-KCNE2 currents were larger with the KCNE2 mutation (fig. 2B–2D). At −110 mV, current densities for KCNQ1-KCNE2 and KCNQ1-KCNE2 R27C were −1.2 ± 0.1 pA/pF (n=30) and −2.4 ± 0.4 pA/pF (n=26) (P=.041 [Student’s t test]), respectively, and, at +30 mV, current densities were 5.4 ± 0.5 pA/pF (n=30) and 14.2 ± 1.1 pA/pF (n=26) (P<.001 [Student’s t test]), respectively (fig. 2D). At physiological temperature (37°C), we also obtained a similar cellular electrophysiological result (data not shown).
Figure 2.
Gain-of-function effect of the KCNE2 R27C mutation. A–C, Representative whole-cell current traces. These were recorded from COS-7 cells transfected with KCNQ1 alone (A), cells cotransfected with KCNQ1 and KCNE2 (B), and cells cotransfected with KCNQ1 and KCNE2 R27C (C). D, Plot of current density versus voltage for cotransfection of KCNQ1 and KCNE2 (▴) (n = 30) or for cotransfection of KCNQ1 and KCNE2 R27C (▪) (n=26) at room temperature. E, Comparison of current density at −110 mV (inward current) and +30 mV (outward current) at room temperature. Q1-T8A represents cells cotransfected with KCNQ1 and KCNE2 T8A (n=15); Q1-Q9E, cells cotransfected with KCNQ1 and KCNE2 Q9E (n=14); Q1-E2, cells cotransfected with KCNQ1 and KCNE2 (n=30); and Q1-R27C, cells cotransfected with KCNQ1 and KCNE2 R27C (n=26). Cells were held at −80 mV before depolarization to various test potentials from −120 mV to +60 mV in 10-mV increments for 2,000 ms and then were held at −40 mV for 500 ms.
In a previous study of familial AF, we showed that the S140G mutation in KCNQ1 had a drastic gain-of-function effect on the KCNQ1-KCNE2 channel as well as on the KCNQ1-KCNE1 channel, leading to permanent AF (Chen et al. 2003). In the present study, we have demonstrated that the R27C mutation in KCNE2 resulted in a qualitatively similar effect—that is, an increase in both outward and inward KCNQ1-KCNE2 currents. The increase of inward potassium current at hyperpolarized potential can stabilize resting membrane potential and can shorten the atrial action potential duration, whereas the amplification of outward potassium current at depolarized potential can shorten the repolarization phase of atrial action potential. These functional alterations of the potassium channel caused by the KCNE2 R27C mutation may create a substrate favorable to a multiple wavelet re-entry, which has been considered to be the dominant conceptual model of AF (Nattel 2002).
There are some differences in phenotype between the KCNQ1 S140G mutation and the KCNE2 R27C mutation; the patients with AF who had the KCNE2 R27C mutation had a less severe phenotype. They exhibited a paroxysmal type instead of a permanent type of AF and had a later age at onset (earliest onset 46 years, as compared with 5 years). The phenotypic differences may be related to the different functional consequences of the mutations on potassium currents: the KCNQ1 S140G mutation had a very strong gain-of-function effect on both KCNQ1-KCNE1 and KCNQ1-KCNE2 currents, whereas the KCNE2 R27C mutation only moderately amplified KCNQ1-KCNE2 currents.
All reported KCNE2 mutations, such as T8A and Q9E, are known to cause LQTS. They have a loss-of-function effect on the HERG-KCNE2 channel, leading to prolongation of action potential duration and the QT interval (Sesti et al. 2000; Splawski et al. 2000; Tinel et al. 2000; Isbrandt et al. 2002; Lu et al. 2003). To exclude the possibility of an association between R27C and LQTS, we analyzed the effect of R27C on HERG-KCNE2 current. When KCNE2 or KCNE2 R27C was coexpressed with HERG in COS-7 cells, there was neither a difference in tail current nor a difference in voltage dependency for activation of the HERG-KCNE2 channel (data not shown). The KCNE2 R27C mutation did not influence the HERG-KCNE2 channel's biophysical properties in CHO cells either. For HERG coexpressed with KCNE2 or KCNE2 R27C, current densities at +40 mV were 43.6 ± 14.5 pA/pF (n=14) and 34.5 ± 5.4 pA/pF (n=20) (P=.61 [Student’s t test]), respectively, and the deactivation time constants at −40 mV were 519.7 ± 37.8 ms (n=14) and 557.3 ± 35.6 ms (n=20) (P=.48 [Student’s t test]), respectively. We also coexpressed T8A or Q9E with KCNQ1 in COS-7 cells. Unlike the R27C substitution, T8A and Q9E significantly inhibited KCNQ1-KCNE2 current (fig. 2D and 2E).
In contrast to all the LQTS-associated KCNE2 mutations, the KCNE2 R27C mutation increased KCNQ1-KCNE2 current. Furthermore, the mutation did not affect HERG-KCNE2 current. Consistent with the clinical findings, these results showed that R27C was not associated with LQTS in these two Chinese families.
