Abstract
Patients with triple negative breast cancer (TNBC) show only modest response rates to immune checkpoint inhibitor immunotherapy, motivating ongoing efforts to identify approaches to boost efficacy. Using an immunocompetent mouse model of TNBC, we investigated combination therapy with an anti-PD-1 immunotherapy antibody plus balixafortide, a cyclic peptide inhibitor of CXCR4. Cell-based assays demonstrated that balixafortide functions as an inverse agonist, establishing a mode of action distinct from most compounds targeting CXCR4. Combination anti-PD-1 plus balixafortide significantly reduced growth of orthotopic tumors and extended overall survival relative to single agent therapy or vehicle. Adding balixafortide to anti-PD-1 increased numbers of tertiary lymphoid structures, a marker of local tumor immune responses associated with favorable response to immunotherapy in TNBC. Single cell RNA sequencing revealed that combination anti-PD-1 plus balixafortide reduced T cell exhaustion and increased markers of effector T cell activity. Combination therapy also reduced signatures of immunosuppressive myeloid derived suppressor cells (MDSCs) in tumors. MDSCs isolated from mice treated with anti-PD-1 plus balixafortide showed reduced inhibition of T cell proliferation following ex vivo stimulation. These studies demonstrate that combining inhibition of CXCR4 with anti-PD-1 to enhances responses to checkpoint inhibitor immunotherapy in TNBC, supporting future clinical trials.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-89882-5.
Keywords: Breast cancer, CXCR4, Checkpoint inhibitor immunotherapy, Myeloid derived suppressor cells
Subject terms: Cancer, Immunology, Medical research, Oncology
Introduction
Recent approval of checkpoint inhibitor immunotherapy targeting the PD-1 pathway for patients with triple negative breast cancer (TNBC) as part of neoadjuvant therapy for locally advanced disease or adjuvant therapy for metastases marked the first success of immunotherapy in breast cancer. However, overall benefits of immunotherapy in patients with triple negative breast cancer remain modest relative to other malignancies1,2. Using current selection criteria, only ∼8–20% of patients with TNBC respond to current immunotherapy regimens1. As a result, most patients receiving immunotherapy for TNBC receive no benefit from treatment while remaining at risk for potentially severe complications of autoimmunity caused by checkpoint inhibitors3,4. Limitations of current immunotherapy treatment regimens for TNBC motivate ongoing efforts to identify approaches to improve efficacy in neoadjuvant and/or adjuvant settings.
Chemokine CXCL12 and receptor CXCR4 regulate multiple aspects of the tumor immune microenvironment in TNBC5,6. CXCL12 secreted from carcinoma associated fibroblasts in breast tumors signals through CXCR4 on cancer cells to promote tumor growth, invasion, and metastasis7–9. CXCL12-CXCR4 signaling controls trafficking of both anti-tumor immune cells and various immunosuppressive cell types. While typically regarded as a chemoattractant, CXCL12 bound to the surface of malignant cells in TNBC and other cancers may suppress motility of T cells and chemotaxis to other chemokines, excluding these key effectors of anti-PD-1 therapy from the tumor interior10,11. Inhibitory effects of CXCL12 on infiltration of immune cells into a tumor creates an immune excluded environment associated with poor response to checkpoint inhibitor immunotherapy12. Immune exclusion also prevents formation of tertiary lymphatic structures (TLS), which comprise non-orthotopic aggregates of immune cells formed in a tumor in response to chronic inflammation13. Lack of TLS in a tumor environment also correlates with poor outcomes and response to immunotherapy.
Beyond excluding anti-tumor immune cells, CXCL12-CXCR4 recruit a variety of immunosuppressive cell types to tumors. These cell types include subsets of myeloid derived suppressor cells, other types of suppressive myeloid cells, and T regulatory cells. All these cell types dampen anti-tumor immunity and correlate with poor prognosis in TNBC and other malignancies14,15. Myeloid derived suppressor cells (MDSCs) comprise a heterogeneous group of immunosuppressive cells ranging from early (immature) forms to more differentiated monocytic or granulocytic types14. MDSCs suppress functions of T cells and other immune cell types, such as NK cells. Mechanisms of immunosuppression include secreted molecules, including arginase 1, IL-10, and TGF-β, and induction of T regulatory cells. Blocking effects of MDSCs can produce a favorable immune response profile and eliminate tumors in pre-clinical models of TNBC16. Inhibiting CXCR4 in pre-clinical models of pancreatic cancer, hepatocellular cancer, and metastatic breast cancer can overcome immunosuppressive effects of CXCL12-CXCR4 signaling, although mechanisms of action remain incompletely understood17–19.
In this study, we investigated effects of inhibiting CXCR4 in combination with checkpoint inhibitor immunotherapy in a mouse model of TNBC. Numerous inhibitors of CXCR4 are under development, and two small molecules have been approved for defined clinical indications: AMD3100 (plerixafor) to mobilize hematopoietic stem cells for transplant and mavorixafor for the rare WHIM immunodeficiency syndrome (warts, hypogammaglobulinemia, infections, and myelokathexis)20. For this study, we used balixafortide, a synthetic cyclic peptide selective for CXCR4, that has been used safely in clinical trials for breast cancer21. Balixafortide combined with anti-PD-1 therapy reduced tumor growth and extended overall survival relative to monotherapy with either agent. Histology revealed that treatment regimens with balixafortide increase formation of TLS in the tumor environment. Single cell RNA sequencing showed that combination anti-PD1 and balixafortide reduced expression of immune checkpoint genes and markers of exhaustion in T-cells, suggesting a decrease in overall myeloid mediated immunosuppression. Sequencing data and ex vivo analysis also demonstrated that combination therapy reduced immunosuppression by myeloid cells infiltrating into tumors. These data provide mechanisms through which inhibiting CXCR4 potentiates immunotherapy in TNBC, supporting future clinical studies of this approach to enhance response to immunotherapy in patients.
