Abstract
The lysosomal protease cathepsin B has been implicated in a variety of pathologies including pancreatitis, tumor angiogenesis, and neuronal diseases. We used a tube formation assay to investigate the role of cathepsin B in angiogenesis. When cultured between two layers of collagen I, primary endothelial cells formed tubes in response to exogenously added VEGF. Overexpressing cathepsin B reduced the VEGF-dependent tube response, whereas pharmacologically or molecularly suppressing cathepsin B eliminated the dependence on exogenous VEGF. However, tube formation still required VEGF receptor activity, which suggested that endothelial cells generated VEGF. Indeed, VEGF mRNA and protein was detectable in cells treated with cathepsin B inhibitor, which correlated with a rise in the level of HIF-1α. In addition to boosting the level of proangiogenic factors, blocking cathepsin B activity reduced the amount of the antiangiogenic protein endostatin. Thus endothelial cells have the intrinsic capacity to generate pro- and antiangiogenic agents. These observations complement and expand our appreciation of how endothelial cell–derived proteases regulate angiogenesis.
INTRODUCTION
Cathepsins are cysteine endopeptidases that belong to the family of papain-like proteolytic enzymes that are principally located in the endosomal/lysosomal compartment (Turk et al., 2000). Although many of the cathepsins are ubiquitously expressed (B, C, F, H, L, O, and Z), some (C, K, S, and W) seem to be predominant in a subset of tissues (Qian et al., 1989; Wolters and Chapman, 2000). The cathepsins were historically thought to contribute to degradation of proteins in the lysosome, and recent evidence indicates additional functions.
Increased cathepsin B expression and/or activity are associated with neuronal diseases and tumor progression (Qian et al., 1989; Buck et al., 1992; Mackay et al., 1997). An imbalance between cathepsin B expression and its endogenous inhibitor cystatin B results in neuronal apoptosis, and thereby contributes to Alzheimer's disease and Unverricht-Lundborg progressive myoclonus epilepsy (Mackay et al., 1997). In the case of tumors, cathepsin B expression correlates with angiogenesis and is thought to promote the remodeling of the extracellular matrix to permit neovascularization (Buck et al., 1992; Mai et al., 2002). Furthermore, overexpression of cathepsin B protein increases the intensity of angiogenesis in primary colon adenocarcinoma (Kruszewski et al., 2004), whereas blockade of cathepsin B expression suppresses angiogenesis in human glioblastoma cells (Yanamandra et al., 2004). Thus cathepsin B is known to be a likely contributor to neuronal diseases and tumor angiogenesis. The cell types and molecular targets that are modulated by cathepsin B have not been identified.
In addition to proteases, there are a number of factors that regulate angiogenesis. Agents that promote angiogenesis include growth factors that act through known cell surface receptors expressed on endothelial cells. The most potent is vascular endothelial growth factor (VEGF), which is essential for both physiological and pathological angiogenesis in many settings (Folkman, 1995; Carmeliet and Jain, 2000). Inactivation of a single Vegf allele in mice results in embryonic lethality with defective vascularization in several organs (Ferrara et al., 1996). Up-regulation of VEGF is also seen in pathological conditions including tumor angiogenesis and proliferative retinopathy secondary to diabetes (Aiello et al., 1994; Ferrara and Davis-Smyth, 1997). VEGF gene expression is regulated by an oxygen-sensing signaling pathway, which determines the stability of a key transcription factor hypoxia-inducible factor-1 (HIF-1; Ferrara et al., 2003). VEGF levels are also regulated by growth factors (Ferrara and Davis-Smyth, 1997), and by protease like matrix metalloproteinase (MMP)-9, which releases VEGF from extracellular reservoirs (Bergers et al., 2000).
There is a growing appreciation of the existence of agents that suppress angiogenesis and thereby provide a counter-balance to the proangiogenic growth factors such as VEGF. For instance, thrombospondin (Tsp) -1 and -2, endostatin and angiostatin are examples of proteins that suppress angiogenesis (Good et al., 1990; O'Reilly et al., 1994, 1997). Tsp-1 acts indirectly, by suppressing MMP-9 and thereby preventing the release of extracellular matrix (ECM)-bound VEGF (Rodriguez-Manzaneque et al., 2001). Tsp-1 can also act directly on the endothelial cells, through CD36 (Dawson et al., 1997). The mechanism of action of endostatin and angiostatin is still under investigation, but it is at least in part at the level of the endothelium (O'Reilly et al., 1994, 1997). Endostatin is generated from the NC1 domain of type XVIII collagen as a result of proteolytic cleavage by enzymes, such as elastase, MMPs, and cathepsins L, B, and K (Wen et al., 1999; Felbor et al., 2000; Ferreras et al., 2000). Taken together, these data indicate that the environment of the endothelium has both positive and negative regulators of angiogenesis, which must be integrated at least in part at the level of the endothelial cells.
The “angiogenic switch” is a functional definition for the transition of a tumor from a small (1–2 mm), avascular state to the vascular and clinically dangerous phase. This concept is usually discussed focusing on the tumor cells themselves, despite the critical input of the host endothelial cells, i.e., the endothelial cells must execute the angiogenic response that results from a change in the balance of angiomodulators. Recent studies have identified some of the molecules that regulate the switch (VEGF, basic fibroblast growth factor [FGF], and Tsp-1; Hanahan and Folkman, 1996), as well as the signaling pathways that govern the expression of these molecules (Watnick et al., 2003; Maxwell, 2004). The role of endothelial cells in the angiogenic switch has not been extensively investigated. In this study we discovered that endothelial cells produce pro- and antiangiogenic factors in a cathepsin B–regulated manner. This finding suggests that endothelial cells contribute to the angiogenic switch and that this input is controlled by proteases such as cathepsin B.
MATERIALS AND METHODS
Antibodies and Reagents
Anti-mouse and anti-rabbit immunoglobulins G conjugated to horseradish peroxidase (HRP) were obtained from Amersham Biosciences (Piscataway, NJ). Rabbit polyclonal anti-endostatin antibody and anti-cathepsin B antibody were obtained from Oncogene (Boston, MA). Mouse monoclonal anti-HIF-1α antibody was obtained from Novus Biologicals (Littleton, CO). The RasGAP antibody was a crude polyclonal rabbit antiserum and was previously described (Valius et al., 1993).
The various inhibitors, cathepsin B inhibitor (CBI), CA-074-Me, cpm-VAD-CHO, GM6001, and (Z)-3-[(2,4-dimethyl-3-(ethoxycarbonyl)pyrrol-5-yl)methylidenyl]indolin-2-one (VEGF receptor 2 inhibitor) were purchased from Calbiochem (San Diego, CA).
Recombinant endostatin was purchased from Calbiochem. All other chemicals and reagents were obtained from Sigma (St. Louis, MO) unless otherwise indicated.
Isolation of Bovine Retinal Endothelial Cells
Retinal endothelial cells were isolated from bovine eyes as described previously (Gitlin and D'Amore, 1983). Briefly, the intact globes were soaked in 20% betadine in phosphate-buffered saline (PBS; GIBCO-BRL, Gaithersburg, MD), the anterior segments were transected, and then the retinas were detached with a spatula and isolated by cutting the optic nerve. The detached retinas were placed in PBS and rinsed to remove retinal pigment epithelium. The retinas were then homogenized transferred onto an 88-μM mesh that was placed over a funnel. The homogenate was washed extensively with PBS and then digested with 1 U/ml collagenase II and 20 U/ml DNase I for 45 min at 37°C with shaking. The cells were collected by centrifugation and then treated with collagenase a second time for 45 min at 37°C with shaking. The cells were pelleted and washed with EBM (Clonetics, Walkersville, MD) containing 10% horse serum (HS). The washed cells were resuspended in EBM supplemented with 10% HS, 80 U/ml penicillin/streptomycin C (Irvine Scientific, Santa Ana, CA), and 12 μg/ml bovine brain extract (BBE; Clonetics). The cells were plated on plastic coated with 50 μg/ml bovine fibronectin and incubated at 37°C with 5% CO2. The purity of the cultures was assessed by von Willebrand factor VIII–related antigen (Novus Biologicals) staining and was greater than 99%. For all the experiments, cells were used between passages 7–10.
Cell Culture
Bovine retinal endothelial cells (BRECs) were maintained in EBM supplemented with 10% HS, 80 U/ml penicillin/streptomycin C, and BBE. The cells were plated on plastic coated with 50 μg/ml bovine fibronectin and incubated at 37°C in 5% CO2. Human umbilical vein endothelial cells (HUVECs) were maintained in EGM-2 with low serum growth factor supplement (Clonetics). For the experiments, HUVECs were used between passages 5–7.
