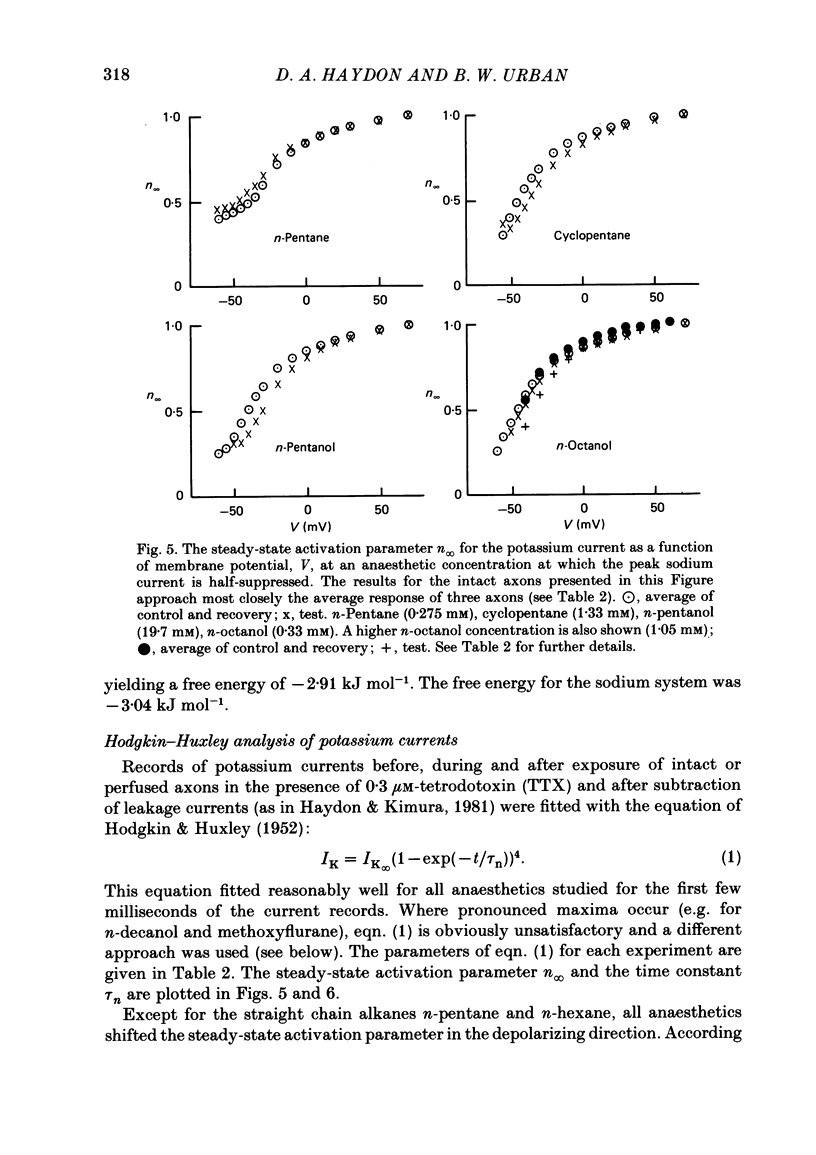
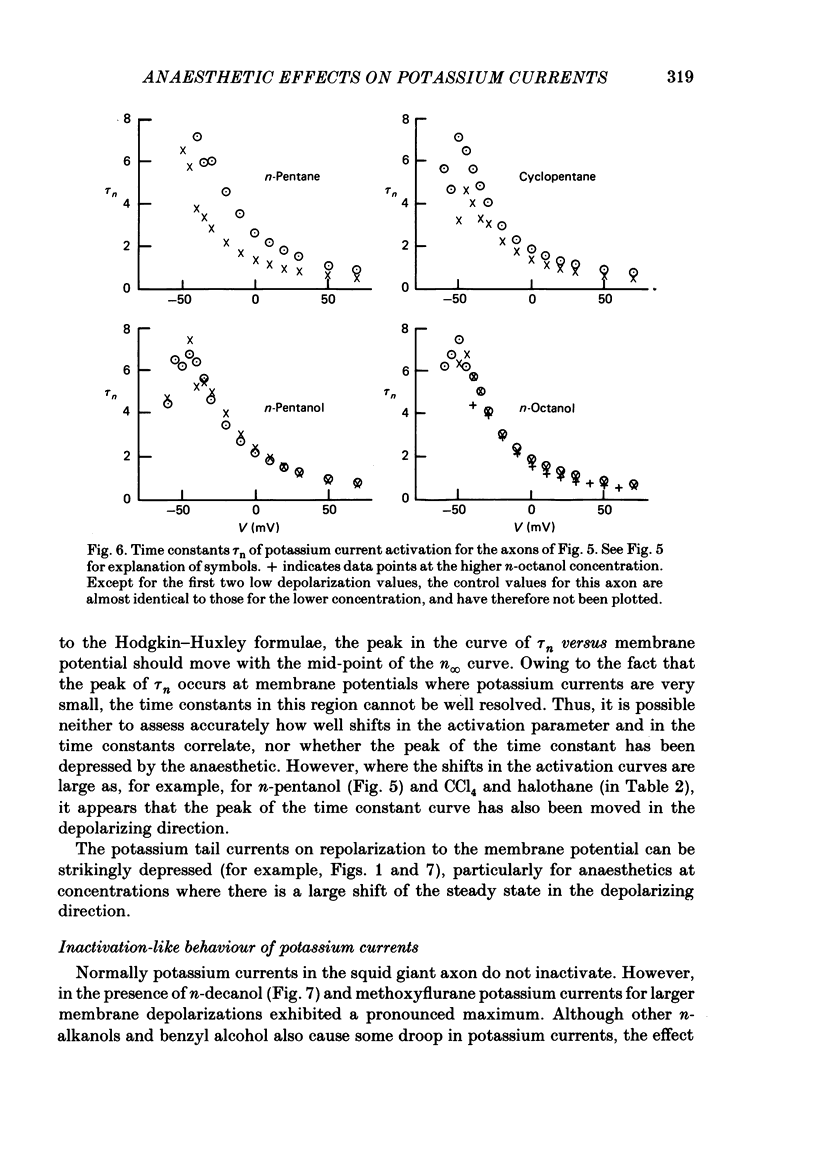
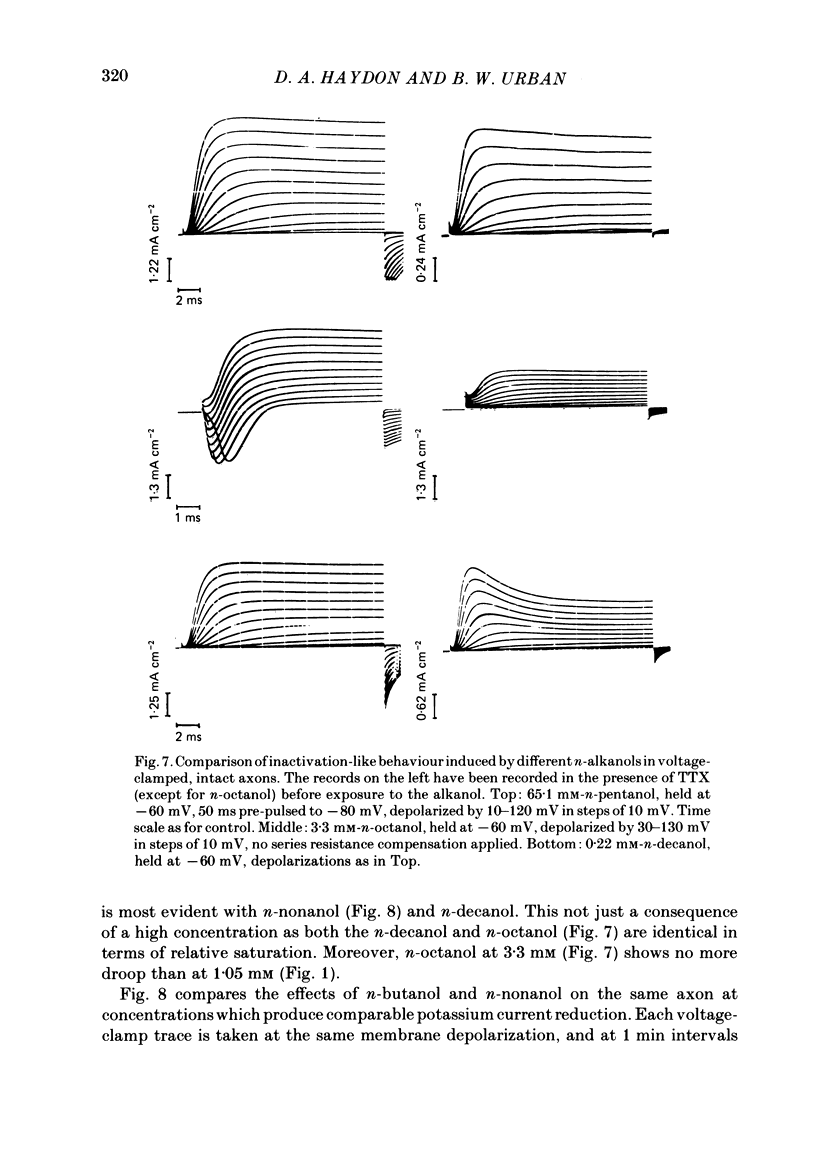
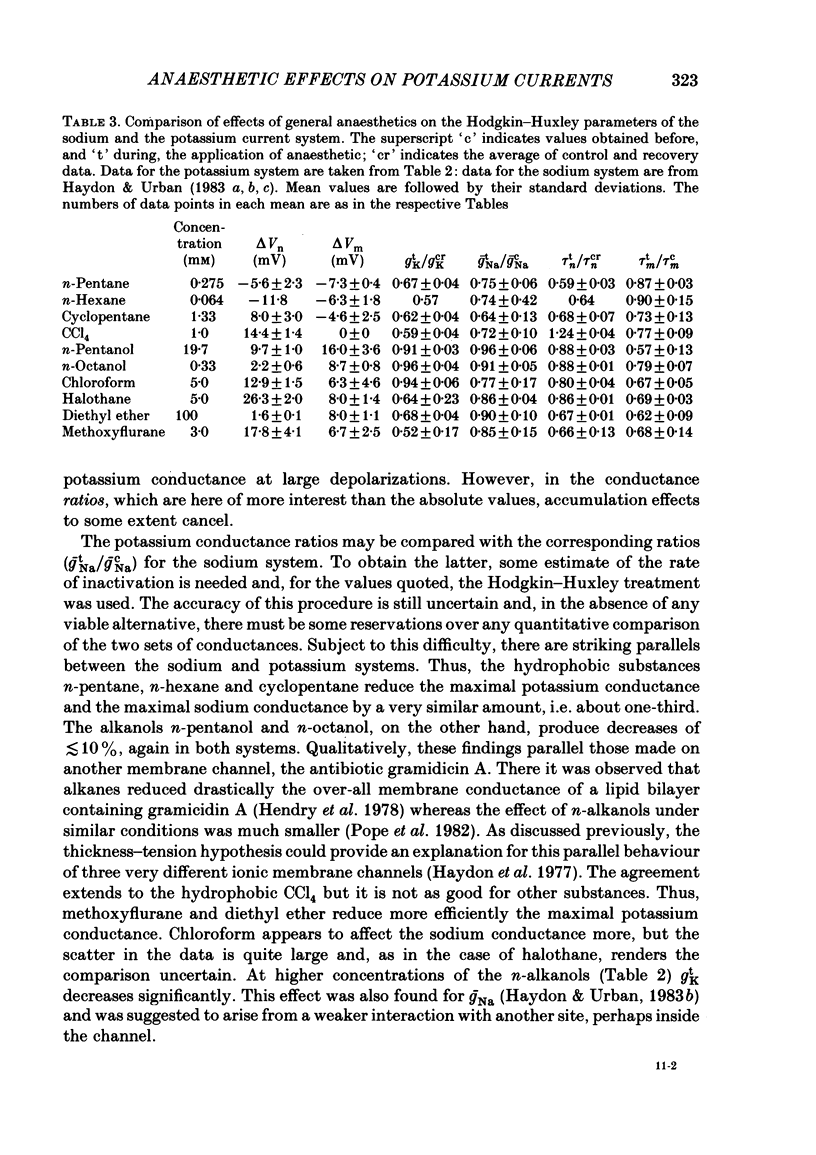
Abstract
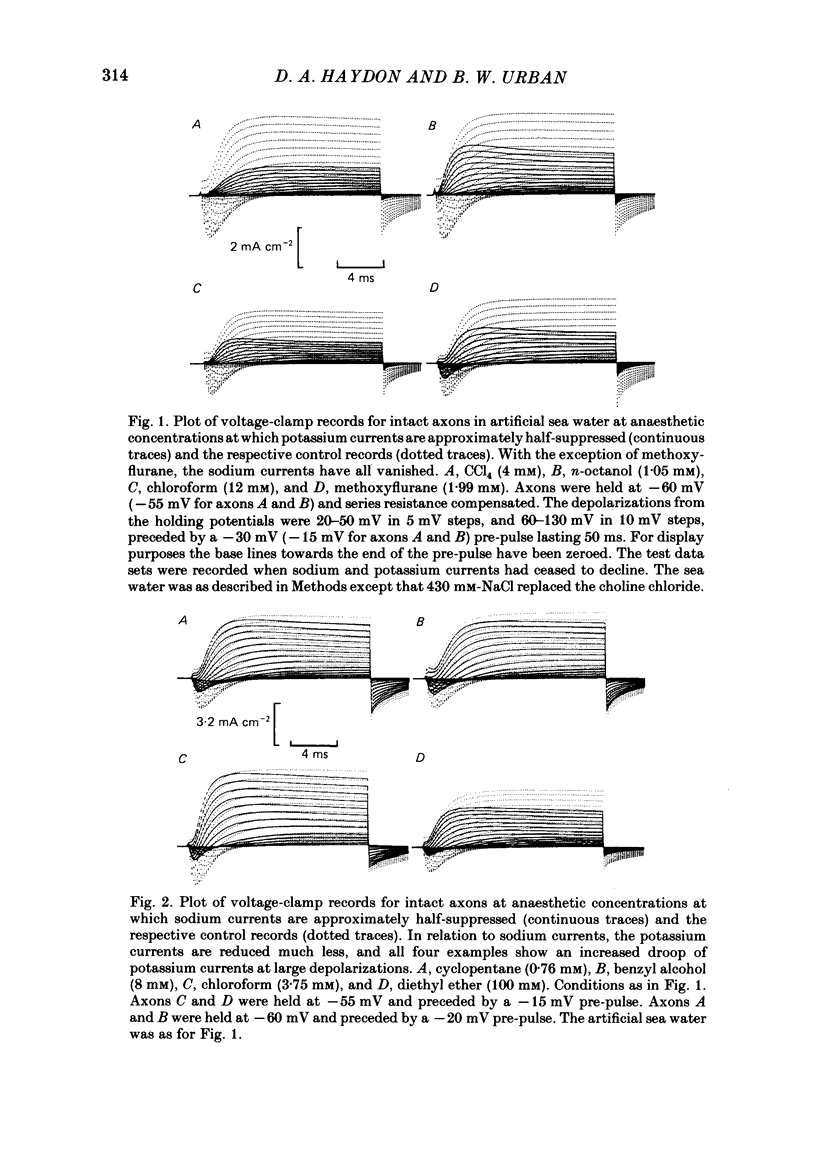
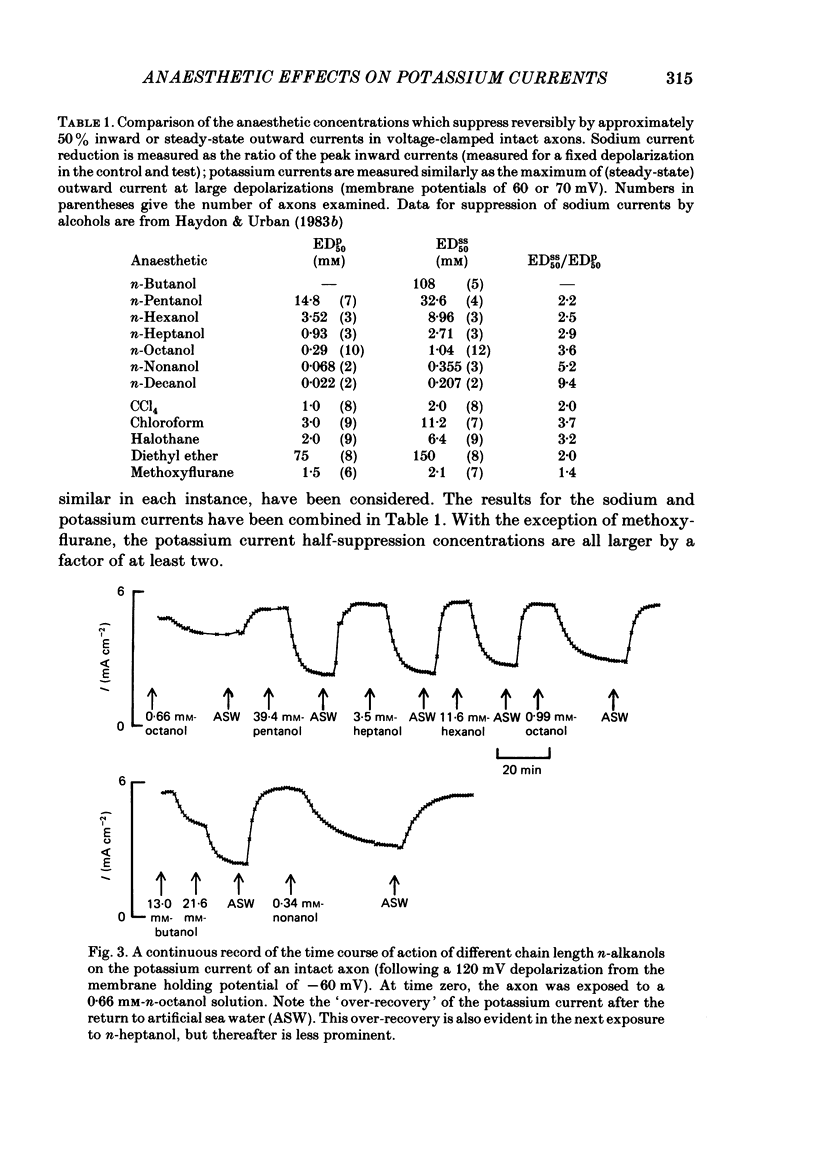
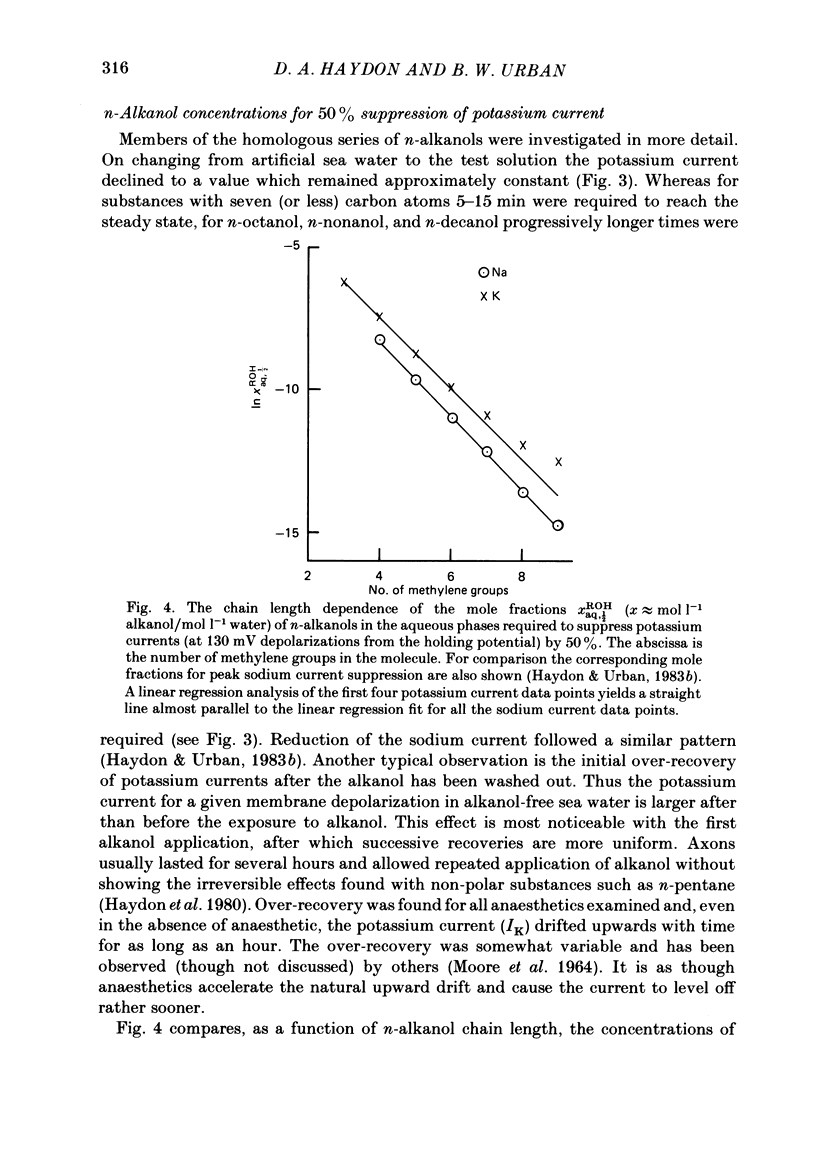
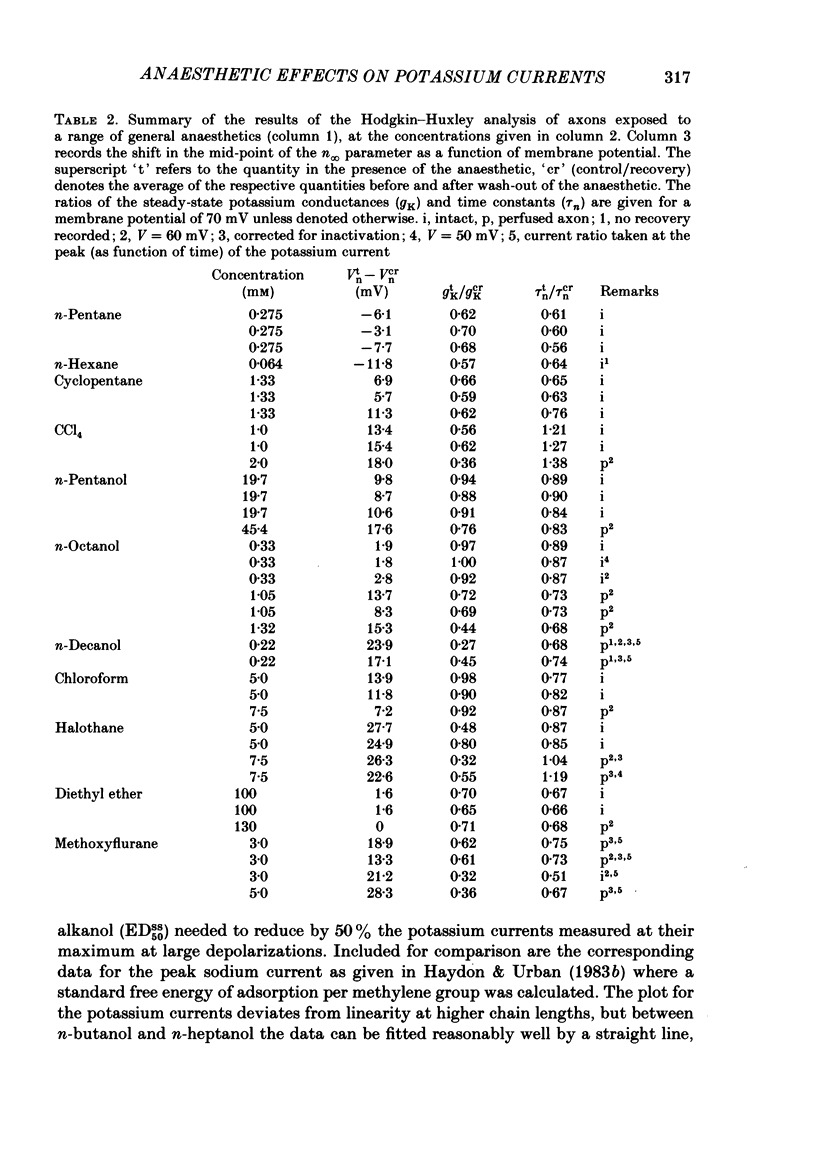
A number of small organic molecules with general anaesthetic action have been examined for their effects on the voltage-dependent potassium current of the squid giant axon. They include representatives of the three classes of anaesthetics examined in previous studies on the sodium current (Haydon & Urban, 1983a, b, c), i.e. the non-polar molecules n-pentane, cyclopentane and CCl4, several n-alkanols and the inhalation anaesthetics chloroform, halothane, diethyl ether and methoxyflurane. Potassium currents under