Results
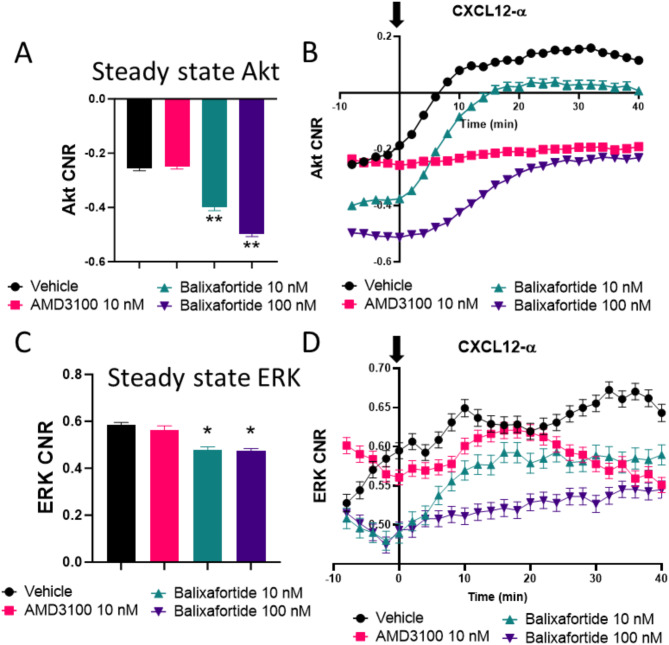
To investigate effects of the peptide-based inhibitor balixafortide on signaling by CXCR4, we quantified activation of Akt and ERK, two kinases activated by this receptor, utilizing fluorescent kinase translocation reporter (KTR) cells developed and used previously by our group22,23. KTRs reversibly translocate between nucleus and cytoplasm based on phosphorylation of a specific kinase substrate for Akt or ERK. These reporters provide dynamic, analog readouts of kinase activity based on the ratio of fluorescence intensities in the cytoplasm (kinase “on”) and nucleus (kinase “off”). We compared balixafortide to the clinically approved CXCR4 inhibitor, AMD3100 (plerixafor). We and others have shown that AMD3100 may function as an antagonist or weak partial agonist for CXCR424. Under steady-state conditions without CXCL12 stimulation, activities of Akt (Fig. 1A) and ERK (Fig. 1C) in cells treated with 1 µM AMD3100 for 30 min remained comparable to vehicle only. By comparison, incubation with 10 or 100 nM balixafortide significantly reduced steady-state levels of Akt and ERK. We note that MDA-MB-231 cells show higher steady-state activity of ERK because of mutations in these cells that constitutively activate the MAPK pathway.
Fig. 1.
Balixafortide functions as an inverse agonist for CXCR4. We measured levels of (A) AKT and C) ERK using kinase translocation reporters (KTRs) in MDA-MB-231 breast cancer cells after treatment with different CXCR4 antagonists as indicated. Bars show mean + SEM values for kinase activity after 30 min of treatment with the listed compounds. Data represent the log2 of cytoplasmic/nuclear fluorescence intensities for each reporter (n > 400 cells per condition). We quantified activation of (B) AKT and D) ERK by KTRs after treatment with 100 ng/ml CXCL12-α added at time 0 (arrow). Data represent mean values for log2 of cytoplasmic/nuclear fluorescence intensities at each time point. We tracked > 300 cells over time for each condition. Error bars denote SEM where visible beyond the symbol. Data for all panels are representative of two independent experiments. *, p < 0.05, **, p < 0.01.
We next quantified effects of AMD3100 to block CXCR4 signaling stimulated by CXCL12. We incubated cells for 30 min with either inhibitor or vehicle control before adding 100 ng/ml CXCL12-α. AMD3100 completed blocked CXCL12-dependent activation of Akt (Fig. 1B) and ERK (Fig. 1D). Although diminishing pre-stimulation activity of Akt, 10 nM balixafortide had minimal effects on fold-change activation of Akt by CXCL12, while 100 nM balixafortide modestly decreased CXCL12-CXCR4 signaling to Akt. Overall, these data indicate that balixafortide functions as an inverse agonist for CXCR4 with concentration-dependent inhibition of CXCL12-CXCR4 signaling.
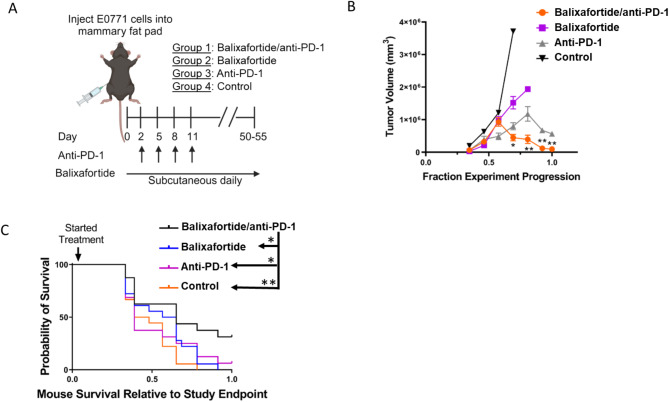
Inhibition of CXCR4 has been investigated as an adjuvant to immunotherapy by functioning at least in part by reducing or modulating the immunosuppressive myeloid compartment of a tumor. Indeed, combining a CXCR4 inhibitor (AMD3100) with checkpoint inhibitor immunotherapy improved treatment efficacy in pre-clinical models of primary hepatocellular and pancreatic ductal adenocarcinoma17,18. To evaluate effects of CXCR4 inhibition to improve checkpoint inhibitor immunotherapy in TNBC, we used murine E0771 TNBC cells, a transplantable tumor model derived from C57BL/6J mice. E0771 tumors exhibit an immunologically active tumor microenvironment like TNBC in humans. We selected a cancer cell model with minimal to no CXCR4 because we and others have shown that inhibiting CXCR4 on cancer cells reduces tumor growth8,25. Therefore, our animal model allows us to focus on effects of balixafortide ± anti-PD-1 on immune cells and other stromal cells in the tumor environment. We orthotopically implanted E0771 cells into C57BL/6J mice and on day 2 randomly assigned animals to one of four different treatment groups: (1) balixafortide and anti-PD-1; (2) balixafortide only; (3) anti-PD-1 only; and (4) vehicle controls. Mice in groups that include anti-PD-1 received four cycles of anti-PD-1 antibody administered at 5 mg/kg per mouse every 3 days, while balixafortide groups received daily subcutaneous injections of the compound throughout the study (Fig. 2A). Monotherapy with balixafortide or anti-PD-1 modestly decreased tumor growth when compared with the control group (Fig. 2B). Notably, combination treatment with balixafortide and anti-PD-1 significantly reduced tumor growth compared with all other groups at later growth times (p < 0.01 by ANOVA) (Fig. 2B).
Fig. 2.
Balixafortide improves anti-PD-1 immunotherapy to reduce tumor growth in mice with TNBC. (A) We implanted mice with E0771 breast cancer cells and randomly assigned mice to listed treatment groups on day 2. Groups 1 and 3 received 4 doses of anti-PD-1 antibody at 5 mg/kg per dose every 3 days for 4 cycles. Groups 1 and 2 received 20 mg/kg balixafortide through subcutaneous injection in the rear flank daily. Control mice received matched vehicles only (n = 10 /group). (B) We calculated tumor volumes by caliper measurements. To account for differences in days required to reach pre-defined study endpoints, we normalized the reported duration of each experiment to fraction experiment progression. Presented data are mean values + SEM combined from 2 different experiments. We compared differences across groups at each time point by ANOVA corrected for multiple comparisons. *, p < 0.05; **, p < 0.01. (C) Graph shows overall survival of mice in different treatment groups with log-rank p-values calculated using a Mantel-Cox test. Asterisks in legend symbolize significance (* <0.05; ** <0.01). We normalized data to fraction of days to reach the study endpoint (defined as 1.0), combining two different experiments with different overall days until endpoint.