Tube Formation Assay
A collagen gel mixture consisting of 80% (by volume) VITROGEN (Cohesion, Palo Alto, CA), 0.02 N NaOH, 20 mM HEPES (GIBCO-BRL), 2 mg/ml NaHCO3, 0.5 μg/ml fibronectin, 0.5 μg/ml laminin, and 10.5 mg/ml RPMI powder (GIBCO-BRL) was prepared. For the lower gel layer, 400 μl of the gel mixture per well was added to each well of the 24-well plates and incubated at 37°C for 2 h. After polymerization, 1 × 105 BRECs were seeded in each well and incubated with medium containing 10% HS and 12 μg/ml BBE for 24 h at 37°C. In the case of HUVECs, 7 × 104 cells were seeded and incubated with culture medium. The medium was removed and 150 μl of the gel mixture was added to each well. To polymerize the upper gel layer, the plates were incubated at 37°C for 2 h. Finally, 500 μl of medium supplemented with 10% HS and 12 μg/ml BBE with or without VEGF (Upstate Biotechnology, Lake Placid, NY) was added. After 5 d, three different fields per well were randomly chosen and photographed using a model Eclipse TE300 inverted phase microscope (Nikon, Tokyo, Japan). Images were captured using Adobe Photoshop (Adobe Systems, San Jose, CA), and the data were imported as a TIFF file into NIH Image. After calibration with a stage micrometer, the total length of all tube within a field was measured and the data were imported into Microsoft Excel. Each experimental condition was assayed in triplicate. The results were expressed as an average, unless otherwise indicated. The error bars indicate the SD of triplicates samples. Experiments were performed on at least three independent occasions. The average tube length in the presence of VEGF or cathepsin B inhibitors was routinely 15–25 mm.
Electron Micrographs
Tubes that formed after 5 d in collagen sandwich gel assay were fixed overnight in 1/2 Karnoversusky solution and then treated with osmium tetroxide and uranyl acetate. After dehydration in ethanol (70, 95, and 100%), tubes were treated with propylene oxide and 1/2 propylene oxide/1/2 epon, 1 h and overnight, respectively. After embedding in epon, thin sections were prepared, stained with toluidine blue, and then examined by Philips 410 electron microscopy (Eindhoven, Netherlands). Representative fields were photographed.
Construction of siRNA-cathepsin B–expressing Vector and Retroviral Infection
Oligonucleotides corresponding to the 379–400 region (region 1, see Figures 3B, 5F, and 6C), 509–531 region (region 2, Figure 3B), and 825–846 region (region 3, Figure 3B) of the bovine cathepsin (L06075) encoding sequences were synthesized and subcloned into the ApaI-EcoRI sites of pLPU6, which is a retroviral version of the previously described vector (Sui et al., 2002). The pLPU6 empty vector and siRNA-cathepsin B/pLPU6 constructs were transfected into 293GPG cells. Supernatant was collected for 5 d, concentrated (25,000 × g, 90 min, 4°C) and used as described previously (Ory et al., 1996). Cells were infected and selected on the basis of proliferation in the presence of puromycin (2 μg/ml). The sequence of siRNA cathepsin B (the region 1) is at the 5′ site GGCGTGTCAACGTGGAGGTGTAAGCTTACACCTCCACGTTGACACGCCCTTTTTG and at the 3′ site AATTCAAAAAGGGCGYGTCAACGTGGTGTAAGCTTACACCTCCACGTTGACATGCC.
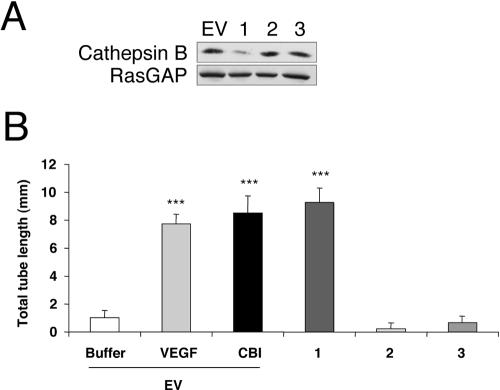
Figure 3.
Gene silencing of cathepsin B induces robust tube formation. (A) BRECs were infected with a retrovirus encoding either the siRNA for one of three regions of cathepsin B or an empty vector (EV). Total cell lysates were made and subjected to Western blot analysis using an anti-cathepsin B antibody. The blot was reprobed with an anti-RasGAP antiserum to verify equivalent protein loading in all the lanes. (B) BRECs infected with either an siRNA for cathepsin B or an EV were plated within a collagen sandwich gel, and then medium containing either 2.5 ng/ml VEGF or 40 μM CBI was added. After 5 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. Error bars, SD of triplicates samples. Similar results were observed in three independent experiments; *** p < 0.001 versus buffer.
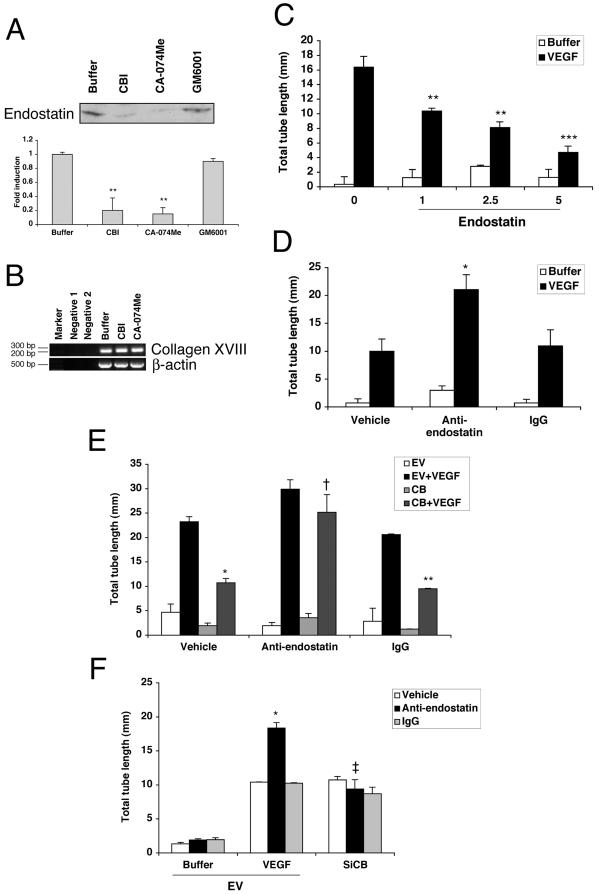
Figure 5.
Cathesin B regulates the level of endostatin, which suppresses tube formation. (A) BRECs were plated in a collagen sandwich gel, and then medium containing either cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) or MMPs inhibitor (1 nM GM6001) was added. After 5 d, the supernatants were collected, immunoprecipitated with an anti-endostatin antibody, and then subjected to Western blot analysis using anti-endostatin antibody. The bar graph below is a fold increase in an intensity of endostatin band compared with the buffer-treated control. Error bars, SD of triplicate samples; 2–3 independent experiments showed similar results; ** p < 0.01 versus buffer. (B) BRECs were plated in a collagen sandwich gel, and then medium containing either buffer or the cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) was added. After 1 d, total RNA was isolated and subjected to RT-PCR using primers directed to bovine collagen XVIII. Bovine β-actin was amplified simultaneously in a separate set of tubes under the same conditions. Negative 1, template alone; Negative 2, primer alone. When fewer PCR cycles were performed, the amount of product was reduced by more than 50% in each of the conditions, i.e., no differences between the samples were detected under limiting PCR conditions. (C) BRECs were plated in a collagen sandwich gel, and then medium containing either buffer or 2.5 ng/ml VEGF was added. The indicated cultures also received recombinant endostatin (1, 2.5, and 5 μg/ml). After 5 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. (** p < 0.01; *** p < 0.001). (D) BRECs were plated in a collagen sandwich gel in the presence of either buffer or VEGF (2.5 ng/ml). An anti-endostatin (2.5 ng/ml), normal rabbit antibody (IgG, 2.5 ng/ml), or vehicle was included in the indicated samples. After 5 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. The tube length of anti-endostatin was significantly different from that of IgG in the presence of VEGF (* p < 0.05). (E) BRECs infected with cathepsin B or an empty vector (EV) were plated within a collagen sandwich gel, and then medium containing either 2.5 ng/ml VEGF or buffer was added. An anti-endostatin (2.5 ng/ml), normal rabbit antibody (IgG, 2.5 ng/ml), or vehicle was included in the indicated settings. After 5 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. Error bars, SD of triplicates samples. Similar results were observed in three independent experiments. In the presence of VEGF, tube length of cathepsin B–overexpressing cells was significantly reduced when compared with that of EV-expressing cells treated with vehicle (* p < 0.05) or IgG (** p < 0.01). In the cathepsin B–overexpressing cells, tube length observed in anti-endostatin and VEGF-treated cultures was significantly increased compared with VEGF-treated cultures that received the control IgG or vehicle († p < 0.01). (F) BRECs infected with either an siRNA for cathepsin B (SiCB) or an EV were plated within a collagen sandwich gel, and then medium containing either 2.5 ng/ml VEGF or buffer was added. An anti-endostatin (2.5 ng/ml) or normal rabbit antibody (IgG, 2.5 ng/ml) or vehicle was included in the indicated settings. After 5 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. Error bars, SD of triplicates samples. Similar results were observed in three independent experiments. In the presence of VEGF, tube length of anti-endostatin antibody-treated cells, which express EV, was significantly increased when compared with that of normal rabbit antibody-treated cells (* p < 0.05). There was a significant drop in tube response to anti-endostatin when cathepsin B levels were reduced (‡ p < 0.01).