KCNE2 can associate with other pore-forming subunits, such as the HCN channel family (Yu et al. 2001). We tested the effect of the R27C mutation on expression of the KCNE2 subunit with HCN1, HCN2, and HCN4 and found no significant effect. Current densities at −100 mV for HCN1-KCNE2 and HCN1-KCNE2 R27C were −34.5 ± 8.9 pA/pF (n=15) and −48.7 ± 16.2 pA/pF (n=11) (P=.42 [Student’s t test]), respectively. The corresponding values were −25.1 ± 6.5 pA/pF (n=17) and −29.5 ± 7.0 pA/pF (n=14) (P=.65 [Student’s t test]) for coexpression with HCN2, and the values were −63.9 ± 16.2 pA/pF (n=16) and –60.3 ± 10.4 pA/pF (n=11) (P=.8 [Student’s t test]) for coexpression with HCN4. The KCNE2 R27C mutation had no significant effects on KCNE2-HCN1,-HCN2, and -HCN4. Thus, it is unlikely that KCNE2 R27C evokes the onset of AF through the HCN channel family.
The carriers of R27C in family 1 (II-1, II-5, and II-6) and in family 2 (II-3 and II-6) did not have AF during a 24-h electrocardiographic monitoring. This may be explained by any of—or any combination of—the reasons that follow.
-
1.
AF occurs as rarely as a few times in a lifetime for some patients with AF (Ryder and Benjamin 1999; Chugh et al. 2001); we performed electrocardiographic monitoring for only 24 h, and a longer duration of monitoring may be required to record paroxysmal AF in these patients.
-
2.
AF occurs in older patients; these carriers may not be old enough to develop the disease.
-
3.
Familial AF caused by the R27C mutation may have a low penetration.
-
4.
R27C may be only a genetic predisposing factor for AF, rather than a direct cause of AF; thus, environmental factors may play a role in the onset of AF.
In all carriers, the premature atrial complexes were recorded during the monitoring. These complexes may be the early signs of development of AF. Paroxysmal AF is frequently initiated by ectopic activity, such as that originating from the pulmonary vein regions and other sites (Jais et al. 1997; Haissaguerre et al. 1998; Itoh et al. 1998; Chen et al. 1999). It must be pointed out that, although the premature atrial complexes of all carriers in the two AF families were basically within normal range, we could not exclude the possibility of association of the KCNE2 R27C mutation with premature atrial complexes.
Onset of AF may be modified by the autonomic nervous system (Zipes et al. 1974; Liu and Nattel 1997). Experimental studies have indicated that vagal stimulation appears to be stronger than sympathetic stimulation for spontaneous initiation of AF, since it decreases atrial ERP and increases inhomogeneity of refractoriness in the atria. Initiation of AF by cholinergic stimulation is modulated by sympathetic tone, and vice versa (Zipes et al. 1974; Liu and Nattel 1997). Further study is needed to investigate the cholinergic effect on the wild-type and KCNQ1-KCNE2 R27C channels. The R27C substitution was not found in 154 patients with lone AF (data not shown), suggesting that, in most patients with AF who do not show evidence of structural heart diseases, linked genes remain to be identified.
In summary, the KCNE2 R27C is a novel mutation in familial AF. The mutation has a gain-of-function effect on potassium current. The findings confirm the hypothesis that ion channels, especially the potassium channel, have a major role in causing AF. An understanding of the molecular mechanisms underlying familial forms of the disease may also provide insight into the pathogenesis of more common acquired forms of AF and may ultimately lead to novel therapeutic drug strategies.