We also analyzed overall survival of mice in various treatment groups (Fig. 2B). Treatment with balixafortide plus anti-PD-1 significantly reduced hazard ratios for death relative to all other groups (p < 0.05) (Fig. 2C). Relative to the combination treatment group, all other groups showed ∼ 2.5-fold or higher risk of death (Supplemental Table 1). Overall, these data establish that adding balixafortide to anti-PD-1 immunotherapy provides additive benefits to anti-PD-1 therapy alone, offering a potential means to improve outcomes in TNBC.
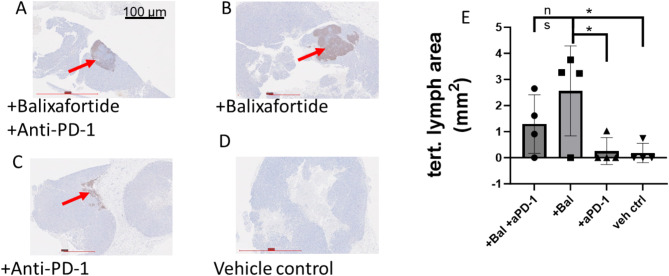
Tertiary lymphatic structures (TLS) are ectopic lymphoid organs that form in peripheral, non-lymphoid organs and tissues at sites of chronic inflammation26. Inflammatory, immune “hot” TNBC tumors commonly form TLS, and these structures represent an important prognostic factor for the success of cancer immunotherapy and improved overall survival27,28. B cells in tumors help form TLS that set the stage for anti-tumor immunity. To assess effects of various treatments on formation and abundance of TLS, we stained tumor sections for B cells with anti-B220 antibody. Tumors from mice treated with vehicle control formed almost no TLS, while anti-PD-1 alone formed only small, disordered TLS in limited numbers of sections (Fig. 3A-D). By comparison, we identified large, organized TLS with dense B cell follicles in in tumors treated with balixafortide alone or in combination with anti-PD-1 antibody. To compare among groups, we quantified the area of each tumor section occupied by B220 staining. Balixafortide treated mice showed the greatest mean area for TLS, although mean areas for TLS did not differ significantly between balixafortide versus balixafortide and anti-PD-1 combination therapy (Fig. 3E). Treatments with balixafortide produced significantly more TLS than anti-PD-1 alone or vehicle (p < 0.05). These data show that balixafortide modulates the local tumor immune environment to increase TLS, a feature associated with successful checkpoint inhibitor immunotherapy.
Fig. 3.
Balixafortide increases area of tertiary lymphatic structures in orthotopic tumors. (A-D) Representative immunohistochemistry images of tumor sections stained by for B lymphocyte marker B220 from mice treated with (A) balixafortide/anti-PD-1, (B) balixafortide, (C) anti-PD-1, and (D) vehicle. Arrows show B cell rich tertiary lymphatic structures. (E) Graph shows mean values and SEM for area occupied by tertiary lymphoid structures on each section. Results are combined data from two independent experiments with slides from 2 separate mice analyzed for each experiment (n = 4 per group). *, p < 0.05 by t-test. NS, not significant.
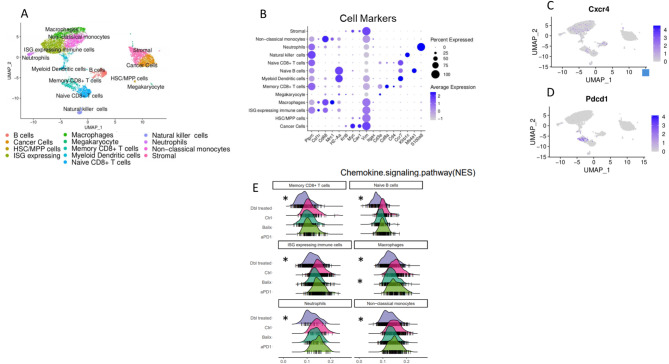
To investigate the transcriptomic profile of immune cells modulated by balixafortide and anti-PD-1 immunotherapy in the E0771 model of TNBC, we performed Drop-seq single cell sequencing with a focus on immune cells in the tumor microenvironment of mice treated as in Fig. 2A. Principal component analysis of single-cell sequencing data revealed 13 distinct Louvain clusters with marker genes present in many of the top variable genes expressed for each cluster (Fig. 4A, Supplemental Fig. 1). Clusters displayed numerous myeloid cell types consisting of macrophages, monocytes, interferon stimulated gene-expressing immune cells (likely transitioning monocytes), and neutrophils. We also identified T cell populations relevant to activation status (memory CD8 T cell and naïve CD8 T cell), naïve B cells, natural killer cells, and dendritic cells. A geographically separate population contained cancer cells and stromal cells. We used representative genes to further verify cancer and immune cell clusters from the scType classifications using singular gene markers for visualization (Fig. 4B). We identified expression of CXCR4 primarily in the large myeloid cell clusters, memory CD8 + T cells, and B cells (Fig. 4C). Expression of Pdcd1 (PD-1 protein) occurred exclusively in the activated ‘memory CD8 + T cell’ cluster as expected (Fig. 4D). To assess the impact of balixafortide on chemokine signaling in cells in the tumor microenvironment, we performed pathway analysis and then compared the score of the chemokine signaling pathway among treatment groups and in each classified cell type. In both the balixafortide or combination treatment groups, we discovered a reduced expression score of the chemokine signaling pathway, especially in myeloid cell and B cell populations (Fig. 4E). These data suggest successful targeting of CXCR4 by balixafortide in vivo. We found balixafortide alone also impacted other pathways related to myeloid immunity. Notably, the score for STAT signaling pathways exhibited a negative shift in macrophages and monocytes in the balixafortide treated groups (Supplemental Fig. 2).
Fig. 4.
Drop-seq transcriptomic analysis reveals modulation of the tumor microenvironment in mice treated with balixafortide and anti-PD-1. We performed single-cell RNA sequencing on tumors from mice treated with balixafortide/anti-PD-1, balixafortide, anti-PD-1, or vehicle (n = 3/group). (A) Clustered UMAP plot shows annotated populations of cells recovered from tumors. (B) We tested representative marker genes for each annotated cluster using DotPlot to verify proper classification of cells through scType. (C, D) Feature plots from the UMAP in panel A show expression of genes targeted by balixafortide (CXCR4) (C) and anti-PD-1 (Pdcd1) (D) therapies showing majority targets in the immune cell block of the UMAP clusters. (E) Ridge plots represent modulation of genes in the chemokine signaling pathway by various treatments. *, p < 0.05 by Kruskal-Wallis test.