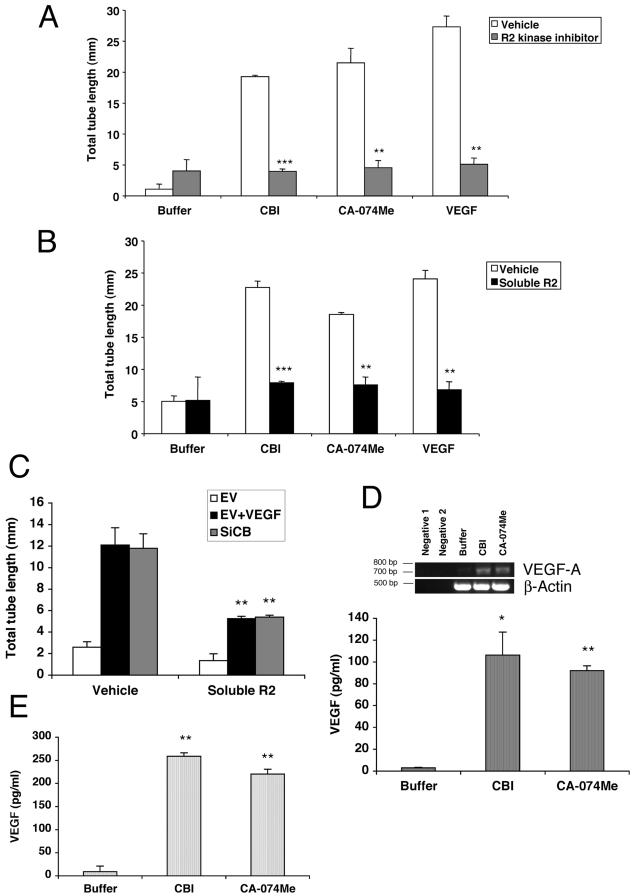
Figure 6.
The CBI-driven tube response is dependent on VEGFR2, and CBI increases endogenous VEGF expression. (A and B) The tube response was monitored in the presence a two different VEGFR2 inhibitors. After 5 d, three randomly selected fields were photographed, and tube lengths were measured. Error bars, SD of triplicates samples. Similar results were observed in three independent experiments. In A, the tube assay was supplemented with vehicle (open bars) or a pharmacological VEGFR2 (R2) kinase inhibitor (gray bars). In B, vehicle (open bars) or a VEGF trap consisting of the extracellular domain of VEGFR2 (Soluble R2, black bars) was added to the tube assay. Similar results were observed in two independent experiments. The tube response induced by either of cathepsin inhibitors or by VEGF was significantly reduced by the addition of either the R2 kinase inhibitor (A) or Soluble R2 (B); *** p < 0.001; ** p < 0.01. (C) BRECs infected with siRNA for cathepsin B (SiCB) or an EV were plated within a collagen sandwich gel, and then medium containing either 2.5 ng/ml VEGF or buffer was added. Vehicle or a VEGF trap consisting of the extracellular domain of VEGFR2 (Soluble R2, black bars) was also added to the tube assay. The tube lengths were significantly decreased in VEGF-treated and SiCB groups when supplemented with Soluble R2 (** p < 0.01). (D) BRECs were plated in a collagen sandwich gel, and then medium containing either buffer or cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) was added. After 1 d, total RNA was isolated and subjected to RT-PCR using primers specific for bovine VEGF-A. Bovine β-actin was amplified simultaneously in a separate set of tubes under the same conditions. In the lower portion of this panel, BRECs were plated in a collagen sandwich gel, and then medium containing either buffer or cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) was added. After 2 d, the cells were harvested and total cell lysates representing 1 × 105 cells were subjected to ELISA that recognize VEGF. The A450 for each sample was determined relative to a VEGF dilution curve made from an internal standard. The VEGF concentration shown is an arbitrary number based on the standard curve using mouse VEGF. Similar results were observed in two independent experiments; * p < 0.05; ** p < 0.01 for the comparison of cathepsin B-versus buffer-treated samples. (E) HUVECs were plated in a collagen sandwich gel, and then medium containing either buffer or cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) was added. After 2 d, the cells were harvested and total cell lysates representing 1 × 105 cells were subjected to ELISA that recognize VEGF. The A450 for each sample was determined relative to a VEGF dilution curve made from an internal standard. Similar results were observed in two independent experiments; ** p < 0.01 for the comparison of cathepsin B-versus buffer-treated samples.
Construction of Cathepsin B Expression Vector and Retroviral Infection
A full-length cDNA of mouse cathepsin B was kindly provided by Dr. Terence S. Dermody (Vanderbilt University, Nashville, TN; Ebert et al., 2002) and used as a PCR template to synthesize a full-length cDNA of cathepsin B flanked by a NotI site at the 5′ end and SalI site at the 3′ end. Two oligonucleotides, corresponding to nucleotides at the 5′ site CCGGCGGCCGCAGGATGTGGTGGTCCTTG and at the 3′ site TCAAGTCGACTTAGAATCTTCCCCAGTA were used as primers. The resulting cDNA was subcloned into pLNCX2. The pLNCX2 empty vector and cathepsin B/pLNCX2 constructs were transfected into 293GPG cells. Supernatant was collected for 5 d, concentrated (25,000 × g, 90 min, 4°C), and used as described previously (Ory et al., 1996). Cells were infected and selected on the basis of proliferation in the presence of G418 (1 mg/ml).
Immunoprecipitation and Western Blot Analysis
BRECs were plated in a collagen sandwich gel and then supplemented with dimethyl sulfoxide (DMSO), 40 μM CBI, 1 μM CA-074 Me, or 1 nM GM6001. After 5 d, conditioned medium was collected and immunoprecipitated with an anti-endostatin antibody in the presence of protease inhibitors (2 μg/ml aprotinin, 5 μg/ml leupeptin, 10 μg/ml phenyl methyl sulfonyl fluoride, and 10 mM sodium fluoride after preclearing the supernatant with nonimmune rabbit IgG; Santa Cruz Biotechnology). Immunocomplexes were collected on protein A/G plus agarose (Santa Cruz Biotechnology) and washed three times with lysis buffer containing 20 mM Tris (pH 8.0), 150 mM NaCl, 1 mM dithiothreitol, 1% deoxycholic acid, 0.5% SDS, 1% Nonidet P-40, and protease inhibitors (as above). The immunoprecipitated proteins were separated on 12.5% SDS-polyacrylamide gels and then electrophoretically transferred onto PVDF membrane (0.2 μm, Millipore, Bedford, MA). Membranes were incubated for 1 h in TBST (10 mM Tris base, pH 8.0, 150 mM NaCl, 0.2% Tween 20) and 5% nonfat dry milk. The anti-endostatin was used for primary antibody, and the secondary antibody was HRP-conjugated goat anti-rabbit antibody. The blot was visualized using the ECL detection system (Amersham Biosciences), and the resulting signals were quantified by densitometric analysis using the Molecular Imager and Quantity One software (Bio-Rad, Hercules, CA).
For Western blot analysis of HIF-1α,2 × 106 cells of BRECs were plated into 10-cm tissue culture plates and incubated for at least 18 h in medium containing 10% HS and 12 μg/ml BBE. The cells were exposed to either 10 nM MG132, 40 μM CBI, 1 μM CA-074-Me, or DMSO for 24 h at 37°C. Total cell lysates were prepared by adding lysis buffer (as above) and protease inhibitors (as above) and incubating 30 min to 1 h on ice. After centrifugation, the supernatants were collected and assayed for protein concentration. Ten to 30 μg of proteins were separated on 10% SDS-polyacrylamide gels, and Western blot analysis was performed as described above. Anti-HIF-1α was used for primary antibody and HRP-conjugated goat anti-mouse antibody was used for secondary antibody. The blot was stripped by incubating for 30 min at 60°C in a buffer containing 6.25 mM Tris-HCl, pH 6.8, 2% SDS, and 100 mM β-mercaptoethanol and then reprobed with anti-RasGAP antibody.
RNA Extraction and RT-PCR
A total of 1 × 106 BRECs were plated in the tube formation assay as described above. After 2 d treatment with 40 μM CBI, 1 μM CA-074Me, or DMSO, total RNA was isolated with RNAgents Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's protocol. The RT-PCR reactions were performed using the Onestep RT-PCR kit (QIAGEN, Valencia, CA). Two oligonucleotides, corresponding to nucleotides at the 5′ site GAAGGAGGGCAGAAACCCCACGAAGTGG and at the 3′ site CCATGAATGCTTCTGCCGGAGCCTCACGC were used as primers for VEGF-A. For HIF-1α, two oligonucleotides corresponding to nucleotides at the 5′ site GGCTTCTGTTATGAGGCTCACCATCAGC and at the 3′ site CCAGACTGTGACGACTGAGAAAAGTCTTGC were used as primers. For collagen XVIII, two oligonucleotides corresponding to nucleotides at the 5′ site CCTACAGGCAGACCATCAGTGTTCC and at the 3′ site CCTTCCCTGTCAGCCACGTAGATGAGCC were used as primers. The first-strand cDNA was reverse-transcribed from total RNA, and the cDNA product was amplified by PCR for 20 or 30 cycles for 30 s at 94°C, 1 min at 55°C, and 1 min at 72°C (for final extension, 10 min at 72°C). The RT-PCR products were separated in 1.0% agarose gels and visualized with ethidium bromide. Bovine β-actin was amplified simultaneously in a separate set of tubes under the same conditions. The 5′ and 3′ primers for β-actin were GCTCAGAGCAAGAGAGGCATCCTGACC and GCAGAGCTTCTCCTTGATGTCACGG, respectively.
