Abstract
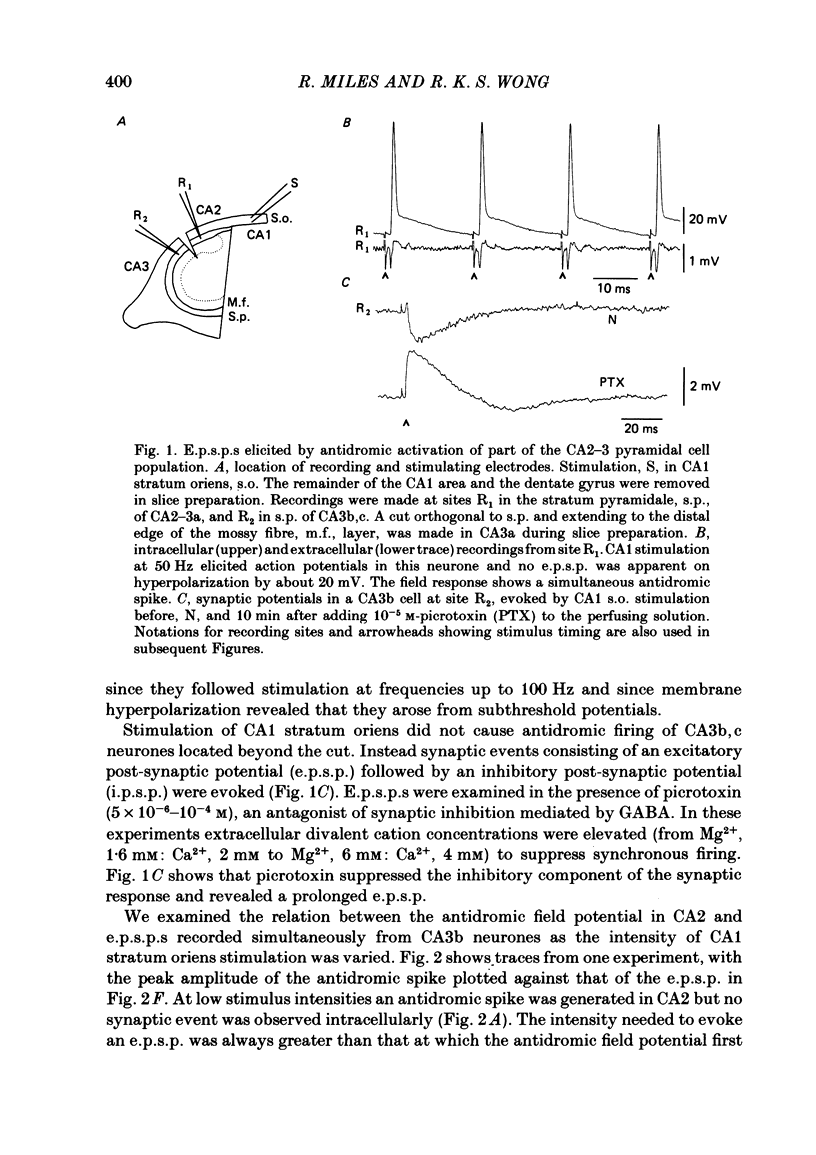