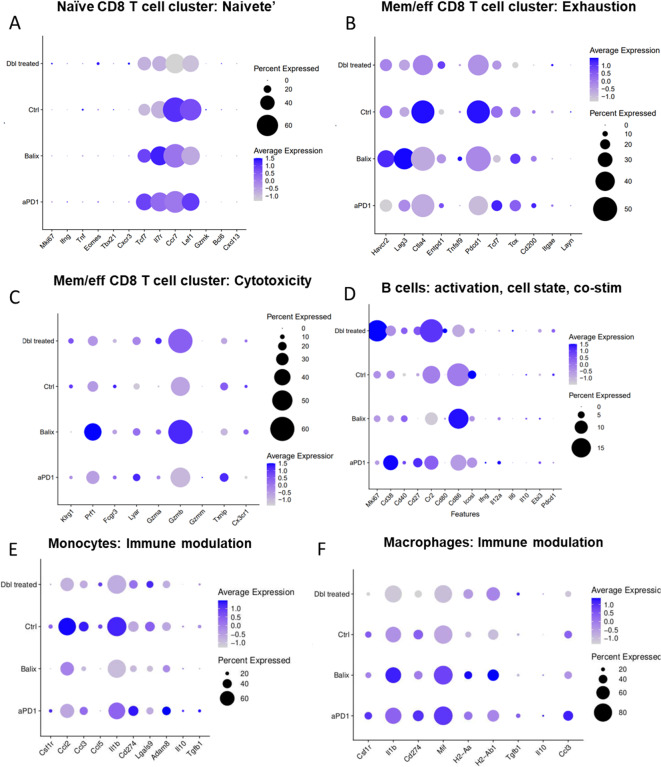
Cytotoxic T cells are a major component of ant-tumor immunity. T cell function and exhaustion are key characteristics to determine to what extent these cells will effectively eliminate breast cancer cells29–31. We compared marker genes that represent cell state or cell function in T cell populations among various treatment groups. In the naïve CD8 + T cell cluster, treatment with balixafortide and anti-PD-1 dramatically reduced two characteristic markers of naïve T cells, Il7r and CCR7, indicating that combination therapy decreased abundance of naïve bystander cells in the tumor (Fig. 5A). Within this population we also verified lack of expression of genes that would implicate activated T cell populations commingling in this naive cluster (Mki67, Ifng, Tnf, Eomes, Tbx21, CXCR3) or genes indicating transitory activated T cell states (Gzmk, Bcl6, CXCL13). When analyzing the activated ‘Memory CD8 + T cell’ cluster, markers for exhaustion in the balixafortide group showed increased checkpoint receptor expression (notably Havcr2, and Lag3) (Fig. 5B). However, combining balixafortide with anti-PD-1 reduced expression of Pdcd1, Ctla4, Havrc2, and Lag3 when compared with the control group (Fig. 5B). The combination treatment group also expressed the lowest level of Tox and CD200, two genes commonly associated with T cell exhaustion (Fig. 5B). In contrast, treatment with balixafortide plus anti-PD-1 produced higher Entpd1 (CD39) expression than all other groups (Fig. 5B). CD39 has been identified as a marker of tumor-reactive T cells in addition to T cell exhaustion32. When assessing genes related to cytotoxic function in the activated ‘memory CD8 + T cell’ population, balixafortide alone produced greatest effects overall with large increases in expression of cytotoxicity effectors Prf1 and Gzmb (Fig. 5C). However, combining balixafortide with anti-PD-1 elevated expression of these genes above control and anti-PD-1 alone treatment groups, indicating that balixafortide treatment may increase the cytotoxic potential of CD8 + T cells in TNBC tumors (Fig. 5C). For intratumoral B cells, treatment with balixafortide and anti-PD-1 resulted in greater proliferation as shown by expression of higher levels of Mki67 (Fig. 5D). Additionally, B cells in tumors from the combination treatment group expressed more CR2 (CD21), a receptor associated with induction of tertiary lymphatic structures in some studies (Fig. 5D)33.
Fig. 5.
Balixafortide and anti-PD-1 reduce markers of T cell exhaustion and immune modulation by myeloid cells. We analyzed single cell RNA sequencing data from Fig. 4 to compare relevant genes that regulate functions of immune cells listed in each panel. Pseudocolor scale in dot plots shows LogFC expression and bubble size represents the percent of cells from the population. (A) T cell Naivete, (B) T cell Exhaustion, (C) T cell cytotoxicity, (D) B cell activation state, (E) monocyte, and (F) macrophage functions.
The types and functions of myeloid cells in the tumor microenvironment regulate anti-tumor immunity or immunosuppression. We analyzed genes involved in suppressing anti-tumor immunity in the annotated monocyte or macrophage clusters. CCL2 is a chemokine known to promote breast cancer metastasis and modulate suppressive myeloid cell populations34. In the monocyte population we found high expression of CCL2 in the control group (Fig. 5E). Treatment with anti-PD-1 alone modestly reduced expression, but balixafortide alone or in combination produced the lowest expression of CCL2 (Fig. 5E). Chemokine CCL3 is an attractant for polymorphonuclear cells that has both pro- and anti-tumoral functions35,36. Treatment with balixafortide, alone or in combination with anti-PD-1, downregulated CCL3 relative to anti-PD-1 alone or control (Fig. 5E). Il1b, a gene associated with immunosuppression by myeloid cells in tumors and increased metastasis in TNBC37, also showed reduced expression in both groups that included treatment with balixafortide (Fig. 5E). In contrast, Lgals9 (Galectin9), the ligand for T cell checkpoint receptor TIM-3, increased in a subpopulation of monocytes treated with balixafortide and anti-PD-1 (Fig. 5E). In the macrophage population, we detected reduced expression of Il1b only in the group of mice treated with both agents. MIF(macrophage inhibitory factor) promotes a M2 phenotype in macrophages; increases myeloid suppression; and functions as a non-cognate ligand for CXCR438. Increased levels of MIF occurred in the anti-PD1 and balixafortide single agent treatments, but combination treatment reduced expression of MIF when compared with all other groups (Fig. 5F). This finding is significant given that inhibition of MIF alone increased response to immune checkpoint inhibitors in melanoma39. All treatments (anti-PD-1, balixafortide, or combination) elevated expression of antigen presentation genes H2-Aa and H2-Ab1 in macrophages (Fig. 5F). Genes that produce functionally immunosuppressive proteins, such as Tgfb1 and Il10, did not show notable changes among treatment groups (Fig. 5F). Overall, these transcriptomic changes in myeloid cells in the tumor microenvironment further validate the findings in which balixafortide complements anti-PD-1 immunotherapy by modulating effectors such as MIF and CCL2 that drive tumor suppression.