voltage clamp were recorded in intact and in intracellularly perfused axons before, during and after exposure to the test substances, and the records were fitted with equations similar to those proposed by Hodgkin & Huxley (1952). Shifts in the curves of the steady-state activation against membrane potential and reductions in the potassium conductance at 60 or 70 mV membrane potential have been tabulated. On the same intact axons, all the anaesthetics with the exception of methoxyflurane reduced potassium currents less than sodium currents by about a factor of two or more. For the n-alkanols, butanol to decanol, the concentrations required to reduce the potassium current at 60 mV membrane potential by 50% were determined. For n-butanol to n-heptanol, the standard free energy per CH2 for adsorption to the site of action was estimated to be -2.91 kJ mol-1 as compared with -3.04 kJ mol-1 for reduction of the sodium current. The magnitude of the free energy decreased for alkanols with longer chain lengths. At anaesthetic concentrations that reduce the sodium current by 50%, the hydrophobic substances n-pentane and cyclopentane reduced the maximal sodium conductance, gNa, and the potassium conductance at 70 mV, gK70, equally by about a third, while the n-alkanols reduced both parameters by less than 10%. By contrast, diethyl ether and methoxyflurane were more effective in reducing the maximal potassium conductance. All of the test substances examined, except n-pentane and n-hexane, shifted the voltage dependence of the potassium steady-state activation in the depolarizing direction. A broad qualitative correlation was found between the shifts in the activation curves for sodium