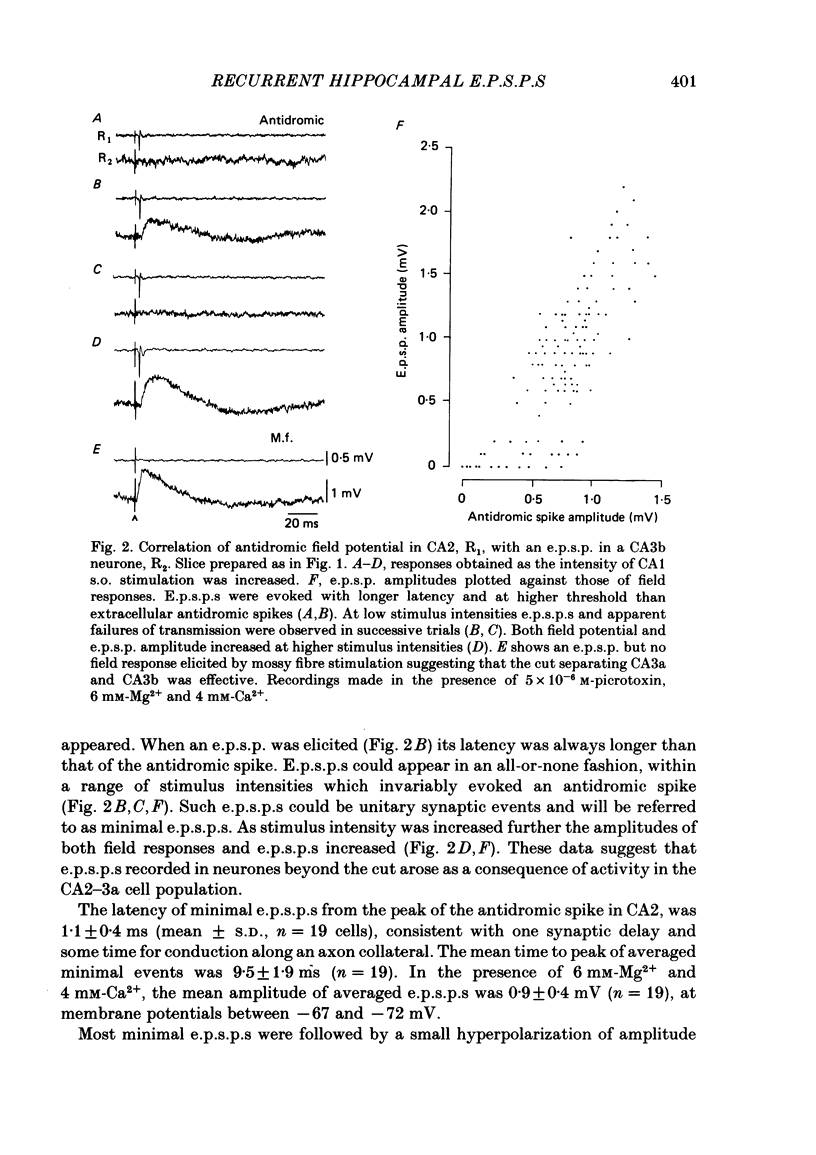