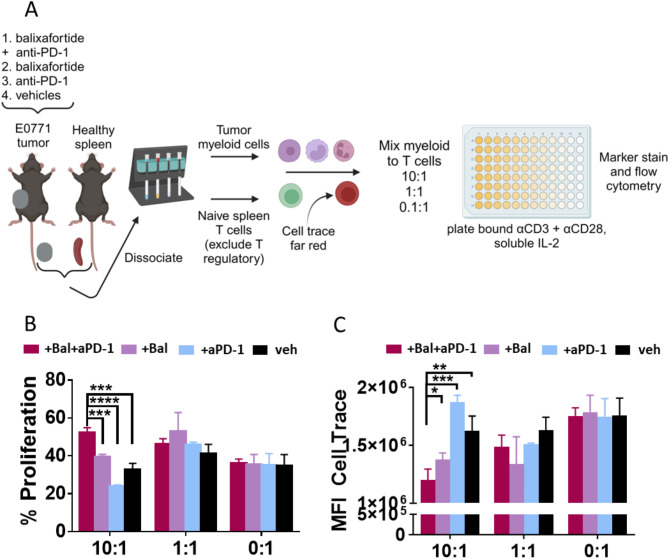
Given the potential impact of CXCR4 antagonism on myeloid cell function in the tumor microenvironment, we employed a tumor suppression assay to assess T cell proliferation (activation) in the presence of myeloid cells isolated from tumors from each of the four treatment groups (Fig. 6A). Suppression of T cell activation occurred only at the 10:1 ratio of myeloid to naïve T cells with this model system. When measuring the total per cent of T cells that proliferated (cells gated below unstimulated control T cell peak), myeloid cells from the control and anti-PD-1 groups reduced T cell proliferation to ∼30% and ∼20%, respectively (Fig. 6B). Myeloid cells from tumors in mice treated with balixafortide alone inhibited proliferation of T cells by ∼60%. Remarkably, 50% of T cells proliferated in the presence of tumor myeloid cells from mice treated with balixafortide and anti-PD-1. This value represents a 10% increase above the baseline 0:1 control with no myeloid cells (Fig. 6B). We observed similar data trends when quantifying dilution of dye in T cells, a marker of proliferation, as mean fluorescence intensity (Fig. 6C) (Supplemental Fig. 3). These data demonstrate that balixafortide alone may rescue T cell proliferation in the presence of immunosuppressive myeloid cells and further elevate proliferation of T cells when combined with anti-PD-1.
Fig. 6.
Treatment with balixafortide and anti-PD-1 reduces immunosuppression by myeloid cell populations. (A) We treated mice with E0771 tumors for two weeks as illustrated in Fig. 1 and recovered myeloid cells from tumors with CD11b + immunomagnetic beads. We isolated T cells from a healthy mouse spleen, excluding T regulatory cells with anti-CD25, and plated indicated ratios of myeloid cells to T cells (10:1, 1:1, 0:1) with 0.1ug/mL insoluble anti-CD3 for activation signal. (B) Graph shows mean values + SEM for percent proliferation measured by taking the percentage of T cells gated below the control T cell peak that received no stimulus and thus did not divide. (C) Graph displays mean value + SEM of mean fluorescence intensity (MFI) of cell trace far red as a complementary way to assess T cell division through dye dilution (lower MFI = more proliferation). *, p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001. Results are representative of two independent experiments.
Discussion
Current immunotherapy treatment regimens for TNBC benefit only modest numbers of patients, motivating ongoing efforts to identify innovative approaches to boost response rates. We demonstrated that combining anti-PD-1 with the CXCR4 inhibitor balixafortide improved response to immunotherapy in a mouse model of TNBC. Relative to single agent therapy, combination anti-PD-1 and balixafortide therapy reduced tumor size and extended overall survival. Adding CXCR4 to anti-PD-1 therapy shifted the tumor immune environment to a more effective anti-tumor state through a variety of complementary mechanisms. Inhibiting CXCR4 increased numbers of TLS in orthotopic tumors, and combination anti-PD-1 and balixafortide reduced gene expression profiles of T cell exhaustion with an associated increase in markers of activation. RNA sequencing data also revealed reduced immunosuppression by myeloid cells, which we validated with ex vivo studies showing that myeloid derived suppressor cells isolated from mice treated with anti-PD-1 plus balixafortide exhibited less effective suppression of T cell proliferation. These data support inhibition of CXCR4 as a potential addition to anti-PD-1 to improve effects of immunotherapy in TNBC.
Past research also supports inhibition of CXCR4 as an approach to improve immunotherapy in challenging malignancies. In a mouse model of metastatic TNBC, combing anti-PD-1 with inhibition of CXCR4 reduced fibrosis in metastases and improved vascular perfusion, resulting in greater influx of T cells into the tumor core19. Mice with hepatocellular carcinoma treated with the multi-kinase inhibitor sorafenib caused hypoxia, a known stimulus for expression and secretion of CXCL1218. Blocking CXCL12-CXCR4 signaling reversed immunosuppressive effects of sorafenib, including recruitment of T regulatory and suppressive myeloid cells. In combination with anti-PD-1, sorafenib plus anti-PD-1 also increased infiltration of T cells into tumors. A phase I clinical study in patients with pancreatic or colorectal cancer also demonstrated with inhibiting CXCR4 for 1 week in combination with anti-PD-1 therapy reduced immunosuppression with a gene expression profile showing greater numbers of tumor infiltrating cytotoxic T cells and natural killer cells10. Our data agree with these past publications showing that inhibiting CXCR4 promotes ant-tumor immunity. Notably, our work extends these findings to therapy in localized TNBC, a recently approved treatment paradigm for this malignancy.
Prior studies of CXCR4 inhibition as part of checkpoint inhibitor immunotherapy focused on AMD3100 (plerixafor), a small molecule antagonist with weak partial agonist activities at higher concentrations40. While approved clinically for mobilization of hematopoietic stem cells, poor pharmacokinetics and toxicity from chronic administration preclude use of this drug to treat solid tumors41,42. We targeted CXCR4 with balixafortide, a synthetic cyclic peptide. Our cell-based studies established that balixafortide functions as an inverse agonist for CXCR4, decreasing steady state signaling to effector kinases Akt and ERK. Inverse agonists stabilize the inactive form of receptor, thereby producing potential pharmacologic benefits of reducing constitutive, ligand-independent activation43. Basal activation of CXCR4 could contribute to ongoing immunosuppression in TNBC tumors and pro-metastatic signaling in cancer cells even in sites of a tumor with low or no CXCL12. While balixafortide combined with the chemotherapy drug eribulin did not improve response rates for patients with metastatic TNBC, clinical studies show an acceptable safety profile for balixafortide, suggesting a path to clinical trials to improve anti-PD-1 immunotherapy21.
Balixafortide without or with anti-PD-1 increased numbers of TLS, organized aggregates of immune cells that resemble secondary lymphoid organs such as lymph nodes, in orthotopic breast tumors. TLS emerge in sites of chronic inflammation and can constitute part of the immune response to cancer. Immune cells including B cells, T cells, and antigen presenting cells aggregate in semi-organized areas of TLS, facilitating antigen presentation and tumor immune responses. High numbers of TLS generally correlate with improved prognosis and response to immunotherapy in TNBC and other malignancies44. Prior work shows that CXCL12-CXCR4 signaling in tumors limits chemotaxis of B cells and other immune cell types toward other chemokines that drive formation of TLS, such as CXCL1310. We propose that balixafortide may block effects of CXCL12-CXCR4 to exclude key effectors of tumor immunity from the tumor environment, facilitating formation of TLS as part of enhanced response to immunotherapy.
