Abstract
Hybridization-induced conformational changes have been successfully used in biosensors for the transduction of DNA-binding events into readily observable optical or electronic signals. Similar signal transduction has not, however, proven of equal utility in proteinbased biosensors. The discrepancy arises because, unlike ssDNA, most proteins do not undergo significant conformational changes upon ligand binding. Here, we describe a solution to this problem. We show that an arbitrarily selected, normally well folded protein can be rationally engineered such that it undergoes ligand-induced folding. The engineered protein responds rapidly (milliseconds) and selectively to its target, and it couples recognition with the largest possible conformational change: folding. These traits suggest that ligand-induced folding could serve as an ideal signal-transduction mechanism. Consistent with this claim, we demonstrate a label-free optical biosensor based on the effect that is sufficiently selective to detect its target even in complex, contaminant-ridden samples such as blood serum.
Keywords: fluorescence, molecular beacon, natively unfolded, quenching
Biosensors promise significant advantages over nonbiological sensing technologies (1). For example, because of the affinity and specificity of biomolecular recognition, biosensors often exhibit impressive sensitivity and selectivity relative to nonbiological approaches (2, 3). Furthermore, because high-affinity proteins can be produced in the laboratory that specifically bind almost any water-soluble ligand (4–6), a generic, protein-based biosensor platform should be highly generalizable. Despite these advantages, however, the application of proteins to commercially viable, real-time electronic or optical biosensors has remained limited, with the glucose oxidase-based glucose sensor being a notable exception (2).
A large number of electronic and optical protein-based sensors have been described in the literature, many of which feature impressive sensitivity and generalizability (2, 3). Because the physical properties of proteins generally do not change significantly upon ligand binding, however, most of these approaches require the addition of multiple reagents or use changes in relatively nonspecific properties, such as mass, charge, or electron density, to generate an observable signal. This limitation renders many of these approaches either time- and reagent-intensive, or sensitive to false positives arising from the nonspecific adsorption of contaminants (3). For example, although immunochemical techniques, such as “protein chips,” are sensitive and highly specific, they are also relatively slow batch processes often requiring cumbersome instrumentation, expensive labile reagents, or previous labeling of the target molecule (7, 8). Other, impressively rapid, sensitive, label-free biosensor technologies, such as surface plasmon resonance (9), field-effect transistor (10), quartz crystal microbalance (3), and microcantilevers (11, 12), rely on binding-induced changes in refractive index, charge, or mass at the sensor surface to produce detectable signals. But because contaminants also have mass, polarizable electron density, and charge, nonspecific adsorption often produces significant background signals when these techniques are used with realistically complex samples such as blood serum (9, 11, 12).
Mechanisms that couple target binding to a large conformational change could circumvent the false positives that have often plagued label-free biosensors. They would do so by linking signaling to a binding-specific physical change in the sensing protein rather than to changes in some less specific property such as increases in adsorbed mass or charge. There are two general approaches to engineering such a linkage (13). The first is to identify those rare proteins that exhibit a large, binding-induced conformational change and then to modify their binding specificity such that the protein recognizes the desired target. Hellinga and coworkers (e.g., ref. 14) have compellingly demonstrated this approach by building a family of sensors based on a naturally occurring, ligand-induced change in maltose-binding protein. Here, in contrast, we adopt the alternative approach. Namely, we demonstrate a means of rationally modifying a small protein of arbitrary binding specificity such that it undergoes ligand-induced folding (see Fig. 1), and we describe a convenient optical means of detecting the effect even in complex, contaminant-ridden samples.
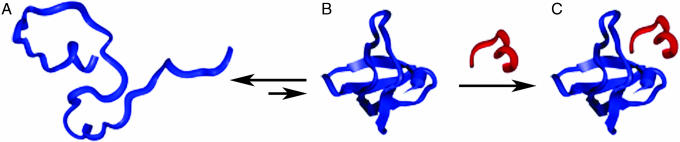
Fig. 1.
A proposed mechanism for ligand-induced folding. A rapid equilibrium exists between the unfolded (A) and folded (B) forms of CΔ4, favoring the unfolded state. CΔ4 molecules transiently sampling the folded state are competent to bind p85α-2 and when bound are “trapped” by the favorable free energy gained through formation of the complex (C).