and potassium currents but, quantitatively, the agreement between the two shifts was poor. In n-decanol and methoxyflurane solutions, the voltage-clamped potassium currents exhibited pronounced inactivation-like behaviour. These currents can be fitted by the Hodgkin-Huxley formalism if an inactivation term analogous to the sodium current inactivation is added.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. THE EFFECTS OF SEVERAL ALCOHOLS ON THE PROPERTIES OF THE SQUID GIANT AXON. J Gen Physiol. 1964 Nov;48:265–277. doi: 10.1085/jgp.48.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Jr, Palti Y., Senft J. P. Potassium ion accumulation in a periaxonal space and its effect on the measurement of membrane potassium ion conductance. J Membr Biol. 1973 Nov 8;13(4):387–410. doi: 10.1007/BF01868237. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Potassium pores of nerve and muscle membranes. Membranes. 1975;3:325–358. [PubMed] [Google Scholar]
- Chabala L. D. The kinetics of recovery and development of potassium channel inactivation in perfused squid (Loligo pealei) giant axons. J Physiol. 1984 Nov;356:193–220. doi: 10.1113/jphysiol.1984.sp015460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A., Hendry B. M. Anaesthetic action of esters and ketones: evidence for an interaction with the sodium channel protein in squid axons. J Physiol. 1984 Sep;354:407–418. doi: 10.1113/jphysiol.1984.sp015384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A., Hendry B. M. Dual effects of internal n-alkyltrimethylammonium ions on the sodium current of the squid giant axon. J Physiol. 