Among several complementary causes for improved response to immunotherapy, our RNA sequencing and ex vivo functional data demonstrated pronounced effects of anti-PD-1 plus balixafortide to reduce functions of suppressive myeloid cells, including myeloid derived suppressor cells (MDSCs). MDSCs are a heterogenous group of innate immune cells that suppress activation and functions of adaptive immune cells. MDSCs expand during cancer and are recruited to tumors in large part through CXCL12-CXCR4 signaling and chemotaxis14. Elevated numbers of MDSCs confer poor prognosis in breast cancer, so blocking recruitment and function of these cells can enhance response to immunotherapy45. Interestingly, in vivo treatment with anti-PD-1 and balixafortide limited suppressive effects of MDSCs on proliferation of T cells even after isolation from the tumor environment and testing ex vivo. These data indicate that effects of balixafortide to inhibit functions of MDSCs persists for at least a limited time even in the absence of the compound and other components of the tumor immune environment. Future studies will establish mechanisms underlying lasting effects of balixafortide to inhibit suppression of T cells by MDSCs.
While our studies focused on effects of balixafortide on tumor immune environments, we and others have shown that inhibiting CXCL12-CXCR4 also directly blocks cancer cell intrinsic functions in tumor growth and metastasis. Effects on cancer cells could contribute to overall improvements in tumor response in survival in cancer immunotherapy. We note that our past ex vivo analysis of E0771 breast cancer cells shows these do not have detectable levels of cell surface CXCR4 by flow cytometry46, although these cancer cells potentially could upregulate CXCR4 in vivo. Based on our RNA sequencing data, we investigated effects of anti-PD-1 plus balixafortide to oppose effects of myeloid suppressor cells through ex vivo testing. However, multiple immune and stromal factors likely contribute to effects of CXCR4 inhibition in immunotherapy. This work focused on a single mouse TNBC cell line known to respond to immunotherapy47, so future studies will investigate effects of inhibiting CXCR4 to enhance effects of anti-PD-1 in nonresponsive breast cancer models.
In summary, our research demonstrated functions of CXCR4 to drive an immunosuppressive tumor environment in TNBC. Inhibiting CXCR4 with a clinically safe inhibitor helped reverse immunosuppression; enhanced activation of T cells; and boosted response to anti-PD-1 in a model of orthotopic TNBC. This work motivates future clinical trials testing combination therapy with a CXCR4 inhibitor and anti-PD-1 checkpoint drug to boost currently modest responses to neoadjuvant and adjuvant immunotherapy in TNBC.
Materials and methods
Cell lines
We cultured mouse E0771 breast cancer cells and C57BL/6J mouse mammary fibroblasts (gift of Harold Moses, Vanderbilt University) in DMEM base media (Gibco, #11965) supplemented with 10% FBS, 1% GlutaMax (ThermoFisher Scientific, #35050061), 1% penicillin-streptomycin (Gibco, #15140148), and 1% plasmocin (InvivoGen). We maintained all cells in a humidified 37⁰C incubator with 5% CO2.
For cell-based assays analyzing inhibitors of CXCR4, we used MDA-MB-231 cells (originally from the ATCC) stably transduced with CXCR4 and kinase translocation reporters (KTRs) that measure changes in AKT and ERK signaling as reported previously22,23. We cultured these cells in the DMEM medium used for E0771 and mouse mammary fibroblasts. We authenticated cell line models by STR analysis.
Cell based assay for CXCR4-targeted compounds
We seed 1 × 104 MDA-MB-231 KTR cells in 96 well glass bottom plates (Cellvis) in complete DMEM medium. One day later, we changed medium to Fluorobrite (ThermoFisher Scientific) with 1% serum for hour and then added AMD3100 (ThermoFisher Scientific) or balixafortide (provided by Spexis) at final concentrations listed in Fig. 1 for 30 min. We then quantified imaged KTRs in MDA-MB-231 cells with an EVOS M7000 Imaging System equipped with a humidified stage top incubator set to 37⁰C with 5% CO2. We acquired all images with a 10X objective.
For studies testing stimulation with CXCL12, we plated cells as described in the prior paragraph, including addition of listed concentrations of AMD3100 or balixafortide. After obtaining images for 10 min, we then added 100 ng/ml CXCL12-α (R&D Systems) to wells and acquired images for an additional 40 min. We quantified data from single cells tracked over time using in house Matlab scripts as reported previously, reporting data for all tracked cells as the mean value for log2 ratio of fluorescence intensities in cytoplasm to nucleus (log2CNR) at each time point22. Higher values for CNR indicate greater kinase activity. We discard data from any cell not tracked throughout the full time course.
Mouse tumor models
The University of Michigan IACUC approved all animal procedures under protocol 100,534. We performed all experiments in accordance with relevant guidelines and regulations. We implanted 5 × 105 E0771 mouse mammary tumor cell lines and 1 × 105 mouse mammary fibroblasts orthotopically into 4th inguinal mammary fat pads of 6–8-week-old female C57BL/6J female mice (The Jackson Laboratory)48. We housed mice in specific pathogen-free housing facilities maintained by the Unit for Laboratory Animal Medicine at the University of Michigan. Two days after implanting cells, we randomly assigned mice (n= 10/group) to treatment with balixafortide (20 mg/kg subcutaneously daily) and anti-PD-1 antibody (5 mg/kg intraperitoneal) (BioXCell, #CD279); balixafortide plus control antibody (BioXCell, #BE0089); anti-PD-1 and PBS vehicle for balixafortide; or both vehicles25. We administered antibodies every three days for four total doses. We measured growth of orthotopic tumors with calipers, quantifying tumor size for each mouse as mm3 by V (volume) = [(width x 2) x (length x 2)] measurements. We euthanized mice by cervical dislocation under isoflurane anesthesia with secondary removal of a vital organ for a pre-determined humane endpoint of tumor size, which defined time of censoring for survival analyses. Study reporting conforms to ARRIVE guidelines.
Immunohistochemistry of tumor sections
At the designated endpoint for experiments, we removed tumors for fixation in 10% formalin. The University of Michigan Unit for Lab Animal Medicine in vivo animal core prepared paraffin embedded sections and performed immunostaining for B cells with an antibody to B220 (RA3-6B2, ThermoFisher Scientific). We used Aperio Imagescope software to annotate dense regions of B220-positive cells with mononuclear morphology and measure the pixel area of each region.
Dropseq and single cell sequencing analysis
We prepared single cell suspensions of tumors from mice in various treatment groups using 30 min of digestion with a gentleMACS Octo dissociator with heater (Miltenyi Biotec) followed by passage through 70 and 40 μm mesh filters (ThermoFisher Scientific). We enriched CD45 + cells with immunomagnetic beads (Miltenyi Biotec, 130-052-301) and combined cells with flow through CD45- cells at a 7:3 ratio. We performed Dropseq high-throughput single-cell barcoding transcriptome sequencing for each cell population from mice treated with balixafortide/anti-PD-1, balixafortide only, anti-PD-1 only, or vehicle (n= 3 tumors per treatment group) as described previously49. We used a Nextera XT kit for library preparation and ran transcriptomic libraries on an Illumina Novaseq 6000 sequencer. We further processed samples in Seurat for UMI > 200 and mitochondrial content lower than 5% for viable cells50. We used Seurat V4 in Rstudio on the University of Michigan Advanced Research Computing cluster for data reduction, differential expression, dimensional heatmaps, and dot plots. We annotated cell clusters using the scType package in R and verified cluster with marker genes shown in Fig. 451. We performed pathway analysis using the scGSVA R package (https://github.com/guokai8/scGSVA), which is a single cell wrapper for the GSVA package52. We compared differences in scGSVA values by Kruskal-Wallis test with p < 0.05 defining significance.