Methods
Mutagenesis and Purification. The coding sequence of the WT SH3 domain of human Fyn tyrosine kinase (FynSH3) was cloned into a pET-15b (Novagen) expression vector, introducing an aminoterminal His6 tag. QuikChange PCR mutagenesis (Stratagene) was used to generate four sequential amino acid truncations from the carboxyl terminus (CΔ1–CΔ4) by introducing successive stop codons; the identity of each truncation mutant was confirmed by DNA sequencing (data not shown). Mutant plasmids were transformed into BL21(DE3)pLysS (Stratagene) Escherichia coli, expressed, and purified by Ni-NTA agarose (Qiagen) affinity chromatography. To minimize loss of CΔ4 mutants to aggregation, the protein was purified in the presence of 2 M guanidine hydrochloride (Gdn·HCl). Free energies of unfolding were determined for each protein to identify the least-truncated mutant, CΔ4, that led to complete unfolding (data not shown). Purified CΔ4 was concentrated to ≈100 μM in an ultrafiltration cell (Amicon). Immediately before use, the protein was rapidly exchanged into 50 mM potassium phosphate, pH 7 (herein referred to as “phosphate buffer”) by Sephadex G25 (Sigma) spin-column gel filtration. The concentration of a stock solution of CΔ4 was determined by absorbance at 280 nm (molar extinction coefficient = 16,500 M-1·cm-1).
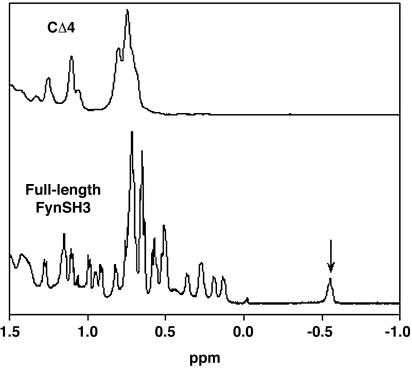
NMR Characterization. The 1D 1H NMR spectra were collected for native, full-length FynSH3 and CΔ4 (see Fig. 2) in phosphate buffer/10% D2O at 25°C on a 500-MHz Bruker AVANCE500 spectrometer (Billerica, MA). The spectra consisted of 128 scans of 1,024 points. The concentrations of FynSH3 and CΔ4 were 500 and 100 μM, respectively.
Fig. 2.
CΔ4 exhibits a conspicuous loss of NMR peak dispersion relative to full-length FynSH3, suggesting the absence of the well defined local chemical environments characteristic of the folded state. Notably, a highly shifted methyl resonance (indicated) is reduced by more than an order of magnitude by the mutation, suggesting that the folding equilibrium lies significantly toward the unfolded state.
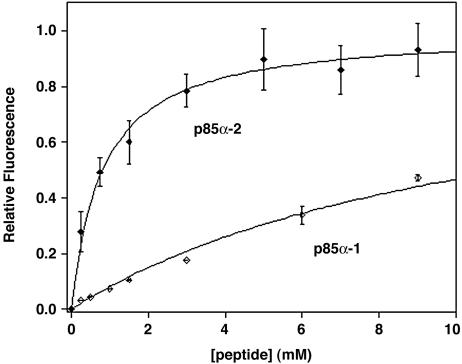
Ligand-Induced Folding. To evaluate the affinity and selectivity of CΔ4, the high-affinity 11-residue peptide p85α-2 and the low-affinity 13-residue peptide p85α-1 were selected as target ligands (15). Both peptides were obtained commercially (United Biochemical Research, Seattle) and used without further purification. The concentrations of stock peptide solutions in phosphate buffer were quantified by amino acid analysis. Fluorescence emission spectra were collected on samples of 1 μM CΔ4 exchanged into phosphate buffer containing concentrations of p85α-2 or p85α-1 ranging from 0 to 12 mM and 0 to 30 mM, respectively (Fig. 3), by using an LS 50 B luminescence spectrometer (Perkin–Elmer) maintained at 25°C. Samples were excited at 280 nm. Dissociation constants were determined by plotting peak fluorescence intensity against ligand concentration and fitting the data by using kaleidagraph (Albeck Software, Reading, PA). Reported values and error estimates represent the mean and SD of multiple measurements.