VEGF Enzyme-linked Immunosorbent Assay
An enzyme-linked immunosorbent assay (ELISA) was performed to measure the level of VEGF (R&D Systems, Minneapolis, MN). For the HUVECs experiments we used a kit that recognizes human VEGF-A. Because there is no ELISA for bovine VEGF, we used the kit that recognizes multiple species. Briefly, the BRECs and HUVECs were plated in the collagen sandwich gel and then supplemented with cathepsin B inhibitor or buffer for 48 h. No VEGF was detected in the conditioned medium. The cells were liberated from the collagen gel by incubation for 30 min at 37°C with 1–2 U of collagenase in Hanks' balanced salt solution. The cells were collected by centrifugation and then sheared in a volume of 100 μl of lysis buffer containing protease inhibitors. Aliquots (5–10 μg) of the resulting lysates were used in the ELISA. The intraassay and interassay variability had coefficients of variance of 8.2 and 8.4%, respectively, as determined by the manufacturer for the range of protein concentrations used in our assays. The A450 for each sample was determined relative to a VEGF dilution curve made from an internal standard.
Mice
Homozygous cathepsin B–deficient mice (Ctsb–/–) on a C57BL6 background were kindly provided by Dr. Hidde Ploegh (Harvard Medical School, Boston, MA). The Ctsb–/–mice and the corresponding wild-type (Cstb+/+) with the same genetic background were housed and cared for according to institutional guidelines. Mice between 6 and 8 mo of age of both sexes were used for experiments.
Preparation of Retinal Explants
The vessel outgrowth procedure was a modification of a previously published protocol (Knott et al., 1999). A collagen gel mixture was prepared as described for the tube formation assay, and 400 μl was place into each well of a 24-well plate and incubated at 37°C for 2 h to allow the gel to solidify. Whole retinas were dissected from enucleated eyes and spread out in a Petri dish containing PBS. The retinas were cut into pieces of ∼1 mm diameter. Individual explants were added to 200 μl of a freshly prepared collagen gel mixture and placed on top of the solidified collagen gel. The plates were incubated at 37°C for 2 h to permit solidification of the top gel. At the end of this incubation 500 μl of medium supplemented with 10% HS, 12 μg/ml BBE, and 25 ng/ml mouse VEGF (R&D Systems) was added. The explants were incubated at 37°C for 1 mo, during which time they were periodically photographed through an Eclipse TE300 inverted phase microscope (Nikon). Images were captured using Adobe Photoshop (Adobe Systems), and the data were imported as a TIFF file into NIH Image. The total vessel length in each explant was measured, and the data were imported into Microsoft Excel. The resulting data were presented as a mean of three replicates from two independent experiments (Ctsb–/–mice, n = 3; Ctsb+/+ mice, n = 4). The error bars indicate the SD of triplicates samples.
Statistics
The Student's t test was used to assess statistical significance; p < 0.05 was significant.
RESULTS
Inhibition of Cathepsin B Promotes Tube Formation of Primary Endothelial Cells
When plated between two layers of collagen 1, primary BRECs organize into cords, provided that VEGF is added to the culture medium (Figure 1A). Electron micrographs of the cords revealed a lumen, defined by 5–7 cells (Figure 1C); we therefore called these structures tubes. These tubes formed over the course of 5 d and achieved an average length of 15–25 mm (Figure 1B). In the continued presence of VEGF, the tubes lengthened for at least 1 mo (unpublished data). Reducing the concentrations of VEGF below 2.5 ng/ml attenuated the response, whereas higher doses of VEGF did not boost the output (unpublished data). This VEGF-dependent in vitro assay is similar to assays reported by other groups (Cai et al., 2003; Ottino et al., 2004), and we used it to identify variables that control in vitro angiogenesis.
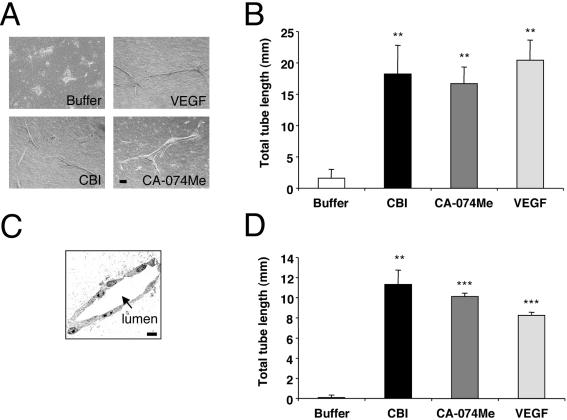
Figure 1.
Cathepsin B inhibitors increase tube formation of primary endothelial cells. BRECs were plated within a collagen sandwich gel, and then medium containing either 2.5 ng/ml VEGF or two different cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) was added. After 5 d in culture at 37°C, three randomly selected fields were photographed (A) and tube lengths were measured. Error bars, SD of triplicate samples. Similar results were observed in five independent experiments. ** p < 0.01. (C) BRECs were cultured in a collagen gel in the presence of 2.5 ng/ml VEGF for 5 d and then fixed with glutaraldehyde and embedded in plastic. Thin sections were prepared and examined by electron microscopy. Endothelial cells are observed surrounding a lumenal space (arrow). Bar, 50 μm. (D) HUVECs were plated within a collagen sandwich gel, and then medium containing either 2.5 ng/ml VEGF or cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) was added. After 1 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. Error bars, SD of triplicates samples. Similar results were observed in three independent experiments; *** p < 0.001; ** p < 0.01 versus buffer.
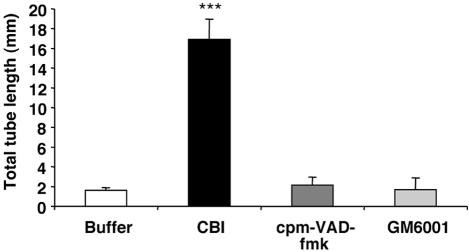
We serendipitously found that cathepsin B inhibitors could substitute for VEGF in the tube assay. Two irreversible, cell permeable, and chemically distinct cathepsin B inhibitors (cathepsin B inhibitor [CBI] and CA-074Me) induced tube formation that was similar to that seen in VEGF-treated cultures (Figure 1, A and B). A dose-response experiment revealed that the optimal concentration was 40 μM for CBI and 1 μM for CA-074Me (Supplementary Figure S1), and the inhibitors were used at these concentrations for the remainder of the experiments. Both of the cathepsin B inhibitors induced tube formation in HUVECs (Figure 1D), indicating that this phenomenon was not restricted to a single primary endothelial cell type. Because cathepsin B is implicated in apoptosis and ECM degradation (Buck et al., 1992; Mackay et al., 1997), we tested if inhibitors of caspases (cpm-VAD-CHO) or MMPs (GM6001) could also promote tube formation in the absence of VEGF. In contrast to the cathepsin B inhibitors, the inhibitors of other proteases failed to substitute for VEGF (Figure 2). The data in Figures 1 and 2 demonstrate that inhibitors of cathepsin B, but not caspases or MMPs, induce primary endothelial cells to form tubes in a collagen 1 sandwich assay. These observations suggest that endothelial cells posses an intrinsic capacity to form tubes, which is protease-regulated.
Figure 2.
Inhibitors of MMPs or caspases fail to promote tube formation. BRECs were plated within a collagen sandwich gel and then supplemented with medium containing either 1 nM MMPs inhibitor (GM6001) or 20 μM caspase inhibitor (cpm-VAD-CHO) or 40 μM CBI. After 5 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. Error bars, SD of triplicates samples. Similar results were observed in two independent experiments; *** p < 0.001 versus buffer.
Reducing Cathepsin B Levels by siRNA Induces Robust Tube Formation
As an alternative approach to abrogate cathepsin B activity, we reduced the level of cathepsin B proteins by stably expressing a cathepsin B siRNA. We targeted three different regions of the coding region of the cathepsin B gene and found that cells expressing oligos directed to one of these regions had a 70% decrease in cathepsin B protein, whereas cathepsin B levels were unchanged by expression of oligos directed to the other two regions (Figure 3A). When we tested the tube response in these cells, we found that the cells that had a reduced level of cathepsin B spontaneously formed tubes, whereas the other two cell lines did not. Furthermore, the magnitude of the response in the cells with a reduced level of cathepsin B was comparable to that triggered by exogenously added VEGF or the cathepsin B inhibitor (Figure 3B). Note that the overall tube response was consistently reduced in this series of experiments (compare with Figures 1, 2, and 4, 5, 6) and may be related to expression of the siRNA expression vector. We conclude that either pharmacologically inhibiting cathepsin B or reducing the overall cellular amount of the enzyme resulted in spontaneous formation of tubes in the absence of exogenously added proangiogenic factors.
Figure 4.