1985 Apr;361:47–64. doi: 10.1113/jphysiol.1985.sp015632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A., Hendry B. M. The asymmetrical effects of some ionized n-octyl derivatives on the sodium current of the giant axon of Loligo forbesi. J Physiol. 1984 May;350:429–445. doi: 10.1113/jphysiol.1984.sp015210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys J. 1981 May;34(2):271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Urban B. W. Some effects of aliphatic hydrocarbons on the electrical capacity and ionic currents of the squid giant axon membrane. J Physiol. 1980 Dec;309:229–245. doi: 10.1113/jphysiol.1980.sp013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of hydrocarbons and carbon tetrachloride on the sodium current of the squid giant axon. J Physiol. 1983 May;338:435–450. doi: 10.1113/jphysiol.1983.sp014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry B. M., Urban B. W., Haydon D. A. The blockage of the electrical conductance in a pore-containing membrane by the n-alkanes. Biochim Biophys Acta. 1978 Oct 19;513(1):106–116. doi: 10.1016/0005-2736(78)90116-5. [DOI] [PubMed] [Google Scholar]
- Inoue I. Activation-inactivation of potassium channels and development of the potassium-channel spike in internally perfused squid giant axons. J Gen Physiol. 1981 Jul;78(1):43–61. doi: 10.1085/jgp.78.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsson A., Keightley C. A., Smith G. A., Richards C. D., Hesketh T. R., Metcalfe J. C. The effect of bilayer thickness and n-alkanes on the activity of the (Ca2+ + Mg2+)-dependent ATPase of sarcoplasmic reticulum. J Biol Chem. 1981 Feb 25;256(4):1643–1650. [PubMed] [Google Scholar]
- Johannsson A., Smith G. A., Metcalfe J. C. The effect of bilayer thickness on the activity of (Na+ + K+)-ATPase. Biochim Biophys Acta. 1981 Mar 6;641(2):416–421. doi: 10.1016/0005-2736(81)90498-3. [DOI] [PubMed] [Google Scholar]
- MOORE J. W., ULBRICHT W., TAKATA M. EFFECT OF ETHANOL ON THE SODIUM AND POTASSIUM CONDUCTANCES OF THE SQUID AXON MEMBRANE. J Gen Physiol. 1964 Nov;48:279–295. doi: 10.1085/jgp.48.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C. G., Urban B. W., Haydon D. A. The influence of n-alkanols and cholesterol on the duration and conductance of gramicidin single channels in monoolein bilayers. Biochim Biophys Acta. 1982 May 21;688(1):279–283. doi: 10.1016/0005-2736(82)90605-8. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Jr Inactivation of potassium current in squid axon by a variety of quaternary ammonium ions. J Gen Physiol. 1981 Mar;77(3):255–271. doi: 10.1085/jgp.77.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]


