Abstract
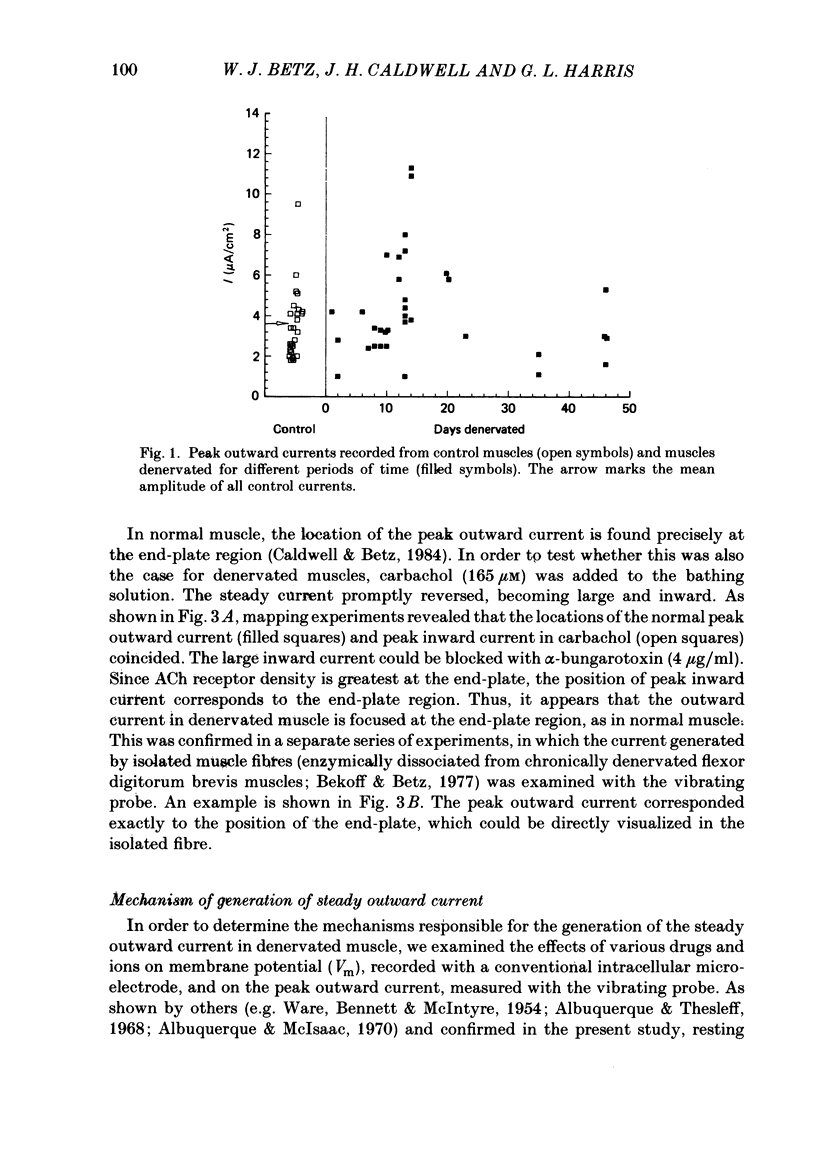
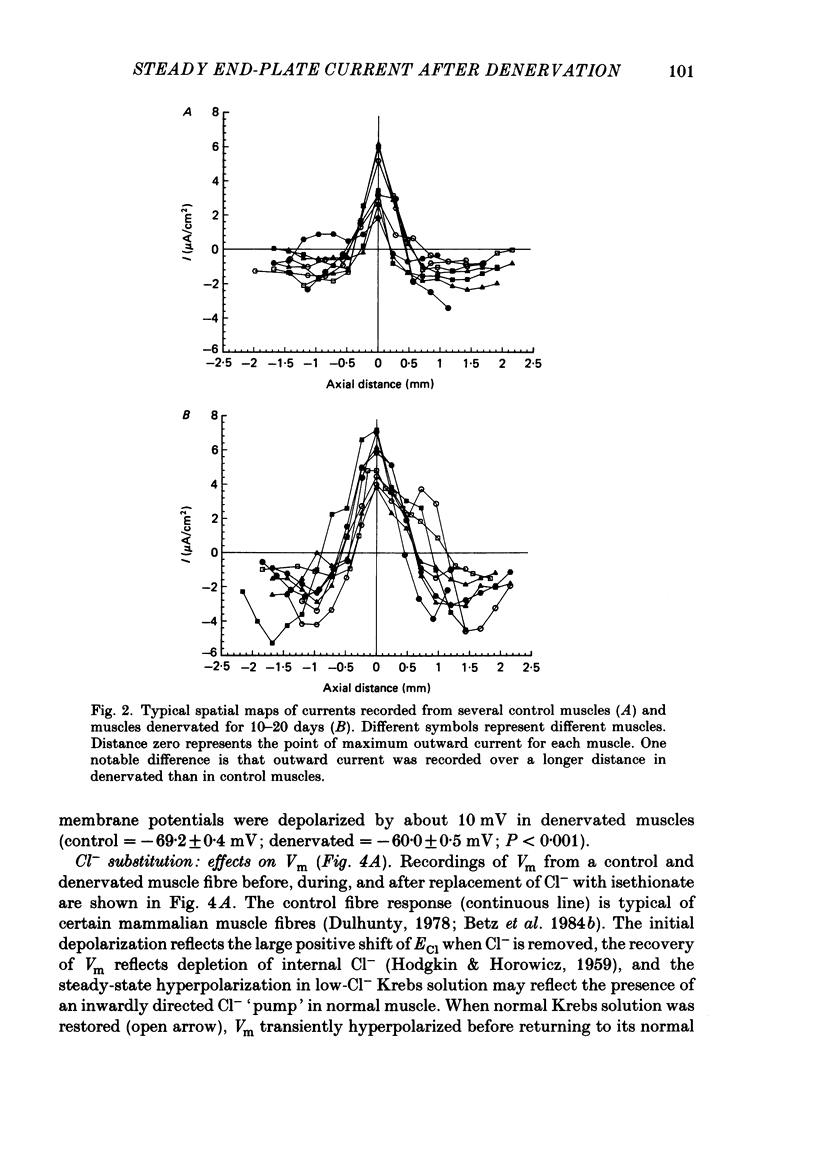
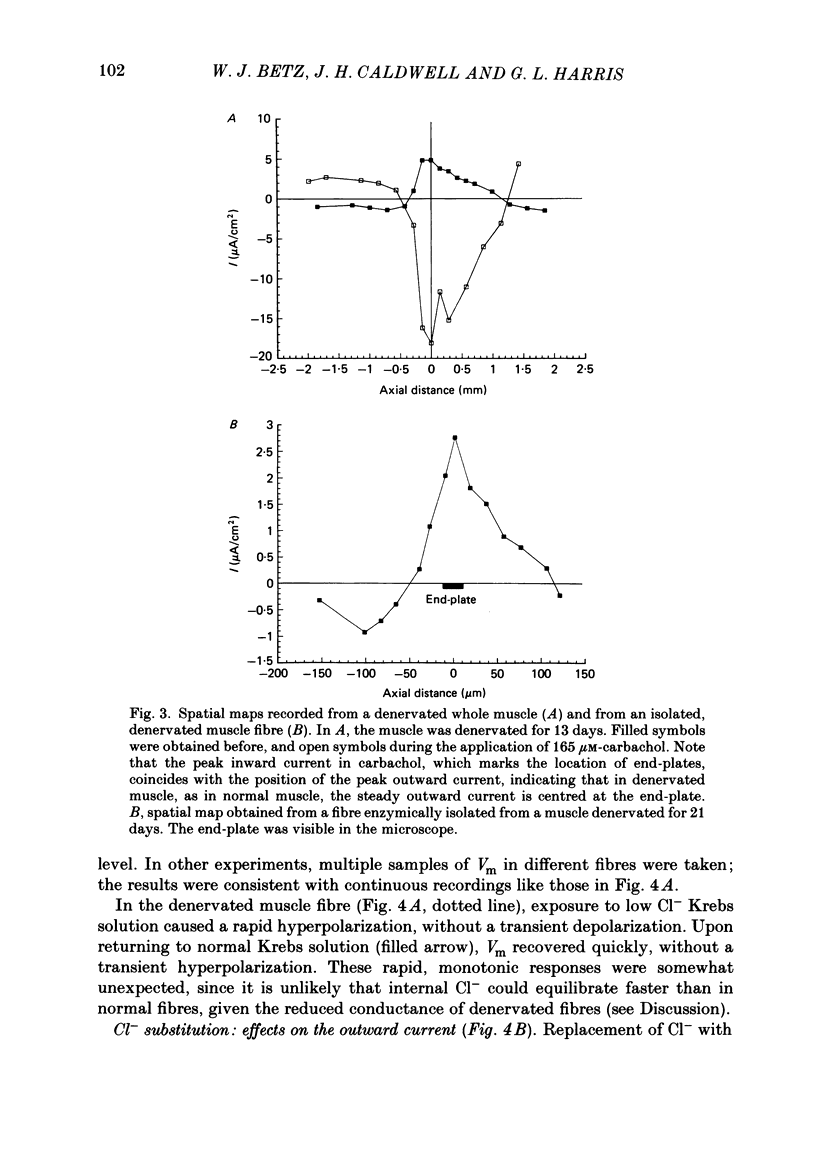
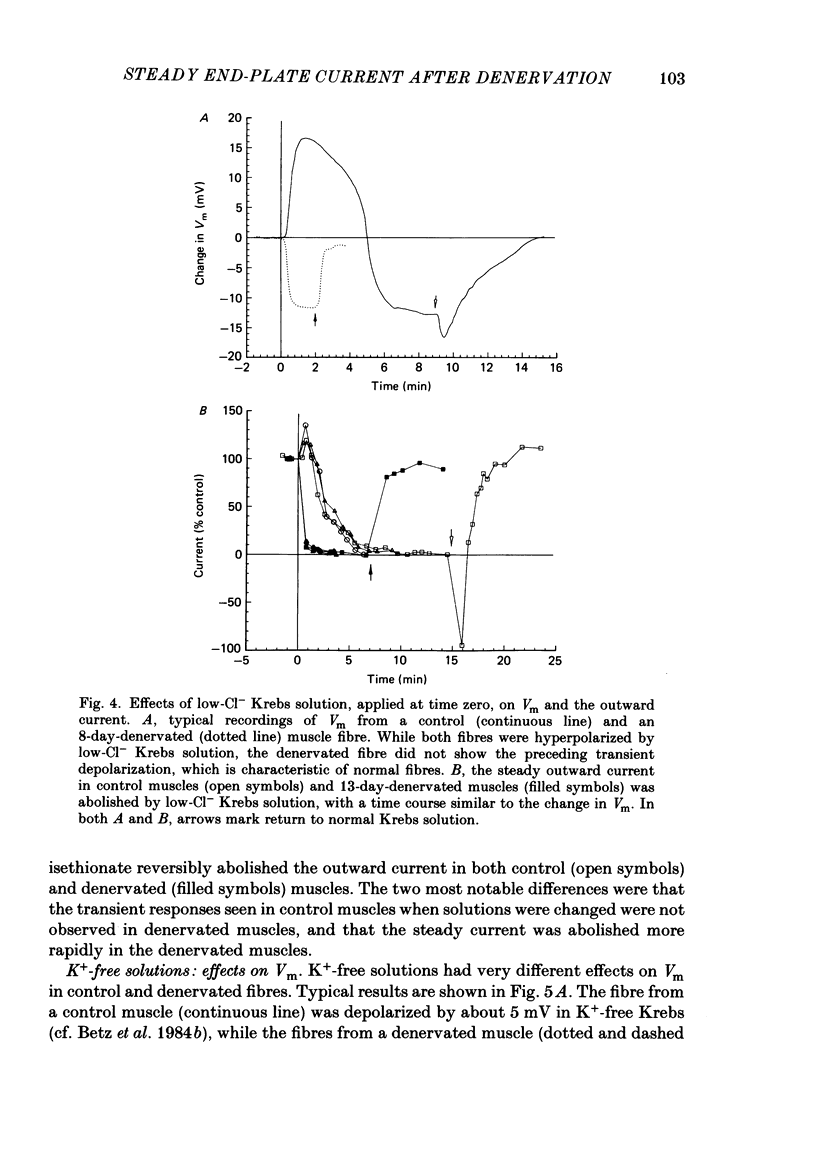
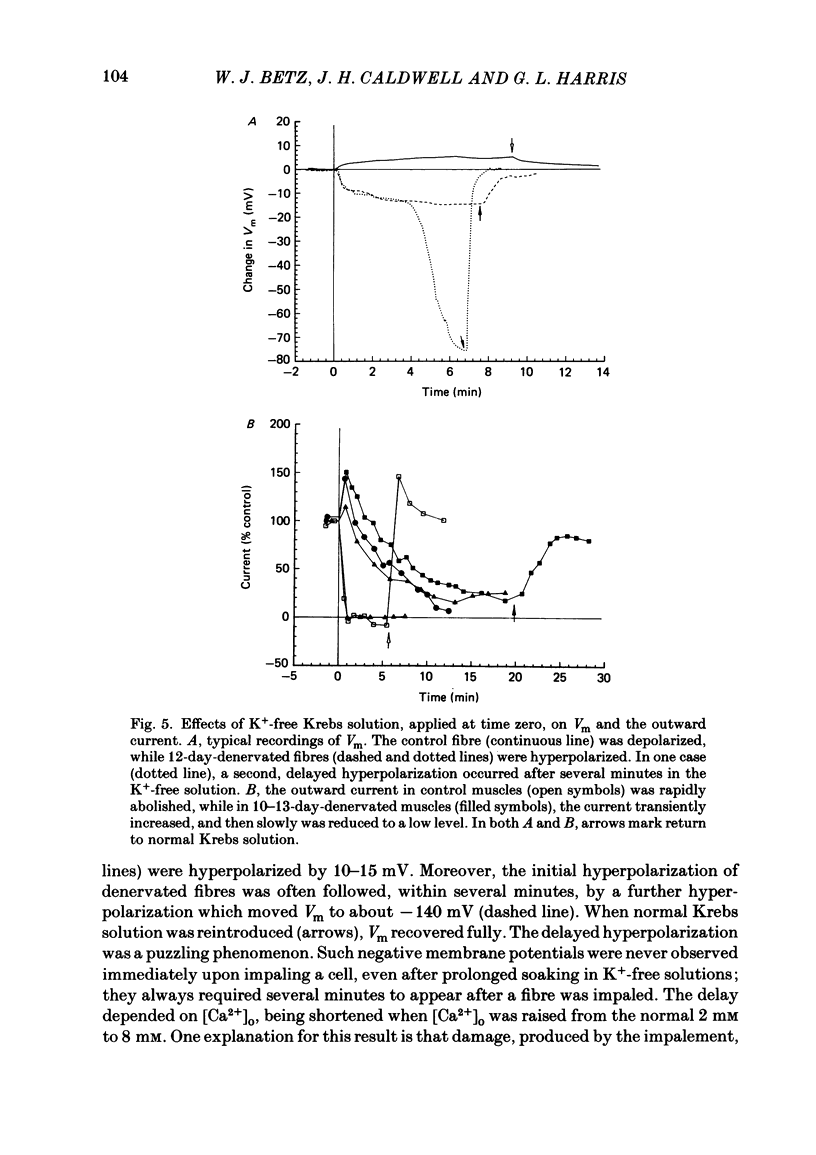
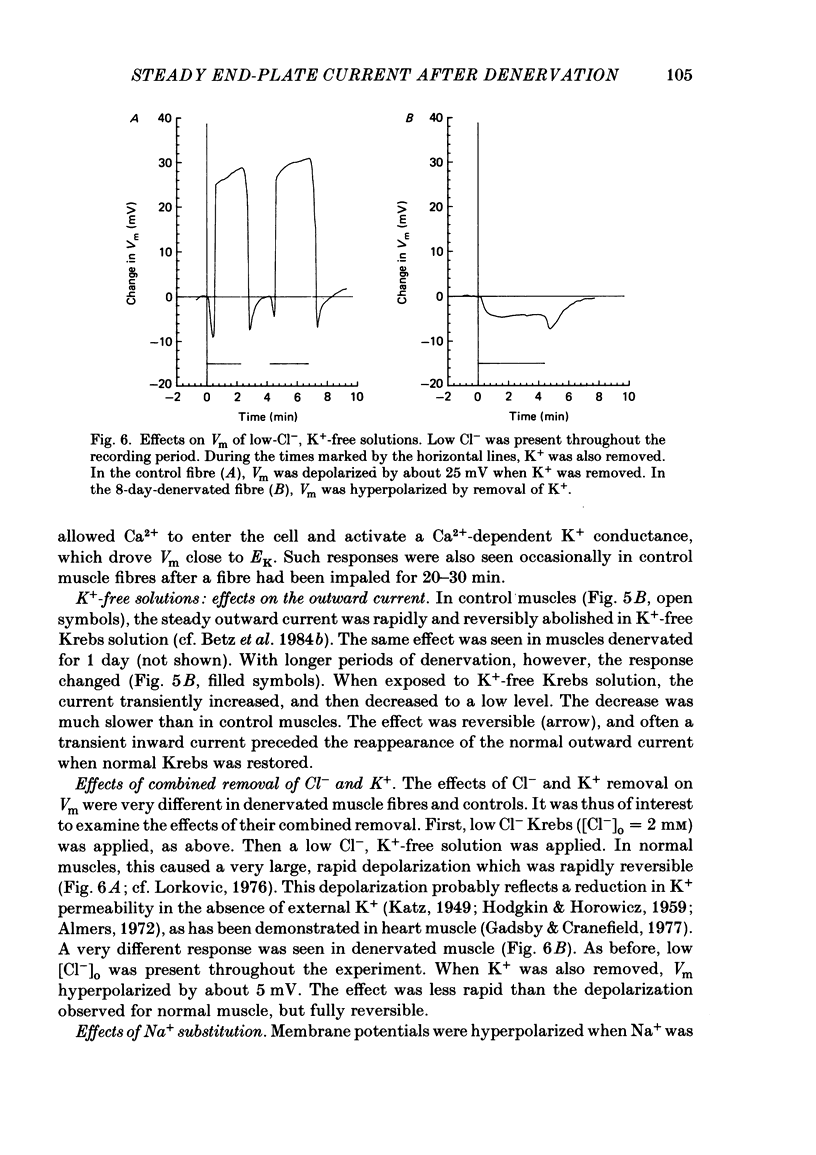
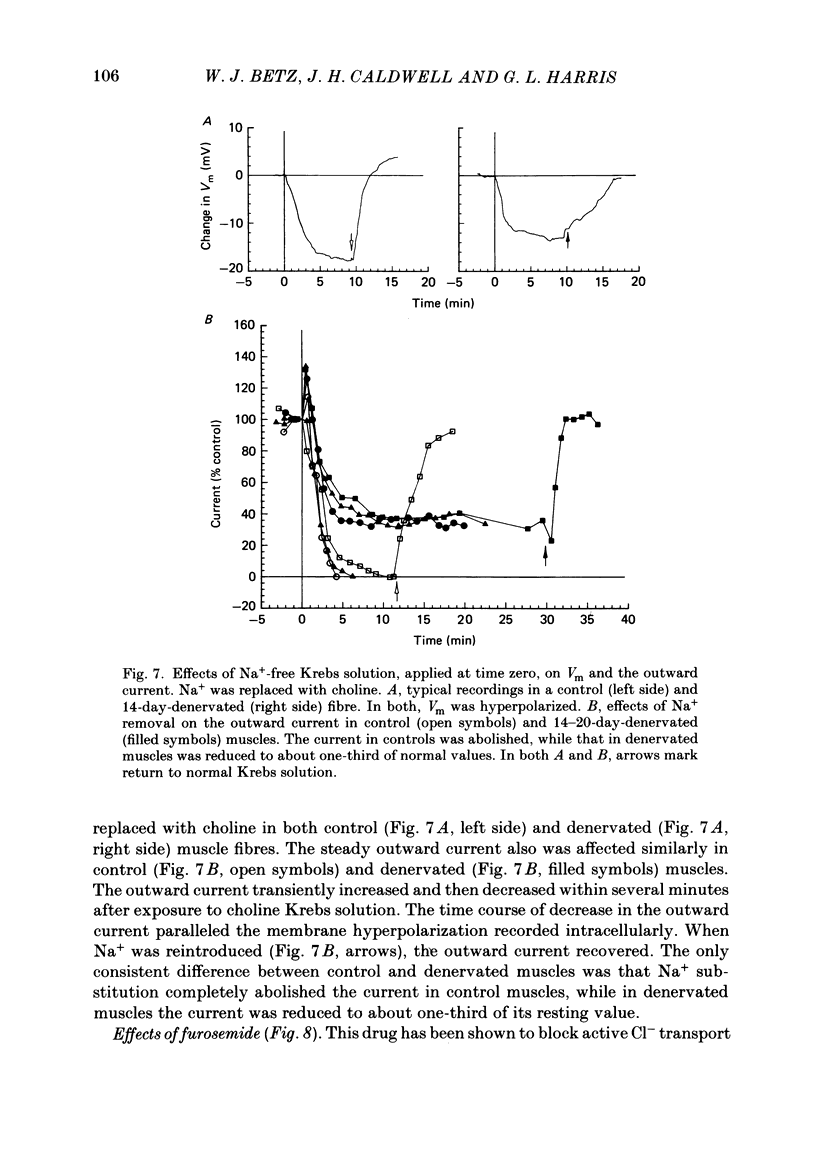
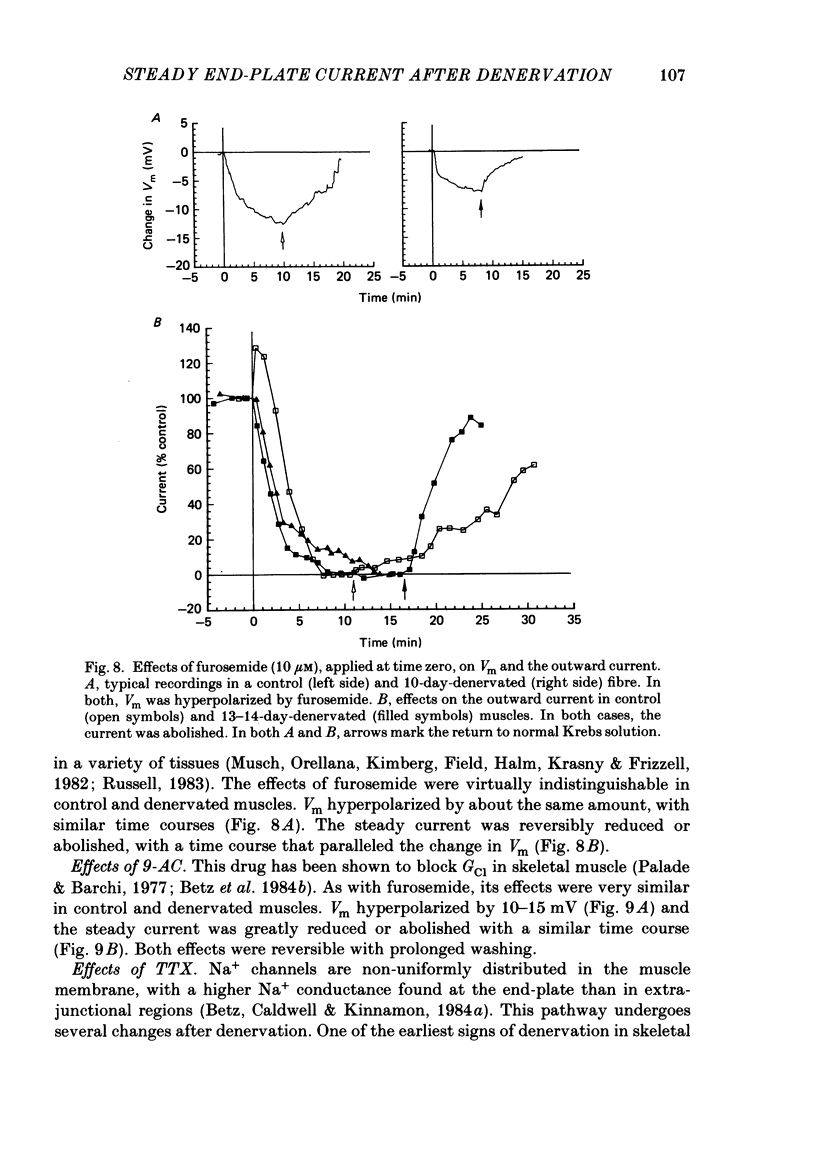
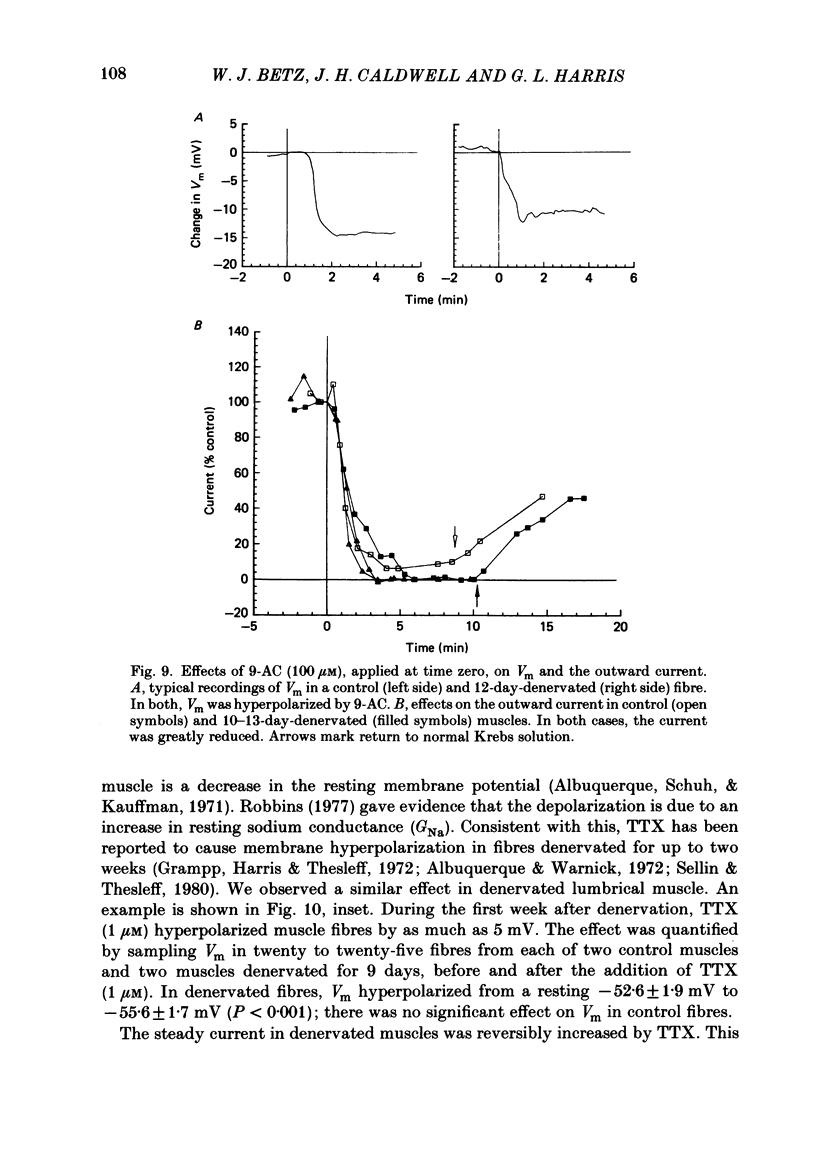
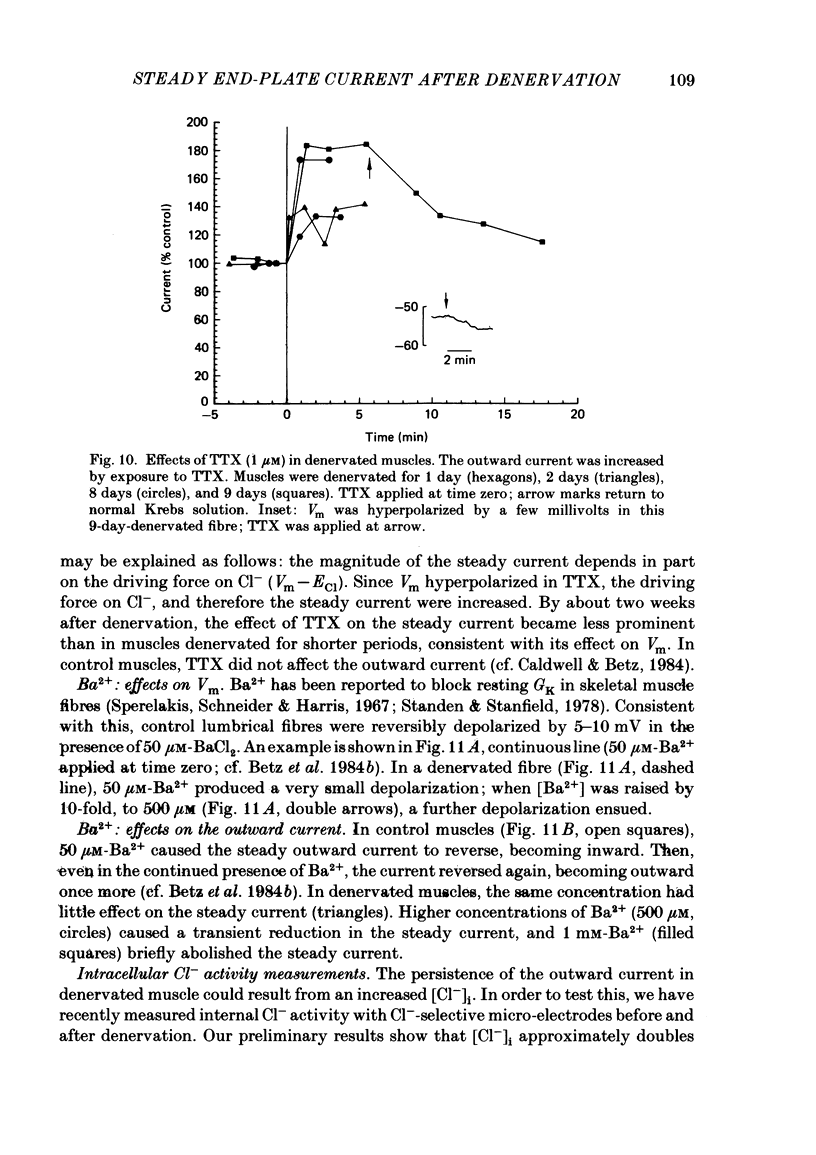
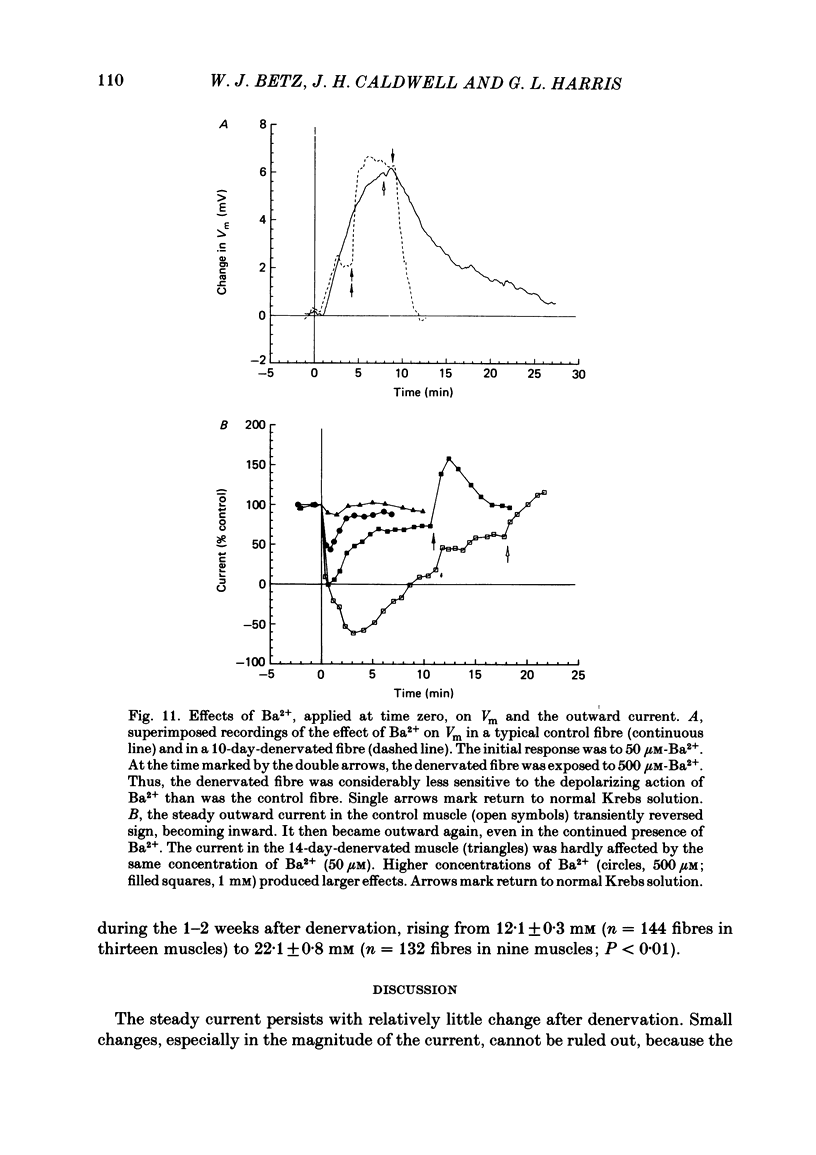
An electric current flows continuously out of the synaptic region of rat lumbrical muscle fibres. It is generated apparently as a result of a non-uniform Cl- conductance (GCl), with GCl being lowest at the end-plate. We investigated the effects of denervation on this current. The current persisted with little change after denervation. This was somewhat unexpected, since GCl falls dramatically after denervation, and in acute experiments on normal muscles, the steady current is greatly reduced by agents which block GCl. The steady current was blocked in denervated muscle, as in normal muscle, by low-Cl- solutions, Na+-free and K+-free solutions, and treatment with