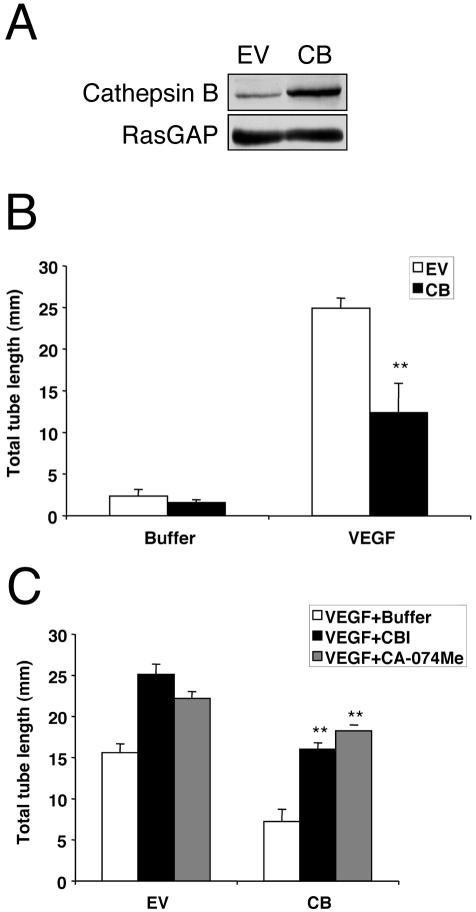
Overexpression of cathepsin B inhibits VEGF-induced tube formation. (A) BRECs were infected with a retrovirus encoding either cathepsin B or an empty vector (EV). Total cell lysates were made and subjected to Western blot analysis using an anti-cathepsin B antibody. The blot was reprobed with an anti-RasGAP antiserum to verify equivalent protein loading in all the lanes. Similar results were observed in two independent experiments. (B) BRECs infected with either cathepsin B (CB) or an empty vector (EV) were plated within a collagen sandwich gel, and then medium containing 2.5 ng/ml VEGF or buffer was added. After 5 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. Error bars, SD of triplicates samples. Similar results were observed in three independent experiments. In the presence of VEGF, tube length of cathepsin B–overexpressing cells was significantly reduced when compared with that of EV-expressing cells (** p < 0.01). (C) BRECs infected with either cathepsin B (CB), or an empty vector (EV) were plated within a collagen sandwich gel, and then medium containing 2.5 ng/ml VEGF and buffer or 40 μM CBI or 1 μM CA-074Me was added. After 5 d in culture at 37°C, three randomly selected fields were photographed, and tube lengths were measured. Error bars, SD of triplicates samples. Similar results were observed in two independent experiments; there was a statistically significant increase compared with that of buffer-treated cells (** p < 0.01).
Overexpression of Cathepsin B Inhibits VEGF-induced Tube Formation
Because either pharmacological inhibition of cathepsin B or siRNA-based reduction of cathepsin B protein promoted tube formation, we tested if increasing the level of cathepsin B would suppress the VEGF-dependent tube response. The mouse cathepsin B cDNA was stably expressed in BRECs as described in Materials and Methods. Western blotting of the resulting cells revealed a 2.4-fold increase in the level of cathepsin (Figure 4A) compared with the empty vector expressing cells (EV). We consistently observed that the cathepsin B–overexpressing cells exhibited a reduced VEGF-stimulated tube response (Figure 4B), which was alleviated by the addition of either of the cathepsin B inhibitors (Figure 4C). This finding demonstrates that cathepsin B suppressed in vitro tube formation and complemented the observations in Figures 1, 2, 3 showing that reducing the cathepsin B input promoted tube formation. Taken together, these data strongly support the idea that cathepsin B regulates the ability of endothelial cells to form tubes.
BRECs Generate Endostatin in a Cathepsin B–dependent Manner
Given that cathepsin B can process collagen XVIII to endostatin (Ferreras et al., 2000), an inhibitor of endothelial cell tubulogenesis (Kuo et al., 2001), we considered the possibility that cathepsin B might control the generation of this antiangiogenic protein. Consistent with this premise, endothelial cells embedded in type I collagen gels expressed collagen XVIII mRNA as detected by RT-PCR (Figure 5B). Further, the generation of endostatin or an endostatin-like material (Ferreras et al., 2000) was identified by immunoblot analysis in the conditioned medium of endothelial cells cultured in the tube formation assay (Figure 5A). As predicted, pretreatment of endothelial cells with either of the cathepsin B inhibitors, but not the MMP inhibitor, reduced strongly the levels of endostatin detected in this system without affecting collagen XVIII mRNA levels (Figure 5, A and B). These findings support the idea that cathepsin B–generated endostatin proteolytically.
We further investigated the potential role of endostatin in the tube response. As expected, recombinant endostatin attenuated the VEGF-induced tube response in a concentration-dependent manner (Figure 5C). Moreover, addition of the anti-endostatin antibody lengthened VEGF-induced tubes (Figure 5D), which suggested that the endogenous levels of endostatin detected in Figure 5A are impacting the tube response. Similarly, the anti-endostatin antibody reversed the suppression of tube formation brought about by overexpressing cathepsin B (Figure 5E). Finally, the antiendostatin antibody failed to promote the tube response when the levels of cathepsin B were reduced by siRNA (Figure 5F). Taken together these findings indicate that BRECs produced endostatin in a cathepsin B–dependent manner and that it regulated the angiogenic capacity of the BRECs.
Cathepsin B Inhibitors Increase VEGF Expression
The cathepsin B–dependent regulation of endostatin (Figure 5) did not fully explain why tubes were forming in response to inhibition of cathepsin B. Tube formation in this assay was dependent on the addition of an exogenous proangiogenic factor such as VEGF (Figure 1). Therefore we anticipated that the cathepsin B inhibitors were also elevating the level of one or more proangiogenic factors such as VEGF.
If VEGF was driving tube formation in the cathepsin B–impaired cells, then blocking the receptors for VEGF would inhibit the response. A VEGF receptor 2 (VEGFR2) kinase activity inhibitor (R2 kinase inhibitor) reduced the tube response stimulated by VEGF, CBI, or CA-074Me by 80, 75, or 80%, respectively (Figure 6A). These studies indicated that VEGFR2 kinase activity was required for tube formation in response to cathepsin B inhibitors.
To independently confirm that tube formation in the cathepsin B inhibitor–treated cells was dependent on VEGF, we used a soluble VEGF receptor comprising the entire extracellular domain of VEGFR2. BRECs in the tube assay were exposed to conditioned medium from NIH3T3 cells expressing the soluble VEGFR2 (Soluble R2) or an empty expression vector. The conditioned medium from empty vector–expressing NIH 3T3 cells had no effect on tube formation under any condition tested (Figure 6B and unpublished data). In contrast, soluble R2 reduced tube formation that was driven by VEGF, CBI, or the CA-074Me by 70, 65, or 55%, respectively (Figure 6B). Moreover, soluble R2 inhibited the tube formation that occurred in response to reducing the level of cathepsin B protein (Figure 6C). These two complementary approaches revealed that the receptor for VEGF played an important role in tube formation in response to cathepsin B inhibitors.
To determine if cathepsin B regulates VEGF expression, VEGF mRNA levels were assessed in the absence or presence of cathepsin B inhibitors. Although low levels of VEGF message were detected in unstimulated cells, the addition of either cathepsin B inhibitor dramatically increased VEGF mRNA levels (Figure 6D). Consistent with these data, VEGF protein levels dramatically increased in cultures treated with the cathepsin B inhibitors (Figure 6D). We extended this analysis to HUVECs and also found that the basal level of VEGF was very low and that either of the cathepsin B inhibitors dramatically increased the amount of VEGF (Figure 6E). No VEGF was present in the conditioned medium of any of the samples (unpublished data). Hence several types of primary endothelial cells express VEGF mRNA and synthesize the protein via a process that is suppressed by cathepsin B.
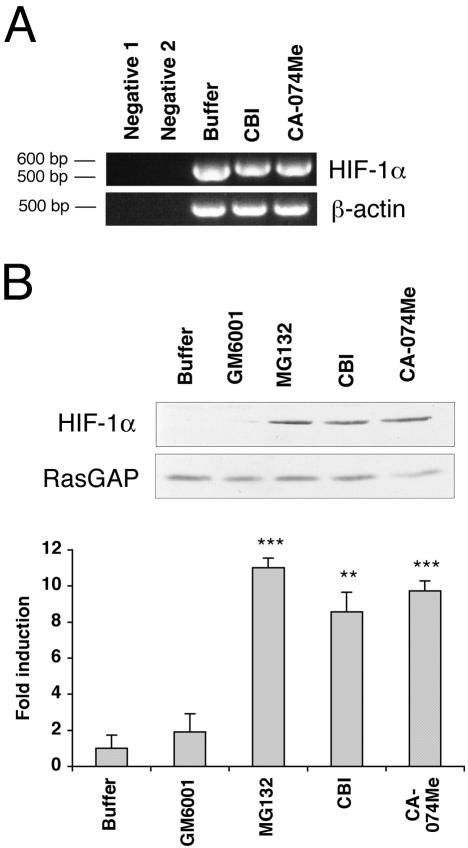
Cathepsin B Inhibitors Increase HIF-1α Expression
To begin to understand how cathepsin B regulated VEGF mRNA and protein levels, we considered the effect of cathepsin B on HIF-1α, which is one of the regulators of VEGF transcription (Forsythe et al., 1996). Although HIF-1α was originally found to modulate VEGF levels in response to hypoxia (Forsythe et al., 1996; Semenza, 2000), recent studies indicate that HIF-1α can control VEGF in hypoxia-independent settings as well (Isaacs et al., 2002). Analysis of HIF-1α mRNA levels revealed that the cathepsin B inhibitors were unable to induce dramatic changes (Figure 7A). In contrast, the inhibitors induced a 9–10-fold increase of HIF-1α protein levels over the very low basal level (Figure 7B). The MMP inhibitor was without effect, and a proteasomal inhibitor (MG132) greatly (11-fold) increased the level of HIF-1α, which is consistent with reports that HIF-1α is rapidly degraded by a proteasome-based pathway (Jaakkola et al., 2001; Isaacs et al., 2002). These findings suggest that cathepsin B can regulate the steady state levels of HIF-1α. Furthermore, the rise in VEGF levels in cathepsin B–treated cells may be at least in part due to the increase the amount of HIF-1α.