Fig. 3.
CΔ4 folds selectively in response to its target ligand. The fluorescence of CΔ4, which is a signature of SH3 domain folding (15), increases with increasing peptide concentrations in a ratio that mirrors the dissociation constants of the full-length protein.
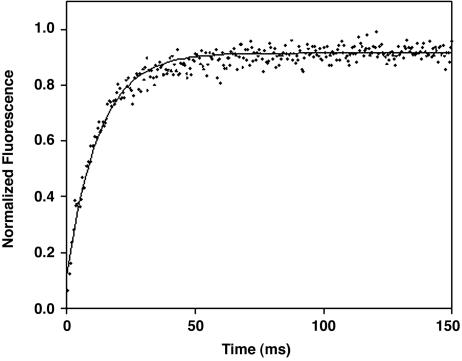
Response Time. The rate with which CΔ4 responds to the high-affinity target was determined by using an SX.18MV stopped-flow fluorimeter (Applied Photophysics, Surrey, U.K.). Kinetic data were obtained by rapidly diluting 110 μMCΔ4 in phosphate buffer, 11-fold, with 1.1 mM p85α-2 also in phosphate buffer. After mixing, p85α-2 binding stabilizes the folded state of CΔ4, giving rise to a rapid, single-exponential increase in tryptophan fluorescence (Fig. 4). Measurements were taken at 25°C, with sample excitation at 280 nm and detection above 320 nm. Data were fitted by using a nonlinear least-squares fitting algorithm in kaleidagraph. Reported values and error estimates represent the mean and SD of four independent measurements.
Fig. 4.
CΔ4 responds rapidly to its target ligand. When CΔ4 is mixed with target, we observe a rapid single-exponential increase in fluorescence with a time constant within error of that reported for the folding of the full-length protein (18).
Readout Mechanism. A serine-to-cysteine substitution (CΔ4/S115C: numbering as per the Protein Data Bank ID 1AVZ) was performed at a position 4 and 5 aa removed, respectively, from the two tryptophan residues of FynSH3 by using QuikChange PCR mutagenesis (Stratagene). The identity of the mutant was confirmed by DNA sequencing. The CΔ4/S115C plasmid was then transformed, expressed, and purified as described above. Immediately before conjugation, 100 μM CΔ4/S115C was rapidly exchanged into phosphate buffer containing 2 M Gdn·HCl by Sephadex G25 (Sigma) spin-column gel filtration. CΔ4/S115C was then reduced for 30 min in the presence of 1 mM tris(2-carboxyethyl)phosphine hydrochloride. BODIPY FL N-(2-aminoethyl)maleimide (BODIPY; Molecular Probes) dissolved in acetonitrile was added dropwise with gentle mixing to a final concentration of 1 mM. The reaction was then placed in the dark and allowed to proceed for 2 h. The pH of the reaction was adjusted to 8 and the mixture was loaded onto a Ni-NTA-agarose (Qiagen) affinity column preequilibrated in phosphate buffer containing 2 M Gdn·HCl. The column was then washed extensively with this same buffer to remove unreacted dye. Bound protein was eluted with equilibration buffer containing 100 mM imidazole. Protein-conjugate-containing fractions were identified by absorbance and then dialyzed extensively against a large volume excess of phosphate buffer containing 2 M Gdn·HCl. The dialyzed pool was stored in the dark at room temperature. An aliquot of the CΔ4/S115C-BODIPY conjugate was diluted 5-fold with phosphate buffer. To remove the remaining Gdn·HCl, the diluted sample was buffer exchanged into phosphate buffer by Sephadex G25 (Sigma) spin-column gel filtration. The concentration of the sensor molecule was determined by subtracting the estimated contribution of BODIPY from the total absorbance at 280 nm, based on its relative peak absorbance at 505 nm (16). Based on the apparent relative concentrations of BODIPY and CΔ4, the conjugation reaction appears to have proceeded to completion (data not shown). We note, however, that any unconjugated CΔ4 would be spectroscopically silent and thus would not affect sensor readout beyond a minimal reduction in the pool of free ligand. To evaluate tryptophan-BODIPY segregation as a readout mechanism, fluorescence emission spectra were collected on 1 μM samples of the sensor molecule in phosphate buffer or in FCS (Sigma) (50% vol/vol in phosphate buffer) containing the appropriate concentration of p85α-2 (Fig. 6). Data were collected on an LS 50 B luminescence spectrometer (Perkin–Elmer) maintained at 25°C. Samples were excited at 480 nm.