T cell suppression assay preparation, reagents, and antibodies
For suppression assays, we isolated T cells from the spleens of wild-type C57BL/6J mice by processing tissue through a 70-micron filter; lysing red blood cells with ACK buffer (ThermoFisher Scientific, A1049201); and sorting cells by negative selection using the Pan T Cell Isolation Kit II from Miltenyi (#130-095-130). During the sorting step, we removed T regulatory cells by adding 3 µg/ml anti-CD25 antibody (clone PC61, Biolegend) to the isolation cocktail We labeled recovered T cells with Cell Trace Far Red (Invitrogen) using the manufacturers protocol. For activation of T cells, we coated 96 well plates with 1 ug/mL anti-CD3ε (145-2C11, Biolegend) and 3 ug/mL anti-CD28 (37.51 Biolegend) antibodies. We also added 5U/ml human IL-2, which cross-reacts with higher affinity than mouse IL-2 with the IL-2 receptor. We used modest levels of soluble IL-2 because the Pan T Cell Isolation Kit enriches for both CD4 and CD8 T cells; upon activation, CD4 cells will produce additional IL-2. For plating the experiment, we used a T cell input of 50,000 cells per well starting with 95–100% viability of recovered cells. The myeloid cell input varied based on ratios shown in Fig. 6. For flow cytometry experiments, antibodies included anti-mouse CD45 (S18009D), TCRβ chain (H57-597), CD3 (17A2), CD8α (53 − 6.7), and CD4 (RM4-5) to identify T cells stained with Cell Trace Red. We used unstimulated T cells labeled with Cell Tracer Far Red as a control for undivided peaks for downstream analysis.
Statistics
We performed all data processing in Prism GraphPad V9. Persons analyzing data were blinded to experimental groups. We used a t test for statistical comparisons among groups of normally distributed samples and Mann-Whitney test for non-parametric means. Asterisks (ns = p > 0.05; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001) indicate p values shown in figure legends. We generated Kaplan–Meier plots and analyzed differences in survival using the Mantel-Cox test and Mantel-Haenszal test. In assessing statistical differences of tumor burden over time between in vivo groups, we used analysis of variance (ANOVA). Similarly, we utilized ANOVA for the suppression assay.
Study approvals
The University of Michigan Institutional Animal Care and Use Committee approved all animal procedures under protocol PRO00010534.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Polyphor, LLC (now Spexis) provided funding for the research. The authors also acknowledge support from United States National Institute of Health grants R01CA238042, R01CA238023, R33CA225549, U24CA237683, R37CA222563, R50CA221807, P30 CA047904, P50 CA272218, and R35 GM150509. We also acknowledge support from the W.M. Keck Foundation.
Author contributions
N.G.C., K.E.L., and G.D.L. designed experiments. N.G.C., A.B., A.F., A.R., KKKY.H., K.E.L., and B.A.H. performed experiments. K.E.L. provided new reagents. E.C.R. and Y-C. C. analyzed RNA sequencing data. N.G.C. and G.D.L. wrote the manuscript. All authors reviewed and edited the manuscript.
Data availability
We deposited single cell RNA sequencing data in the Gene Ontology Omnibus (GEO) as GSE270613. Other data are available upon reasonable request to the corresponding author.
Declarations
Competing interests
Spexis (formerly Polyphor LLC) provided partial funding to G.D.L. for this research. No other authors have a competing interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Brock A. Humphries, Email: brhu@umich.edu
Gary D. Luker, Email: gluker@umich.edu
References
- 1.Solinas, C. et al. Targeting immune checkpoints in breast cancer: An update of early results. ESMO Open2, e000255. 10.1136/esmoopen-2017-000255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruosso, T. et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J. Clin. Invest.129, 1785–1800. 10.1172/jci96313 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, K. K. & Bass, A. R. Autoimmune complications of immunotherapy: Pathophysiology and management. BMJ369, m736. 10.1136/bmj.m736 (2020). [DOI] [PubMed]
- 4.Okwundu, N., Grossman, D., Hu-Lieskovan, S., Grossmann, K. F. & Swami, U. The dark side of immunotherapy. Ann. Transl. Med.9, 1041. 10.21037/atm-20-4750 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luker, G. D. et al. At the Bench: Pre-clinical evidence for multiple functions of CXCR4 in cancer. J. Leukoc. Biol.109, 969–989. 10.1002/jlb.2bt1018-715rr (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mezzapelle, R. et al. CXCR4/CXCL12 activities in the tumor microenvironment and implications for tumor immunotherapy. Cancers14. 10.3390/cancers14092314 (2022). [DOI] [PMC free article] [PubMed]
- 7.Orimo, A. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell121, 335–348 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Smith, M. et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res.64, 8604–8612 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature410, 50–56 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Biasci, D. et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc. Natl. Acad. Sci. U. S. A.117, 28960–28970. 10.1073/pnas.2013644117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearon, D. T. & Janowitz, T. AMD3100/Plerixafor overcomes immune inhibition by the CXCL12-KRT19 coating on pancreatic and colorectal cancer cells. Br. J. Cancer125, 149–151. 10.1038/s41416-021-01315-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, M. M., Coupland, S. E., Aittokallio, T. & Figueiredo, C. R. Resistance to immune checkpoint therapies by tumour-induced T-cell desertification and exclusion: Key mechanisms, prognostication and new therapeutic opportunities. Br. J. Cancer129, 1212–1224. 10.1038/s41416-023-02361-4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaghjiani, R. G. & Skitzki, J. J. Tertiary lymphoid structures as mediators of immunotherapy response. Cancers14. 10.3390/cancers14153748 (2022). [DOI] [PMC free article] [PubMed]
- 14.Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol.21, 485–498. 10.1038/s41577-020-00490-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santagata, S. et al. Targeting CXCR4 reverts the suppressive activity of T-regulatory cells in renal cancer. Oncotarget8, 77110–77120. 10.18632/oncotarget.20363 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehdizadeh, R., Shariatpanahi, S. P., Goliaei, B. & Rüegg, C. Targeting myeloid-derived suppressor cells in combination with tumor cell vaccination predicts anti-tumor immunity and breast cancer dormancy: An in silico experiment. Sci. Rep.13, 5875. 10.1038/s41598-023-32554-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. U. S. A.110, 20212–20217. 10.1073/pnas.1320318110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen, Y. et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology61, 1591–1602. 10.1002/hep.27665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, I. X. et al. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl. Acad. Sci. U. S. A.116, 4558–4566. 10.1073/pnas.1815515116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao, R. et al. Recent advances in CXCL12/CXCR4 antagonists and nano-based drug delivery systems for cancer therapy. Pharmaceutics14. 10.3390/pharmaceutics14081541 (2022). [DOI] [PMC free article] [PubMed]