furosemide and 9-anthracene-carboxylic acid. The current in denervated muscle appears to be generated by the same general mechanism as in normal muscle. The results suggest that the [Cl-]i is significantly higher in denervated than in normal muscle fibres. Preliminary experiments with Cl- -selective micro-electrodes have confirmed this: [Cl-]i rises from about 12 mM to about 23 mM after denervation. This has the effect of moving the Cl- equilibrium potential (ECl) in a positive direction, so that the driving force for passive Cl- efflux is increased. The increased driving force compensates for the reduced GCl, allowing the steady current to persist in denervated fibres.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Thesleff S. A comparative study of membrane properties of innervated and chronically denervated fast and slow skeletal muscles of the rat. Acta Physiol Scand. 1968 Aug;73(4):471–480. doi: 10.1111/j.1365-201x.1968.tb10886.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E. The pharmacology of batrachotoxin. IV. Interaction with tetrodotoxin on innervated and chronically denervated rat skeletal muscle. J Pharmacol Exp Ther. 1972 Mar;180(3):683–697. [PubMed] [Google Scholar]
- Almers W. Potassium conductance changes in skeletal muscle and the potassium concentration in the transverse tubules. J Physiol. 1972 Aug;225(1):33–56. doi: 10.1113/jphysiol.1972.sp009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A., Betz W. J. Physiological properties of dissociated muscle fibres obtained from innervated and denervated adult rat muscle. J Physiol. 1977 Sep;271(1):25–40. doi: 10.1113/jphysiol.1977.sp011988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Kinnamon S. C. Increased sodium conductance in the synaptic region of rat skeletal muscle fibres. J Physiol. 1984 Jul;352:189–202. doi: 10.1113/jphysiol.1984.sp015286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Kinnamon S. C. Physiological basis of a steady endogenous current in rat lumbrical muscle. J Gen Physiol. 1984 Feb;83(2):175–192. doi: 10.1085/jgp.83.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H. Mapping electric currents around skeletal muscle with a vibrating probe. J Gen Physiol. 1984 Feb;83(2):143–156. doi: 10.1085/jgp.83.