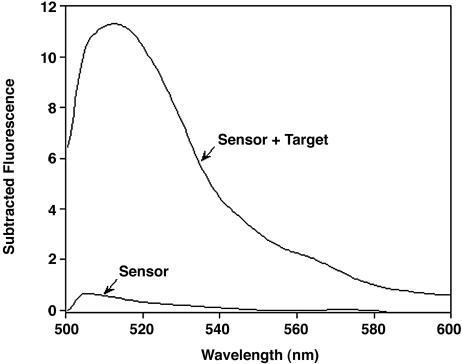
Fig. 6.
A highly selective optical biosensor based on ligand-induced folding. The sensor is sufficiently selective that we can detect its target peptide even in blood serum, a realistically complex and contaminant-ridden clinical material. Shown are background-subtracted fluorescence spectra of 1 μM sensor in 50% FCS and in serum doped with 8 mM target peptide.
Results
Ligand-induced folding occurs when a favorable binding free energy overcomes an unfavorable folding free energy, producing a folded complex (Fig. 1). Thus, if a protein is mutated such that its folding free energy is unfavorable, but by an amount less than its binding free energy, it should exhibit this property. Moreover, because the folding of small, single-domain proteins is extremely cooperative (17), any mutation that destabilizes the native state sufficiently should produce a fully unfolded molecule. We have tested these claims using FynSH3. This 57-residue, predominantly β-sheet protein is normally well folded (Fig. 2) and binds a short proline-rich peptide with micromolar affinity (15).
Literature precedent (18–21) suggested to us that the removal of several residues from either terminus of the FynSH3 domain should destabilize the protein sufficiently to lead to complete unfolding. We thus attempted to unfold the protein by sequentially removing each of the last few residues from its carboxyl terminus. In doing so we find that FynSH3 unfolds only after four carboxyl-terminal residues have been removed (data not shown). The four-residue carboxyl-terminal deletion (residues 138–141 in the Protein Data Bank file 1AVZ), which we term CΔ4, is soluble at >100 μM in simple aqueous buffers (data not shown). As revealed by the large-scale loss of NMR chemical shift dispersion, CΔ4 also appears to be unfolded (Fig. 2). We should note that we assume that there is nothing special about the carboxyl terminus and that amino-terminal deletions or any of a wide range of other mutations would also lead to unfolding; we chose carboxyl-terminal deletions because of the ease of systematically removing additional residues (by the introduction of stop codons) until the desired level of destabilization is achieved.
The engineered protein folds in response to its specific ligand. The folding of SH3 domains is readily monitored by changes in tryptophan fluorescence, which increases upon solvent exclusion (15). Consistent with the hypothesized ligand-induced folding, the fluorescence of CΔ4 increases significantly upon the addition of the high-affinity target peptide, p85α-2 (Fig. 3). Not surprisingly, given that the mutation site is relatively far from the binding pocket, the binding specificity of CΔ4 mirrors that of the full-length protein (Fig. 3). For example, CΔ4 binds p85α-2 with a Kd of 0.8 ± 0.1 mM and the lower-affinity peptide p85α-1 with a Kd of 7.9 ± 1.2 mM, a ratio of selectivity within error of that reported for the full-length protein (15). The Kd values of both peptides, however, are reduced ≈100-fold relative to that of the full-length protein (15). This diminished affinity is consistent with the NMR spectrum of CΔ4, in which a highly shifted methyl resonance present in the native protein is reduced by more than an order of magnitude; binding must now overcome the unfavorable free energy of folding that this represents and thus affinity is reduced.