Figure 7.
The cathepsin B inhibitors stabilize HIF-1α. (A) BRECs were plated in a collagen sandwich gel, and then medium containing either buffer or cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) was added. After 1 d, total RNA was isolated and subjected to RT-PCR using primers specific for bovine HIF-1α. Bovine β-actin was amplified simultaneously in a separate set of tubes under the same conditions. (B) The BRECs were plated in a collagen sandwich gel, and then medium containing either buffer or cathepsin B inhibitors (40 μM CBI or 1 μM CA-074Me) or a proteasome inhibitor (10 nM MG132) or an MMP inhibitor (1 nM GM6001) was added. After 8 h in culture at 37°C, the cells were harvested. Total cell lysates were made and subjected to Western blot analysis using an anti-HIF-1α antibody. The blot was reprobed with an anti-RasGAP antibody to determine the level of protein loaded in each lane. The bar graph below is a fold increase in an intensity of HIF-1α band compared with buffer-treated control. Error bars, SD of three independent experiments. For the comparison of inhibitor-versus buffer-treated samples: ** p < 0.01; *** p < 0.001.
Retinal Vessel Outgrowth Is Enhanced by the Absence of Cathepsin B
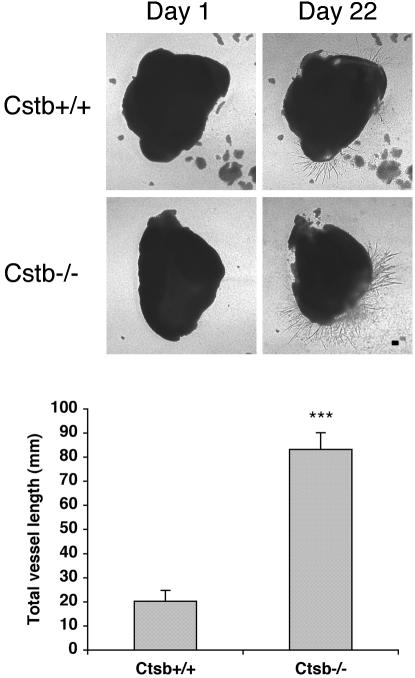
To begin to address the relevance of our findings in the collagen sandwich gel model system, we tested the contribution of cathepsin B on the outgrowth of retinal vessels in an ex vivo setting. Nullizygous cathepsin B mice are viable and fertile (Halangk et al., 2000). We isolated the retina from both Ctsb–/– and Ctsb+/+ mice and embedded 1-mm pieces within our standard collagen sandwich gel. Outgrowth of vessels was first detected on day 12 and continued for at least 1 mo (unpublished data). At all of the times during this period the response was stronger from the Ctsb–/–retinas (Figure 8). One way to quantitate the difference was to measure the total length of vessels, and we consistently found that they were more vessels in the retinas isolated from the null animals (Figure 8). We also compared vessel growth from aortic rings and found the same trend; the response was substantially stronger from the aortas isolated from the in Ctsb–/–compared with the Ctsb+/+ controls. We conclude that vessel outgrowth in this ex vivo setting is suppressed by cathepsin, which is consistent with our observations in the tube formation assay.
Figure 8.
The outgrowth of retinal vessels in an ex vivo setting is enhanced by the absence of the cathepsin B gene. Retinal explants were isolated from Ctsb+/+ and Ctsb–/–mice and plated in a collagen sandwich gel and supplemented with the medium containing either buffer or 25 ng/ml mouse VEGF. On the days indicated in the figure, the sprouting vessels were photographed and the total length of the vessels was measured. The bar graph shows the means of three replicates from two independent experiments, and the error bars indicate the SD of triplicates samples. Ctsb–/–mice (n = 3); Ctsb+/+ mice (n = 4). *** p < 0.001. Bar, 200 μm.
DISCUSSION
There are two major findings in this report: First, the observation that cathepsin B has the potential to suppress angiogenesis. This was a surprising discovery because previous studies suggested that cathepsin B promoted angiogenesis. Second, that endothelial cells have the intrinsic capacity to synthesize both pro- and antiangiogenic factors. These findings advance our understanding of the cell biology of endothelial cells and suggest they are capable of playing an active role in pathophysiology.
How Can Cathepsin B Both Promote and Inhibit Angiogenesis?
Cathepsins were first appreciated for their contribution to lysosomal protein degradation, but recent studies suggest a series of additional roles for these enzymes. For instance, cathepsin B contributes to tumor progression by virtue of its ability to degrade proteins in the extracellular matrix and basement membrane (Buck et al., 1992). In primary adenocarcinomas, overexpression of cathepsin B protein correlates with enhanced angiogenesis (Kruszewski et al., 2004). Similarly, cathepsin B is elevated at the leading edge of invading tumors of the breast (Keppler et al., 1996). Blocking cathepsin B expression in a glioblastoma cell line reduces a number of angiogenic indicators (Yanamandra et al., 2004). Similarly, blocking cathepsin activity affects multiple stages of tumor formation, including a reduction in vascularity (Joyce et al., 2004). These studies suggest that the proangiogenic activity of cathepsin B relates to either its direct action on the tumor cells or, alternatively, to the remodeling of extracellular matrix proteins.
Many of these studies have used the well characterized, commercially available cathepsin B inhibitors. For instance, CBI, also known as benzyloxycarbonyl-Phe-Ala-fluoromethylketone (z-FA-fmk), functions by irreversibly alkylating the active site cysteine of cathepsin B (Rasnick, 1985). It has been suggested to be a potential treatment for inflammatory joint diseases such as rheumatoid arthritis (Ahmed et al., 1992; Esser et al., 1993). Furthermore, CBI is useful for lymphocytes self-protection and cytokine production of macrophage by inhibiting cathepsin B (Schotte et al., 2001; Balaji et al., 2002). The effects of CBI and CA-074Me in our studies could be duplicated by a range of other cathepsin B inhibitors (unpublished data).
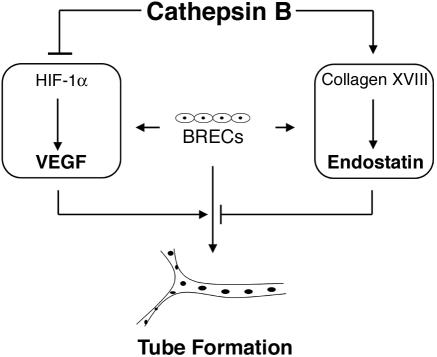
By focusing on the role of cathepsin B in endothelial cells (instead of in tumors), we have come to the conclusion that cathepsin B also has the capacity to suppress an angiogenic response. Although most of our findings were limited to the in vitro setting, it is noteworthy that cathepsin B plays a similar role in an ex vivo model, thus suggesting that our findings likely reflect at least certain facets of the in vivo situation. Taken together, our findings support a model (Figure 9) wherein cathepsin B maintains the endothelium in a nonangiogenic state by increasing endostatin generation while decreasing VEGF expression. Thus cathepsin B may have an important role in normal physiology (as a suppressor of angiogenesis acting at the level of the endothelium), in addition its putative contribution to pathology (where it acts on tumor cells and/or the extracellular matrix to promote angiogenesis).
Figure 9.
Model for how cathepsin B suppresses the intrinsic angiogenic switch. When cultured between two layers of collagen, BRECs are capable of producing both proangiogenic factors (VEGF) and antiangiogenic factors (endostatin). Cathepsin B functions to suppress production of VEGF and boost levels of endostatin. Under these conditions, tube formation is low, but can be induced by exogenously added VEGF. Blocking cathepsin B activity releases this endogenous control mechanism and VEGF levels increase while endostatin levels fall. The net result is spontaneous tube formation.
Possible Mechanisms by which Cathepsin B Governs the Level of HIF-1α and Endostatin
The level of HIF-1α is regulated by oxygen tension via the prolyl hydroxylase/von Hippel-Lindau tumor suppressor protein (VHL)/proteasome pathway (Jaakkola et al., 2001). Under normoxic conditions, prolyl hydroxylase activity is high and it modifies HIF-1α, i.e., it hydroxylates several proline residues. This increases the association of HIF-1α with VHL, which enhances its ubiquitination and thereby targets HIF-1α for proteasomal degradation. In contrast, a drop in the oxygen level reduces the activity of prolyl hydroxylase and thereby stabilizes HIF-1α. Thus the level of HIF-1α is controlled by proteasomal proteases.