Acknowledgments
We thank Huaizhi Chen for assistance in statistical analysis, Marie-Madeleine Larroque for assistance with the electrophysiological study, and the families with AF for their participation. This work was supported by grants from the National Natural Science Foundation of China (to Yihan Chen), the Major Program Fund by the Ministry of Education of China (to Yihan Chen), the Shanghai Science and Technology Development Fund of China (to Yihan Chen), the Shanghai Dawn Scholar Fund of China (to Yihan Chen), the Centre National de la Recherche Scientifique (to J.B.), the Association Française contre les Myopathies (to J.B.), the American Heart Association Established Investigator Award, and the U.S.-Israel Binational Science Foundation (to J.C.).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/GenBank/ (for sequence information for KCNQ1 [accession number AJ006345], HERG [accession number NT_007914], KCNE1 [accession number AP001720], KCNE2 [accession number NM_005136], KCNE3 [accession number XM_208561], KCNE4 [accession number NT_005403], KCNE5 [accession number NM_012282], and KCNJ2 [accession number NT_035430])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for AF and LQTS)
References
- Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME (2003) Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clin Proc 78:1479–1487 [DOI] [PubMed] [Google Scholar]
- Brugada R, Tapscott T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L, Brugada J, Girona J, Domingo A, Bachinski LL, Roberts R (1997) Identification of a genetic locus for familial atrial fibrillation. N Engl J Med 336:905–911 [DOI] [PubMed] [Google Scholar]
- Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, Hsu TL, Ding YA, Chang MS (1999) Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation 100:1879–1886 [DOI] [PubMed] [Google Scholar]
- Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W (2003) KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 299:251–254 [DOI] [PubMed] [Google Scholar]
- Chiang CE, Roden DM (2000) The long QT syndromes: genetic basis and clinical implications. J Am Coll Cardiol 36:1–12 [DOI] [PubMed] [Google Scholar]
- Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ (2001) Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol 37:371–378 [DOI] [PubMed] [Google Scholar]
- Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, Hammill SC, Packer DL, Olson TM (2003) Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol 41:2185–2192 [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Shin JT, Moore RK, Yoerger DM, MacRae CA (2003) Locus for atrial fibrillation maps to chromosome 6q14-16. Circulation 107:2880–2883 [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J (1998) Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339:659–666 [DOI] [PubMed] [Google Scholar]
- Isbrandt D, Friederich P, Solth A, Haverkamp W, Ebneth A, Borggrefe M, Funke H, Sauter K, Breithardt G, Pongs O, Schulze-Bahr E (2002) Identification and functional characterization of a novel KCNE2 (MiRP1) mutation that alters HERG channel kinetics. J Mol Med 80:524–532 [DOI] [PubMed] [Google Scholar]
- Itoh T, Tanaka T, Nagai R, Kamiya T, Sawayama T, Nakayama T, Tomoike H, Sakurada H, Yazaki Y, Nakamura Y (1998) Genomic organization and mutational analysis of HERG, a gene responsible for familial long QT syndrome. Hum Genet 102:435–439 [DOI] [PubMed] [Google Scholar]
- Jais P, Haissaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, Clementy J (1997) A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 95:572–576 [DOI] [PubMed] [Google Scholar]
- Liu L, Nattel S (1997) Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol 273:H805–H816 [DOI] [PubMed] [Google Scholar]
- Lu Y, Mahaut-Smith MP, Huang CL, Vandenberg JI (2003) Mutant MiRP1 subunits modulate HERG K+ channel gating: a mechanism for pro-arrhythmia in long QT syndrome type 6. J Physiol 551:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J (1904) The inception of the rhythm of the heart by the ventricle as the cause of continuous irregularity of the heart. Br Med J 5:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V (2003) Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature 421:634–639 [DOI] [PubMed] [Google Scholar]
- Nattel S (1999) Atrial electrophysiological remodeling caused by rapid atrial activation: underlying mechanisms and clinical relevance to atrial fibrillation. Cardiovasc Res 42:298–308 [DOI] [PubMed] [Google Scholar]
- ——— (2002) New ideas about atrial fibrillation 50 years on. Nature 415:219–226 [DOI] [PubMed] [Google Scholar]
- Nattel S, Li D, Yue L (2000) Basic mechanisms of atrial fibrillation—very new insights into very old ideas. Annu Rev Physiol 62:51–77 [DOI] [PubMed] [Google Scholar]
- Ryder KM, Benjamin EJ (1999) Epidemiology and significance of atrial fibrillation. Am J Cardiol 84:131R–138R [DOI] [PubMed] [Google Scholar]
- Sesti F, Abbott GW, Wei J, Murray KT, Saksena S, Schwartz PJ, Priori SG, Roden DM, George AL Jr, Goldstein SA (2000) A common polymorphism associated with antibiotic-induced cardiac arrhythmia. Proc Natl Acad Sci USA 97:10613–10618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT (2000) Spectrum of mutations in long-QT syndrome genes: KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation 102:1178–1185 [DOI] [PubMed] [Google Scholar]
- Tinel N, Diochot S, Borsotto M, Lazdunski M, Barhanin J (2000) KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J 19:6326–6330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristani-Firouzi M, Jensen JL, Donaldson MR, Sansone V, Meola G, Hahn A, Bendahhou S, Kwiecinski H, Fidzianska A, Plaster N, Fu YH, Ptacek LJ, Tawil R (2002) Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J Clin Invest 110:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner DR, Nerbonne JM (2000) Molecular basis of electrical remodeling in atrial fibrillation. J Mol Cell Cardiol 32:1101–1117 [DOI] [PubMed] [Google Scholar]
- Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, Pan Z, Wang H, Shi W, Robinson RB, El-Maghrabi MR, Benjamin W, Dixon J, McKinnon D, Cohen IS, Wymore R (2001) MinK-related peptide 1: a β subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res 88:E84–E87 [DOI] [PubMed] [Google Scholar]
- Zipes DP, Mihalick MJ, Robbins GT (1974) Effects of selective vagal and stellate ganglion stimulation of atrial refractoriness. Cardiovasc Res 8:647–655 [DOI] [PubMed] [Google Scholar]