The engineered protein responds rapidly to its target ligand; when CΔ4 is mixed with p85α-2, we observe a single-exponential increase in tryptophan fluorescence with a 10.0 ± 0.4 ms time constant (Fig. 4). This value is within error of the time constant reported for the folding of the full-length protein (22). This, in turn, further supports the proposed mechanism for ligand-induced folding, wherein folded and unfolded CΔ4 are in rapid equilibrium, with the unfolded state dominating (Fig. 1). CΔ4 molecules transiently sampling the folded state bind the p85α-2 peptide and are “trapped” by the favorable free energy gained through complex formation (see, by analogy, ref. 23).
By coupling binding to such a significant conformational change, ligand-induced folding could provide a robust signal-transduction mechanism for sensor applications. However, because of the cumbersome, costly techniques traditionally used to monitor protein folding (24) (and the difficulty of monitoring folding in the presence of other, contaminating proteins), such sensors must rely on exogenous, nonbiological reporter groups to signal the folding event. Using CΔ4, we have constructed just such an optical biosensor. We have done so by introducing a cysteine four residues from the protein's two adjacent tryptophan residues, to which we conjugated the fluorescent dye BODIPY. Because ligand-free CΔ4 is unfolded, the flexibility of the polypeptide chain allows the tryptophans to contact the BODIPY, quenching its fluorescence (16). Because the tryptophans are relatively inaccessible in the folded complex, folding inhibits contact and thus significantly increases BODIPY fluorescence (Fig. 5).
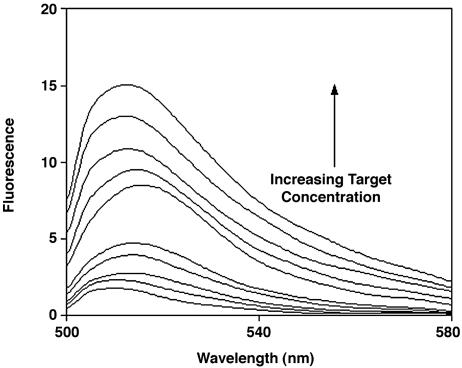
Fig. 5.
A signal transduction mechanism for optical biosensors. Here the target ligand of FynSH3 is detected by folding-linked changes in the emission of a CΔ4-BODIPY conjugate. In the absence of target, the flexibility of the unfolded CΔ4 allows the two tryptophan residues of the protein to quench the fluorescence of the covalently attached BODIPY. Upon ligand binding the tryptophan residues are sequestered, resulting in a large increase in emission. Ligand concentrations are (from bottom up) 0, 0.2, 0.5, 0.8, 1, 2, 5, 8, 10, and 12 mM.
Because their signaling mechanism is based on a specific, binding-induced conformational change, folding-based sensors should be relatively insensitive to the presence of contaminants. To test this prediction, we have shown that we can detect the FynSH3 target ligand even in blood serum (a realistically complex and contaminant-ridden clinical material), albeit with reduced signal gain relative to the sensor response in buffer. The reduced gain, however, occurs because of the significant background fluorescence of serum at the BODIPY excitation wavelength. This background is readily subtracted, recovering the order of magnitude signal gain observed in buffer (Fig. 6).
Discussion
We have demonstrated a means by which ligand-induced folding can be rationally engineered into a normally well folded protein. The engineered protein responds rapidly and selectively to its target ligand and couples recognition with the largest possible conformational change: folding. We have also shown that this ligand-induced folding can serve as a robust signal-transduction mechanism, producing an order of magnitude signal gain without the need to label the target and by using technically facile, visible-light excitation. Furthermore, because their signaling is not based on changes in nonspecific properties such mass or charge, folding-based sensors are sufficiently selective for use in complex, contaminant-ridden samples. Last, although unfolded proteins are sometimes susceptible to aggregation [especially β-sheet proteins (25) such as FynSH3], the sensitivity of fluorescence detection permits the practical use of protein in a concentration regime in which aggregation remains minimal; e.g., although CΔ4 is soluble at >100 μM, our sensor application uses submicromolar concentrations. The successful engineering of proteins with enhanced solubility suggests, moreover, that even aggregation-prone proteins can be adapted for our approach (26). A remaining drawback of rationally engineered ligand-induced folding is that, in coupling recognition to an unfavorable folding free energy, binding affinity is reduced by a like amount. Nevertheless, given that biomolecules often exhibit nanomolar affinities, and many clinically relevant targets are present in micromolar or even millimolar concentrations, it appears that there are many applications for which folding-based sensors will be well suited (2).