Because HIF-1α levels are regulated by proteases, it is possible that cathepsin B is a second class of proteases that govern the level of HIF-1α. Cathepsin B is generally assumed to be both an endopeptidase and an exopeptidase (Aronson and Barrett, 1978; Takahashi et al., 1986), and it prefers substrates that include either X-X-R/F-G/R-L-X at the very end of the C-terminus or an internal R-R (Barrett and Kirschke, 1981). Such motifs are present in HIF-1α, and so HIF-1α may be a direct substrate of cathepsin B. Alternatively, cathepsin B may control the level of HIF-1α indirectly, by degrading proteins such as Hsp90, which are required for stabilizing HIF-1α (Isaacs et al., 2002). Finally, degradation of HIF-1α by the proteasome may involve a cathepsin B–dependent step because cathepsin B inhibitors were comparably effective at stabilizing HIF-1α, as was the proteasomal inhibitor (Figure 7B)
As with HIF-1α, we postulate that the protease activity of cathepsin B is responsible for governing the level of endostatin. Endostatin is produced proteolytically from collagen XVIII (O'Reilly et al., 1997), and cathepsin B is one of the proteases that can generate endostatin from collagen XVIII in an in vitro setting (Felbor et al., 2000; Ferreras et al., 2000). These observations suggest that cathepsin B may be creating endostatin or endostatin-like proteins in vivo. This possibility is further supported by that fact that the consensus cathepsin B cleavage sites are present in collagen XVIII.
It is also possible that cathepsin B indirectly facilitates the degradation of collagen XVIII and HIF-1α by activating proteases that act directly on these proteins. A quintessential feature of members of the cathepsin family is that they interact with and activate urokinase and MMPs, which have also capable of proteolyzing many proteins, including collagen XVIII (Kobayashi et al., 1991; Ferreras et al., 2000; Mai et al., 2002).
The question of subcellular location is an important variable when considering the likelihood of substrate/enzyme interaction. The generation of endostatin from collagen XVIII is likely to occur on the outside of the cell because collagen XVIII is secreted and endostatin is expected to function extracellularly. Even though cathepsin B is a lysosomal enzyme, it is generally assumed that cathepsin B acts outside the invading cell to degrade extracellular matrix components adjacent to the cell surface (Linebaugh et al., 1999; Mai et al., 2002). With respect to HIF-1α, which is a nuclear protein, cathepsin B is found in the cytoplasm (Schotte et al., 1998; Guicciardi et al., 2000) and may degrade HIF-1α before its import into the nucleus. Alternatively, the findings that cathepsin B is nuclear (Riccio et al., 2001) raises the possibility that cathepsin B can enter the nucleus to degrade HIF-1α. In summary, although cathepsin B is a lysosomal enzyme, it has access to the subcellular compartments in which the interaction with collagen XVIII and HIF-1α is most likely to occur.
Mice that are null for cathepsin B develop normally and have no apparent phenotype (Halangk et al., 2000). This suggests that within the context of embryogenesis and normal physiological function, either there are mechanisms to compensate for the loss of cathepsin B or the cathepsin B–dependent routes governing the level of HIF-1α and collagen XVIII are dispensable. Mice that lack collagen XVIII are also grossly normal; however, they do experience a delay in the regression of hyaloid vessels and abnormal outgrowth of retinal vessel (Fukai et al., 2002).
Endothelial Cells Harbor an Angiogenic Switch
The “angiogenic switch” is an angiogenesis-based explanation for the transition of a noninvasive, nonvascular tumor to the more dangerous invasive and vascularized stage (Hanahan and Folkman, 1996). The tumor itself undergoes the “switch,” which must be manifest by the host endothelium. More specifically, the tumor alters the balance of pro- and antiangiogenic factors to favor angiogenesis. For instance, the tumor increases secretion of VEGF and/or FGF, or decreases the production of antiangiogenic factors such as Tsp-1 (Hanahan and Folkman, 1996).
Although the tumor is the focus of most angiogenic switch discussions, the endothelium must respond to the change in the balance of angiomodulators by executing the angiogenic program. Because the endothelium has the capacity to contribute to the overall balance of pro- and antiangiogenic substances, it is possible that the endothelium is contributing to the angiogenic switch. This may be a substantial contribution because both an increase in proangiogenic factors and a decrease in antiangiogenic factors occurs in the endothelial cells when cathepsin B is blocked. At least in some model systems, both changes are needed for the formation and/or persistence of vascularized tumors (Watnick et al., 2003). We conclude that the endothelium not only integrates and responds to changes in angiomodulators, but also has the capacity to make an impact on the balance.
This previously unappreciated feature of the endothelium addresses the intriguing observation that angiogenic responses are localized despite a global elevation of angiomodulators. Upregulation of VEGFR within the angiogenic site has previously been reported and is suggested as a possible explanation (Plate et al., 1992; Ortega et al., 1997). The findings presented herein contribute additional insight. The local concentration of angiomodulators in the area undergoing angiogenesis may be different from the global levels because of the contributions of the endothelium. Our identification of cathepsin B as a regulator of the concentration of angiomodulators at the level of the endothelium provides an opportunity and guidance for future efforts to understand how angiogenesis is regulated.
Supplementary Material
Acknowledgments
We deeply appreciate for the generous contribution of reagents (Dr. Terence S. Dermody [Vanderbilt University, Nashville, TN] for the cathepsin B cDNA and Dr. Hidde Ploegh [Harvard Medical School, Boston, MA] for the Ctsb–/–mice), animal husbandry (Dr. Dimitra Skondra [Harvard Medical School]), and help isolating tissue from the control and knock-out mice (Drs. Hetian Lei, Yong Seek Park, and Dimitra Skondra [Harvard Medical School]). We also thank Ms. Patricia Pearson (Schepens Eye Research Institute) for help with electron microscopy. Finally, we thank the following individuals for their critical input to this manuscript and/or project: Drs. Lena Claesson-Welsh, Patricia D'Amore, Mara Lorenzi, Ali Hafezi-Moghadam, Yong Seek Park, and Sang Hoon Rhee.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–11–1029) on May 18, 2005.
Abbreviations used: BBE, bovine brain extract; BRECs, bovine retinal endothelial cells; CBI, cathepsin B inhibitor; EV, the empty vector expressing cells: HS, horse serum; HUVECs, human umbilical vein endothelial cells; R2, VEGFR2.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Ahmed, N. K., Martin, L. A., Watts, L. M., Palmer, J., Thornburg, L., Prior, J., and Esser, R. E. (1992). Peptidyl fluoromethyl ketones as inhibitors of cathepsin B. Implication for treatment of rheumatoid arthritis. Biochem. Pharmacol. 44, 1201–1207. [DOI] [PubMed] [Google Scholar]
- Aiello, L. P. et al. (1994). Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 331, 1519–1520. [DOI] [PubMed] [Google Scholar]
- Aronson, N. N., Jr., and Barrett, A. J. (1978). The specificity of cathepsin B. Hydrolysis of glucagon at the C-terminus by a peptidyldipeptidase mechanism. Biochem. J. 171, 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji, K. N., Schaschke, N., Machleidt, W., Catalfamo, M., and Henkart, P. A. (2002). Surface cathepsin B protects cytotoxic lymphocytes from self-destruction after degranulation. J. Exp. Med. 196, 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, A. J., and Kirschke, H. (1981). Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 80(Pt C), 535–561. [DOI] [PubMed] [Google Scholar]
- Bergers, G. et al. (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2, 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, M. R., Karustis, D. G., Day, N. A., Honn, K. V., and Sloane, B. F. (1992). Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem. J. 282, 273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, J., Ahmad, S., Jiang, W. G., Huang, J., Kontos, C. D., Boulton, M., and Ahmed, A. (2003). Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes 52, 2959–2968. [DOI] [PubMed] [Google Scholar]
- Carmeliet, P., and Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature 407, 249–257. [DOI] [PubMed] [Google Scholar]
- Dawson, D. W., Pearce, S. F., Zhong, R., Silverstein, R. L., Frazier, W. A., and Bouck, N. P. (1997). CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J. Cell Biol. 138, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert, D. H., Deussing, J., Peters, C., and Dermody, T. S. (2002). Cathepsin L and cathepsin B mediate reovirus disassembly in murine fibroblast cells. J. Biol. Chem. 277, 24609–24617. [DOI] [PubMed] [Google Scholar]
- Esser, R. E., Watts, L. M., Angelo, R. A., Thornburg, L. P., Prior, J. J., and Palmer, J. T. (1993). The effects of fluoromethyl ketone inhibitors of cathepsin B on adjuvant induced arthritis. J. Rheumatol. 20, 1176–1183. [PubMed] [Google Scholar]
- Felbor, U., Dreier, L., Bryant, R. A., Ploegh, H. L., Olsen, B. R., and Mothes, W. (2000). Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 19, 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara, N., Carver-Moore, K., Chen, H., Dowd, M., Lu, L., O'Shea, K. S., Powell-Braxton, L., Hillan, K. J., and Moore, M. W. (1996). Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442. [DOI] [PubMed] [Google Scholar]