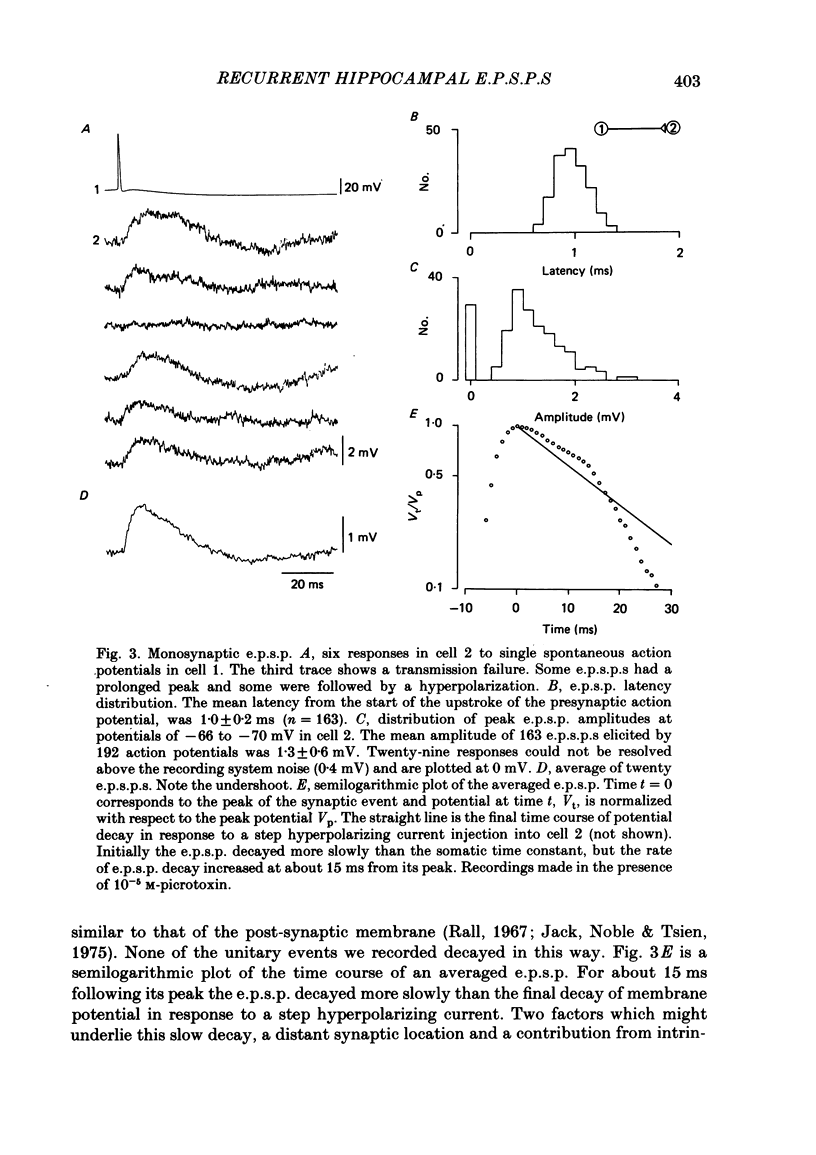
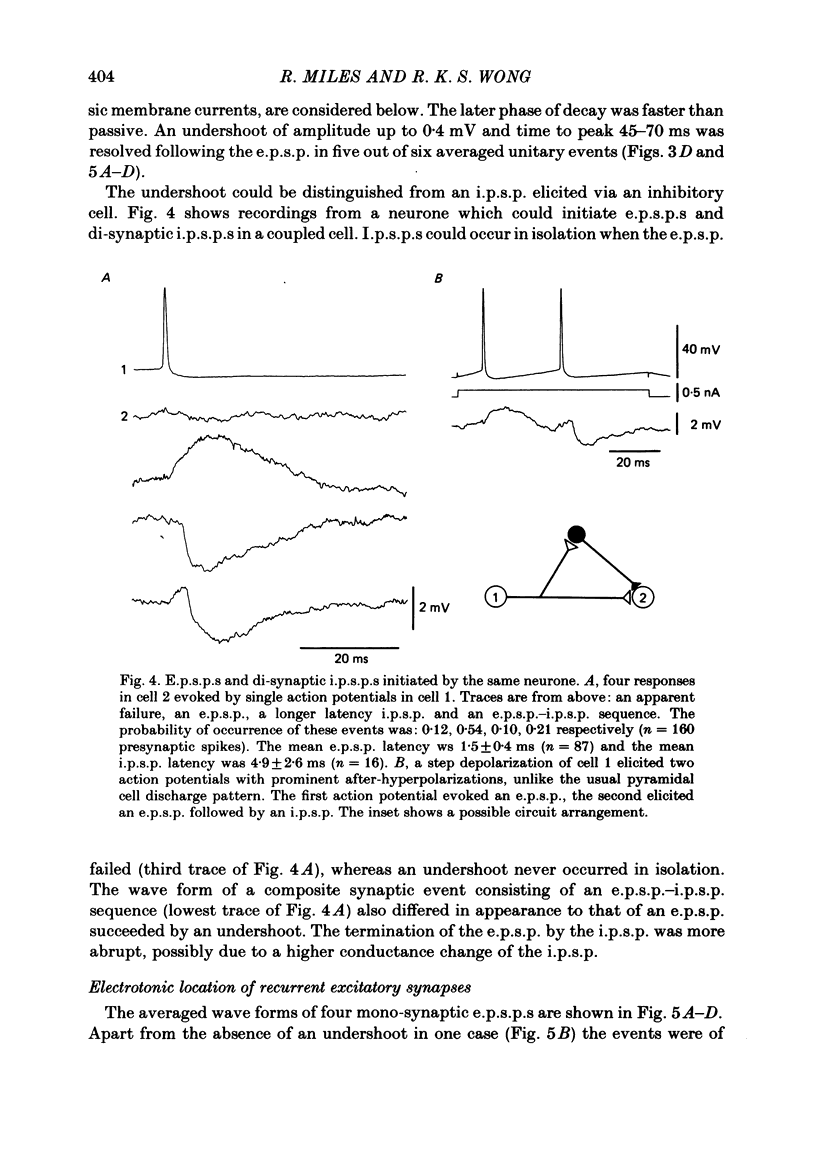