Our ability to rationally engineer ligand-induced folding may be fairly general. For example, other single-domain proteins have been reported that populate soluble, highly unfolded states that are competent to refold (18–21). Among these are a terminal deletion mutant of staphylococcal nuclease (Δ131Δ) and the drkSH3 domain, both of which have been shown to undergo ligand-induced folding (18, 27). The nonintuitive observation that even short truncations produce near-complete unfolding reflects the cooperativity with which naturally occurring single-domain proteins fold. In contrast, the folding of larger proteins tends to be multistate (17), which would likely preclude the sharp on/off transition required for our approach. Despite these limitations, however, the availability of well established immunochemical and in vitro selection techniques (4–6, 28) for the production of novel, single-domain proteins of arbitrary specificity suggests that our approach to protein-based optical biosensors may be fairly general.
The BODIPY/tryptophan readout mechanism we have demonstrated is well suited in terms of signal gain and the manageably low background fluorescence produced by visible light excitation. [Of note, other tryptophan-quenched fluorophores have been reported that excite at longer wavelengths (16), further reducing the background fluorescence of serum and other complex samples.] The currently described readout is limited, however, to proteins that can sequester tryptophan upon folding. Nevertheless, the availability of other optical reporters for the monitoring of protein folding, such as excimer formation (29) and resonance energy transfer (20, 30), suggests that ligand-induced folding provides a general signal-transduction mechanism. Alternatively, it may be possible to monitor ligand-induced folding electronically by folding-induced changes in through-protein electron transfer (31). Analogous methods for the detection of binding-linked conformational changes in DNA are now well established (32).
Acknowledgments
We thank Miguel de los Rios and Blake Gillespie for providing the original clone and assistance with the 1D NMR, respectively. This work was supported by National Institutes of Health Grant EB002046 (to K.W.P.) and funds from the Cottage Hospital Research Fund, the UCBioSTAR program, and CMS, Inc.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FynSH3, SH3 domain of human Fyn tyrosine kinase; CΔ4, four-residue, carboxyl-terminal deletion mutant of FynSH3; BODIPY, BODIPY FL N-(2-aminoethyl)maleimide; Gdn·HCl, guanidine hydrochloride.
References
- 1.Fan, C., Plaxco, K. W. & Heeger, A. J. (2005) Trends Biotechnol. 23, 186-192. [DOI] [PubMed] [Google Scholar]
- 2.Castillo, J., Gaspar, S., Leth, S., Niculescu, M., Mortari, A., Bontidean, I., Soukharev, V., Dorneanu, S. A., Ryabov, A. D. & Csoregi, E. (2004) Sens. Actuators B Chem 102, 179-194. [Google Scholar]
- 3.Cooper, M. A. (2003) Anal. Bioanal. Chem. 377, 834-842. [DOI] [PubMed] [Google Scholar]
- 4.Vaughan, T. J., Osbourn, J. K. & Tempest, P. R. (1998) Nat. Biotechnol. 16, 535-539. [DOI] [PubMed] [Google Scholar]
- 5.Wilson, D. R. & Finlay, B. B. (1998) Can. J. Microbiol. 44, 313. [PubMed] [Google Scholar]
- 6.Mathonet, P. & Fastrez, J. (2004) Curr. Opin. Struct. Biol. 14, 505-511. [DOI] [PubMed] [Google Scholar]
- 7.Murray, P. R., Rosenthal, K. W., Kobayashi, G. & Pfaller, M. A. (2002) Medical Microbiology (Mosby, New York), 4th Ed.