- Ferrara, N., and Davis-Smyth, T. (1997). The biology of vascular endothelial growth factor. Endocr. Rev. 18, 4–25. [DOI] [PubMed] [Google Scholar]
- Ferrara, N., Gerber, H. P., and LeCouter, J. (2003). The biology of VEGF and its receptors. Nat. Med. 9, 669–676. [DOI] [PubMed] [Google Scholar]
- Ferreras, M., Felbor, U., Lenhard, T., Olsen, B. R., and Delaisse, J. (2000). Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 486, 247–251. [DOI] [PubMed] [Google Scholar]
- Folkman, J. (1995). Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1, 27–31. [DOI] [PubMed] [Google Scholar]
- Forsythe, J. A., Jiang, B. H., Iyer, N. V., Agani, F., Leung, S. W., Koos, R. D., and Semenza, G. L. (1996). Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 16, 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai, N. et al. (2002). Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 21, 1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin, J. D., and D'Amore, P. A. (1983). Culture of retinal capillary cells using selective growth media. Microvasc. Res. 26, 74–80. [DOI] [PubMed] [Google Scholar]
- Good, D. J., Polverini, P. J., Rastinejad, F., Le Beau, M. M., Lemons, R. S., Frazier, W. A., and Bouck, N. P. (1990). A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc. Natl. Acad. Sci. USA 87, 6624–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi, M. E., Deussing, J., Miyoshi, H., Bronk, S. F., Svingen, P. A., Peters, C., Kaufmann, S. H., and Gores, G. (2000). Cathepsin B contributes to TNFalpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106, 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halangk, W., Lerch, M. M., Brandt-Nedelev, B., Roth, W., Ruthenbuerger, M., Reinheckel, T., Domschke, W., Lippert, H., Peters, C., and Deussing, J. (2000). Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Invest. 106, 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D., and Folkman, J. (1996). Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364. [DOI] [PubMed] [Google Scholar]
- Isaacs, J. S., Jung, Y. J., Mimnaugh, E. G., Martinez, A., Cuttitta, F., and Neckers, L. M. (2002). Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J. Biol. Chem. 277, 29936–29944. [DOI] [PubMed] [Google Scholar]
- Jaakkola, P. et al. (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472. [DOI] [PubMed] [Google Scholar]
- Joyce, J. A., Baruch, A., Chehade, K., Meyer-Morse, N., Giraudo, E., Tsai, F. Y., Greenbaum, D. C., Hager, J. H., Bogyo, M., and Hanahan, D. (2004). Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 5, 443–453. [DOI] [PubMed] [Google Scholar]
- Keppler, D., Sameni, M., Moin, K., Mikkelsen, T., Diglio, C.A., and Sloane, B. F. (1996). Tumor progression and angiogenesis: cathepsin B & Co. Biochem. Cell Biol. 74, 799–810. [DOI] [PubMed] [Google Scholar]
- Knott, R. M., Robertson, M., Muckersie, E., Folefac, V. A., Fairhurst, F. E., Wileman, S. M., and Forrester, J. V. (1999). A model system for the study of human retinal angiogenesis: activation of monocytes and endothelial cells and the association with the expression of the monocarboxylate transporter type 1 (MCT-1). Diabetologia 42, 870–877. [DOI] [PubMed] [Google Scholar]
- Kobayashi, H., Schmitt, M., Goretzki, L., Chucholowski, N., Calvete, J., Kramer, M., Gunzler, W. A., Janicke, F., and Graeff, H. (1991). Cathepsin B efficiently activates the soluble and the tumor cell receptor-bound form of the proenzyme urokinase-type plasminogen activator (Pro-uPA). J. Biol. Chem. 266, 5147–5152. [PubMed] [Google Scholar]
- Kruszewski, W. J., Rzepko, R., Wojtacki, J., Skokowski, J., Kopacz, A., Jaskiewicz, K., and Drucis, K. (2004). Overexpression of cathepsin B correlates with angiogenesis in colon adenocarcinoma. Neoplasma 51, 38–43. [PubMed] [Google Scholar]
- Kuo, C. J. et al. (2001). Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/endostatin domain. J. Cell Biol. 152, 1233–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linebaugh, B. E., Sameni, M., Day, N. A., Sloane, B. F., and Keppler, D. (1999). Exocytosis of active cathepsin B enzyme activity at pH 7.0, inhibition and molecular mass. Eur. J. Biochem. 264, 100–109. [DOI] [PubMed] [Google Scholar]
- Mackay, E. A. et al. (1997). A possible role for cathepsins D, E, and B in the processing of beta-amyloid precursor protein in Alzheimer's disease. Eur. J. Biochem. 244, 414–425. [DOI] [PubMed] [Google Scholar]
- Mai, J., Sameni, M., Mikkelsen, T., and Sloane, B. F. (2002). Degradation of extracellular matrix protein tenascin-C by cathepsin B: an interaction involved in the progression of gliomas. Biol. Chem. 383, 1407–1413. [DOI] [PubMed] [Google Scholar]
- Maxwell, P. H. (2004). HIF-1's relationship to oxygen: simple yet sophisticated. Cell Cycle 3, 156–159. [PubMed] [Google Scholar]
- O'Reilly, M. S., Boehm, T., Shing, Y., Fukai, N., Vasios, G., Lane, W. S., Flynn, E., Birkhead, J. R., Olsen, B. R., and Folkman, J. (1997). Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 88, 277–285. [DOI] [PubMed] [Google Scholar]
- O'Reilly, M. S., Holmgren, L., Shing, Y., Chen, C., Rosenthal, R. A., Moses, M., Lane, W. S., Cao, Y., Sage, E. H., and Folkman, J. (1994). Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 79, 315–328. [DOI] [PubMed] [Google Scholar]
- Ortega, N., Jonca, F., Vincent, S., Favard, C., Ruchoux, M. M., and Plouet, J. (1997). Systemic activation of the vascular endothelial growth factor receptor KDR/flk-1 selectively triggers endothelial cells with an angiogenic phenotype. Am. J. Pathol. 151, 1215–1224. [PMC free article] [PubMed] [Google Scholar]
- Ory, D. S., Neugeboren, B. A., and Mulligan, R. C. (1996). A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 93, 11400–11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottino, P., Finley, J., Rojo, E., Ottlecz, A., Lambrou, G. N., Bazan, H. E., and Bazan, N. G. (2004). Hypoxia activates matrix metalloproteinase expression and the VEGF system in monkey choroid-retinal endothelial cells: Involvement of cytosolic phospholipase A2 activity. Mol. Vis. 10, 341–350. [PubMed] [Google Scholar]
- Plate, K. H., Breier, G., Weich, H. A., and Risau, W. (1992). Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359, 845–848. [DOI] [PubMed] [Google Scholar]
- Qian, F., Bajkowski, A. S., Steiner, D. F., Chan, S. J., and Frankfater, A. (1989). Expression of five cathepsins in murine melanomas of varying metastatic potential and normal tissues. Cancer Res. 49, 4870–4875. [PubMed] [Google Scholar]
- Rasnick, D. (1985). Synthesis of peptide fluoromethyl ketones and the inhibition of human cathepsin B. Anal Biochem. 149, 461–465. [DOI] [PubMed] [Google Scholar]
- Riccio, M., Di Giaimo, R., Pianetti, S., Palmieri, P. P., Melli, M., and Santi, S. (2001). Nuclear localization of cystatin B, the cathepsin inhibitor implicated in myoclonus epilepsy (EPM1). Exp. Cell Res. 262, 84–94. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque, J. C., Lane, T. F., Ortega, M. A., Hynes, R. O., Lawler, J., and Iruela-Arispe, M. L. (2001). Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc. Natl. Acad. Sci. USA 98, 12485–12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte, P., Schauvliege, R., Janssens, S., and Beyaert, R. (2001). The cathepsin B inhibitor z-FA.fmk inhibits cytokine production in macrophages stimulated by lipopolysaccharide. J. Biol. Chem. 276, 21153–21157. [DOI] [PubMed] [Google Scholar]
- Schotte, P. et al. (1998). Cathepsin B-mediated activation of the proinflammatory caspase-11. Biochem. Biophys. Res. Commun. 251, 379–387. [DOI] [PubMed] [Google Scholar]
- Semenza, G. L. (2000). HIF-1 and human disease: one highly involved factor. Genes Dev. 14, 1983–1991. [PubMed] [Google Scholar]
- Sui, G., Soohoo, C., Affar el, B., Gay, F., Shi, Y., Forrester, W. C., and Shi, Y. (2002). A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 5515–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, T., Dehdarani, A. H., Yonezawa, S., and Tang, J. (1986). Porcine spleen cathepsin B is an exopeptidase. J. Biol. Chem. 261, 9375–9381. [PubMed] [Google Scholar]
- Turk, B., Turk, D., and Turk, V. (2000). Lysosomal cysteine proteases: more than scavengers. Biochim. Biophys. Acta 1477, 98–111. [DOI] [PubMed] [Google Scholar]
- Valius, M., Bazenet, C., and Kazlauskas, A. (1993). Tyrosines 1021 and 1009 are phosphorylation sites in the carboxy terminus of the platelet-derived growth factor receptor beta subunit and are required for binding of phospholipase C gamma and a 64-kilodalton protein, respectively. Mol. Cell Biol. 13, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick, R. S., Cheng, Y. N., Rangarajan, A., Ince, T. A., and Weinberg, R. A. (2003). Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell 3, 219–231. [DOI] [PubMed] [Google Scholar]
- Wen, W., Moses, M.A., Wiederschain, D., Arbiser, J. L., and Folkman, J. (1999). The generation of endostatin is mediated by elastase. Cancer Res. 59, 6052–6056. [PubMed] [Google Scholar]
- Wolters, P. J., and Chapman, H. A. (2000). Importance of lysosomal cysteine proteases in lung disease. Respir. Res. 1, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra, N., Gumidyala, K. V., Waldron, K. G., Gujrati, M., Olivero, W. C., Dinh, D. H., Rao, J. S., and Mohanam, S. (2004). Blockade of cathepsin B expression in human glioblastoma cells is associated with suppression of angiogenesis. Oncogene 23, 2224–2230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.