- 21.Pernas, S. et al. Balixafortide plus Eribulin in HER2-negative metastatic breast cancer: A phase 1, single-arm, dose-escalation trial. Lancet Oncol.19, 812–824. 10.1016/s1470-2045(18)30147-5 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Spinosa, P. C. et al. Short-term cellular memory tunes the signaling responses of the chemokine receptor CXCR4. Sci. Signal.12, eaaw4204. 10.1126/scisignal.aaw4204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckley, S. S. et al. Short-term environmental conditioning enhances tumorigenic potential of triple-negative breast cancer cells. Tomography5, 346–357. 10.18383/j.tom.2019.00019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg, E. M. Jr. et al. Characterization, dynamics, and mechanism of CXCR4 antagonists on a constitutively active mutant. Cell. Chem. Biol.26, 662–673e667. 10.1016/j.chembiol.2019.01.012 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang, J. et al. CXCR4 protein epitope mimetic antagonist POL5551 disrupts metastasis and enhances chemotherapy effect in triple-negative breast cancer. Mol. Cancer Ther.14, 2473–2485. 10.1158/1535-7163.mct-15-0252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher, T. N. & Thommen, D. S. Tertiary lymphoid structures in cancer. Science375, eabf9419. 10.1126/science.abf9419 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Wang, B. et al. The presence of tertiary lymphoid structures provides new insight into the clinicopathological features and prognosis of patients with breast cancer. Front. Immunol.13, 868155. 10.3389/fimmu.2022.868155 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narvaez, D. et al. The emerging role of tertiary lymphoid structures in breast cancer: A narrative review. Cancers16. 10.3390/cancers16020396 (2024). [DOI] [PMC free article] [PubMed]
- 29.Criscitiello, C., Esposito, A., Trapani, D. & Curigliano, G. Prognostic and predictive value of tumor infiltrating lymphocytes in early breast cancer. Cancer Treat. Rev.50, 205–207. 10.1016/j.ctrv.2016.09.019 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Zhou, M., Sacirbegovic, F., Zhao, K., Rosenberger, S. & Shlomchik, W. D. T. Cell exhaustion and a failure in antigen presentation drive resistance to the graft-versus-leukemia effect. Nat. Commun.11, 4227. 10.1038/s41467-020-17991-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo, L. et al. Tumoral PD-1hiCD8 + T cells are partially exhausted and predict favorable outcome in triple-negative breast cancer. Clin. Sci.134, 711–726. 10.1042/cs20191261 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun.9, 2724. 10.1038/s41467-018-05072-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien, R. M., Cannon, A., Reynolds, J. V. & Lysaght, J. Lynam-Lennon, N. Complement in tumourigenesis and the response to cancer therapy. Cancers13. 10.3390/cancers13061209 (2021). [DOI] [PMC free article] [PubMed]
- 34.Kitamura, T. et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med.212, 1043–1059. 10.1084/jem.20141836 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo, A. et al. Myeloid-derived suppressor cells recruited by chemokine (C-C Motif) Ligand 3 promote the progression of breast cancer via phosphoinositide 3-kinase-protein kinase B-mammalian target of rapamycin signaling. J. Breast Cancer23, 141–161. 10.4048/jbc.2020.23.e26 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheng, D. et al. Ccl3 enhances docetaxel chemosensitivity in breast cancer by triggering proinflammatory macrophage polarization. J. Immunother. Cancer10. 10.1136/jitc-2021-003793 (2022). [DOI] [PMC free article] [PubMed]
- 37.Kaplanov, I. et al. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc. Natl. Acad. Sci. U. S. A.116, 1361–1369. 10.1073/pnas.1812266115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noe, J. T. & Mitchell, R. A. MIF-dependent control of tumor immunity. Front. Immunol.11, 609948. 10.3389/fimmu.2020.609948 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Azevedo, R. A. et al. MIF inhibition as a strategy for overcoming resistance to immune checkpoint blockade therapy in melanoma. Oncoimmunology9, 1846915. 10.1080/2162402x.2020.1846915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim, H. Y. et al. The CXCR4 antagonist AMD3100 has dual effects on survival and proliferation of myeloma cells in vitro. Cancer Res. Treat.42, 225–234. 10.4143/crt.2010.42.4.225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi, H. Y., Yong, C. S. & Yoo, B. K. Plerixafor for stem cell mobilization in patients with non-Hodgkin’s lymphoma and multiple myeloma. Ann. Pharmacother.44, 117–126. 10.1345/aph.1M380 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Hendrix, C. W. et al. Safety, pharmacokinetics, and antiviral activity of AMD3100, a selective CXCR4 receptor inhibitor, in HIV-1 infection. J. Acquir. Immune Defic. Syndr.37, 1253–1262. 10.1097/01.qai.0000137371.80695.ef (2004). [DOI] [PubMed] [Google Scholar]
- 43.Sato, J., Makita, N. & Iiri, T. Inverse agonism: The classic concept of GPCRs revisited. Endocr. J.63, 507–514. 10.1507/endocrj.EJ16-0084 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Zhang, N. N. et al. Prognostic impact of tertiary lymphoid structures in breast cancer prognosis: A systematic review and meta-analysis. Cancer Cell Int.21. 10.1186/s12935-021-02242-x (2021). [DOI] [PMC free article] [PubMed]
- 45.Liu, H., Wang, Z., Zhou, Y. & Yang, Y. MDSCs in breast cancer: An important enabler of tumor progression and an emerging therapeutic target. Front. Immunol.14, 1199273. 10.3389/fimmu.2023.1199273 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stacer, A. et al. Endothelial CXCR7 regulates breast cancer metastasis. Oncogene35, 1716–1724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Triplett, T. A. et al. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat. Biotechnol.36, 758–764. 10.1038/nbt.4180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fenner, J. et al. Macroscopic stiffness of breast tumors predicts metastasis. Sci. Rep.4, 5512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen, Y. C. et al. Single-cell RNA-sequencing of migratory breast cancer cells: Discovering genes associated with cancer metastasis. Analyst144, 7296–7309. 10.1039/c9an01358j (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol.33, 495–502. 10.1038/nbt.3192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ianevski, A., Giri, A. K. & Aittokallio, T. Fully-automated and ultra-fast cell-type identification using specific marker combinations from single-cell transcriptomic data. Nat. Commun.13. 10.1038/s41467-022-28803-w (03/10/2022). [DOI] [PMC free article] [PubMed]
- 52.Hänzelmann, S., Castelo, R. & Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform.14. 10.1186/1471-2105-14-7 (2013). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We deposited single cell RNA sequencing data in the Gene Ontology Omnibus (GEO) as GSE270613. Other data are available upon reasonable request to the corresponding author.