- 8.Zhu, H. & Snyder, M. (2003) Curr. Opin. Chem. Biol. 7, 55-63. [DOI] [PubMed] [Google Scholar]
- 9.Homola, J. (2003) Anal. Bioanal. Chem. 377, 528-539. [DOI] [PubMed] [Google Scholar]
- 10.Katz, E. & Willner, I. (2003) Electroanalysis 15, 913-947. [Google Scholar]
- 11.Viani, M. B., Pietrasanta, L. I., Thompson, J. B., Chand, A., Gebeshuber, I. C., Kindt, J. H., Richter, M., Hansma, H. G. & Hansma, P. K. (2000) Nat. Struct. Biol. 7, 644-647. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler, C. (2004) Anal. Bioanal. Chem. 379, 946-959. [DOI] [PubMed] [Google Scholar]
- 13.Hellinga, H. W. & Marvin, J. S. (1998) Trends Biotechnol. 16, 183-189. [DOI] [PubMed] [Google Scholar]
- 14.Marvin, J. S. & Hellinga, H. W. (2001) Proc. Natl. Acad. Sci. USA 98, 4955-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viguera, A. R., Arrondo, J. L. R., Musacchio, A., Saraste, M. & Serrano, L. (1994) Biochemistry 33, 10925-10933. [DOI] [PubMed] [Google Scholar]
- 16.Marme, N., Knemeyer, J. P., Sauer, M. & Wolfrum, J. (2003) Bioconjug. Chem. 14, 1133-1139. [DOI] [PubMed] [Google Scholar]
- 17.Chan, H. S., Shimizu, S. & Kaya, H. (2004) Methods Enzymol. 380, 350-379. [DOI] [PubMed] [Google Scholar]
- 18.Alexandrescu, A. T., Abeygunawardana, C. & Shortle, D. (1994) Biochemistry 33, 1063-1072. [DOI] [PubMed] [Google Scholar]
- 19.de Prat Gay, G., Ruiz-Sanz, J., Neira, J. L., Corrales, F. J., Otzen, D. E., Ladurner, A. G. & Fersht, A. R. (1995) J. Mol. Biol. 254, 968-979. [DOI] [PubMed] [Google Scholar]
- 20.Neira, J. L. & Fersht, A. R. (1999) J. Mol. Biol. 285, 1309-1333. [DOI] [PubMed] [Google Scholar]
- 21.Neira, J. L. & Fersht, A. R. (1999) J. Mol. Biol. 287, 421-432. [DOI] [PubMed] [Google Scholar]
- 22.Plaxco, K. W., Guijarro, J. I., Morton, C. J., Pitkeathly, M., Campbell, I. D. & Dobson, C. M. (1998) Biochemistry 37, 2529-2537. [DOI] [PubMed] [Google Scholar]
- 23.Wildes, D. & Marquesee, S. (2005) Protein Sci. 14, 81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plaxco, K. W. & Dobson, C. M. (1996) Curr. Opin. Struct. Biol. 6, 630-636. [DOI] [PubMed] [Google Scholar]
- 25.Fink, A. L. (1998) Folding Des. 3, 9-23. [Google Scholar]
- 26.Cabantous, S., Terwilliger, T. C. & Waldo, G. S. (2004) Nat. Biotech. 23, 102-107. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, O. & Forman-Kay, J. D. (1997) Biochemistry 36, 3959-3970. [DOI] [PubMed] [Google Scholar]
- 28.Bessette, P. H., Rice, J. J. & Daugherty, P. S. (2004) Protein Eng. Des. Sel. 17, 731-739. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer, S. S. (1997) Methods Enzymol. 278, 286-295. [DOI] [PubMed] [Google Scholar]
- 30.Schuler, B., Lipman, E. A. & Eaton, W. A. (2002) Nature 419, 742-747. [DOI] [PubMed] [Google Scholar]
- 31.Doerr, A. J. & McLendon, G. L. (2004) Inorg. Chem. 43, 7916-7925. [DOI] [PubMed] [Google Scholar]
- 32.Fan, C. H., Plaxco, K. W. & Heeger, A. J. (2003) Proc. Natl. Acad. Sci. USA 100, 9134-9137. [DOI] [PMC free article] [PubMed] [Google Scholar]