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Vaughan-Jones R. D. Continuous direct measurement of intracellular chloride and pH in frog skeletal muscle. J Physiol. 1977 Sep;270(3):801–833. doi: 10.1113/jphysiol.1977.sp011983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Betz W. J. Properties of an endogenous steady current in rat muscle. J Gen Physiol. 1984 Feb;83(2):157–173. doi: 10.1085/jgp.83.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino D., Bryant S. H. Effects of denervation and colchicine treatment on the chloride conductance of rat skeletal muscle fibers. J Neurobiol. 1976 May;7(3):221–228. doi: 10.1002/neu.480070305. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. J Physiol. 1978 Mar;276:67–82. doi: 10.1113/jphysiol.1978.sp012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C. A., Nuccitelli R. Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J Cell Biol. 1984 Jan;98(1):296–307. doi: 10.1083/jcb.98.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Two levels of resting potential in cardiac Purkinje fibers. J Gen Physiol. 1977 Dec;70(6):725–746. doi: 10.1085/jgp.70.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grampp W., Harris J. B., Thesleff S. Inhibition of denervation changes in skeletal muscle by blockers of protein synthesis. J Physiol. 1972 Mar;221(3):743–754. doi: 10.1113/jphysiol.1972.sp009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle L., McCaig C. D., Robinson K. R. The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J Physiol. 1981 May;314:121–135. doi: 10.1113/jphysiol.1981.sp013695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. An ultrasensitive vibrating probe for measuring steady extracellular currents. J Cell Biol. 1974 Nov;63(2 Pt 1):614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorković H. Effect of sodium on voltage-current relationships in rat muscles. Arch Int Physiol Biochim. 1976 Dec;84(5):939–954. doi: 10.3109/13813457609069456. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Poo M. M. The role of electro-osmosis in the electric-field-induced movement of charged macromolecules on the surfaces of cells. Biophys J. 1981 Apr;34(1):85–93. doi: 10.1016/S0006-3495(81)84838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch M. W., Orellana S. A., Kimberg L. S., Field M., Halm D. R., Krasny E. J., Jr, Frizzell R. A. Na+-K+-Cl- co-transport in the intestine of a marine teleost. Nature. 1982 Nov 25;300(5890):351–353. doi: 10.1038/300351a0. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. On the inhibition of muscle membrane chloride conductance by aromatic carboxylic acids. J Gen Physiol. 1977 Jun;69(6):879–896. doi: 10.1085/jgp.69.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. B., Poo M. M. Perturbation of the direction of neurite growth by pulsed and focal electric fields. J Neurosci. 1984 Dec;4(12):2939–2947. doi: 10.1523/JNEUROSCI.04-12-02939.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Poo M. M. Orientation of neurite growth by extracellular electric fields. J Neurosci. 1982 Apr;2(4):483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Lam J. W., Orida N., Chao A. W. Electrophoresis and diffusion in the plane of the cell membrane. Biophys J. 1979 Apr;26(1):1–21. doi: 10.1016/S0006-3495(79)85231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins N. Cation movements in normal and short-term denervated rat fast twitch muscle. J Physiol. 1977 Oct;271(3):605–624. doi: 10.1113/jphysiol.1977.sp012017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. M. Cation-coupled chloride influx in squid axon. Role of potassium and stoichiometry of the transport process. J Gen Physiol. 1983 Jun;81(6):909–925. doi: 10.1085/jgp.81.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. H., Brown H. M. Liquid and solid-state Cl- -sensitive microelectrodes. Characteristics and application to intracellular Cl- activity in Balanus photoreceptor. J Gen Physiol. 1977 Oct;70(4):507–530. doi: 10.1085/jgp.70.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin L. C., Thesleff S. Alterations in membrane electrical properties during long-term denervation of rat skeletal muscles. Acta Physiol Scand. 1980 Mar;108(3):243–246. doi: 10.1111/j.1748-1716.1980.tb06529.x. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F., Harris E. J. Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J Gen Physiol. 1967 Jul;50(6):1565–1583. doi: 10.1085/jgp.50.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump R. F., Robinson K. R. Xenopus neural crest cell migration in an applied electrical field. J Cell Biol. 1983 Oct;97(4):1226–1233. doi: 10.1083/jcb.97.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Chloride activity and its control in skeletal and cardiac muscle. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):537–548. doi: 10.1098/rstb.1982.0150. [DOI] [PubMed] [Google Scholar]
- WARE F., Jr, BENNETT A. L., McINTYRE A. R. Membrane resting potential of denervated mammalian skeletal muscle measured in vivo. Am J Physiol. 1954 Apr;177(1):115–118. doi: 10.1152/ajplegacy.1954.177.1.115. [DOI] [PubMed] [Google Scholar]
- Westgaard R. H. Influence of activity on the passive electrical properties of denervated soleus muscle fibres in the rat. J Physiol. 1975 Oct;251(3):683–697. doi: 10.1113/jphysiol.1975.sp011116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Miyata Y. Changes in the distribution of the extrajunctional acetylcholine sensitivity along muscle fibers during development and following cordotomy in the rat. Neuroscience. 1983 Jun;9(2):437–443. doi: 10.1016/0306-4522(83)90306-8. [DOI] [PubMed] [Google Scholar]