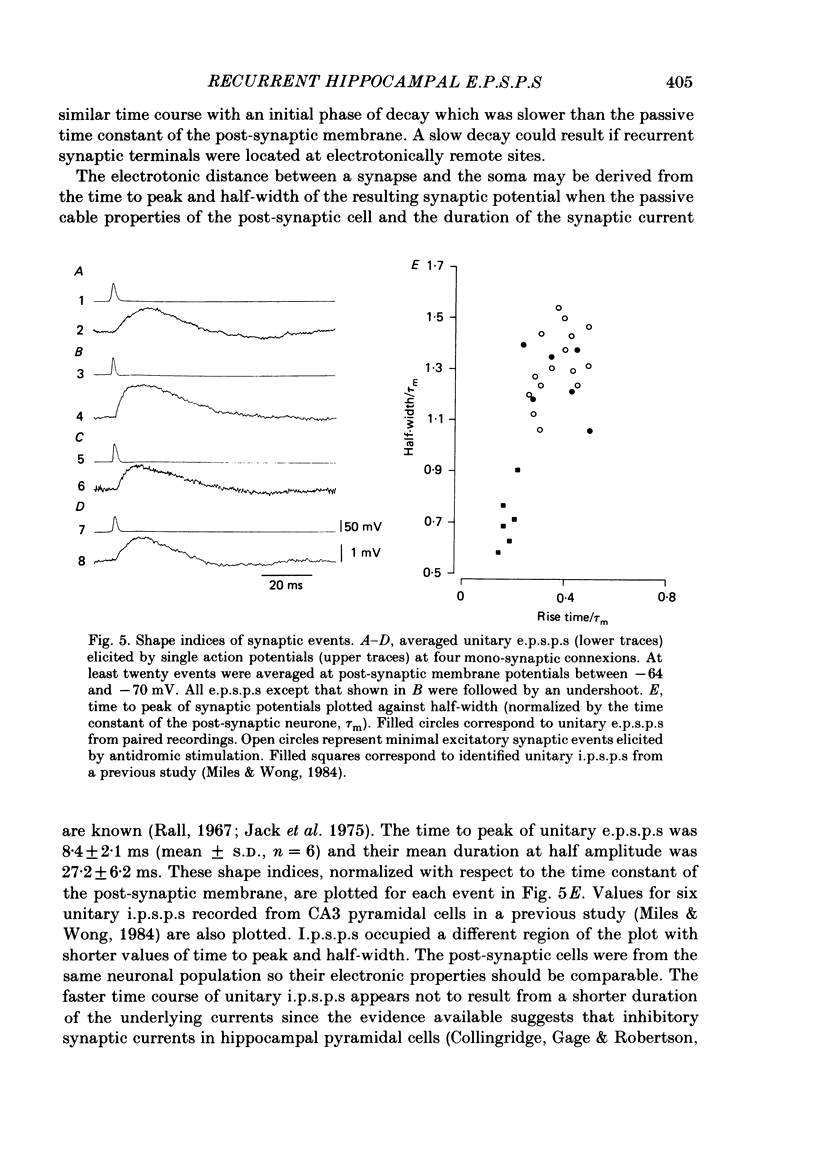
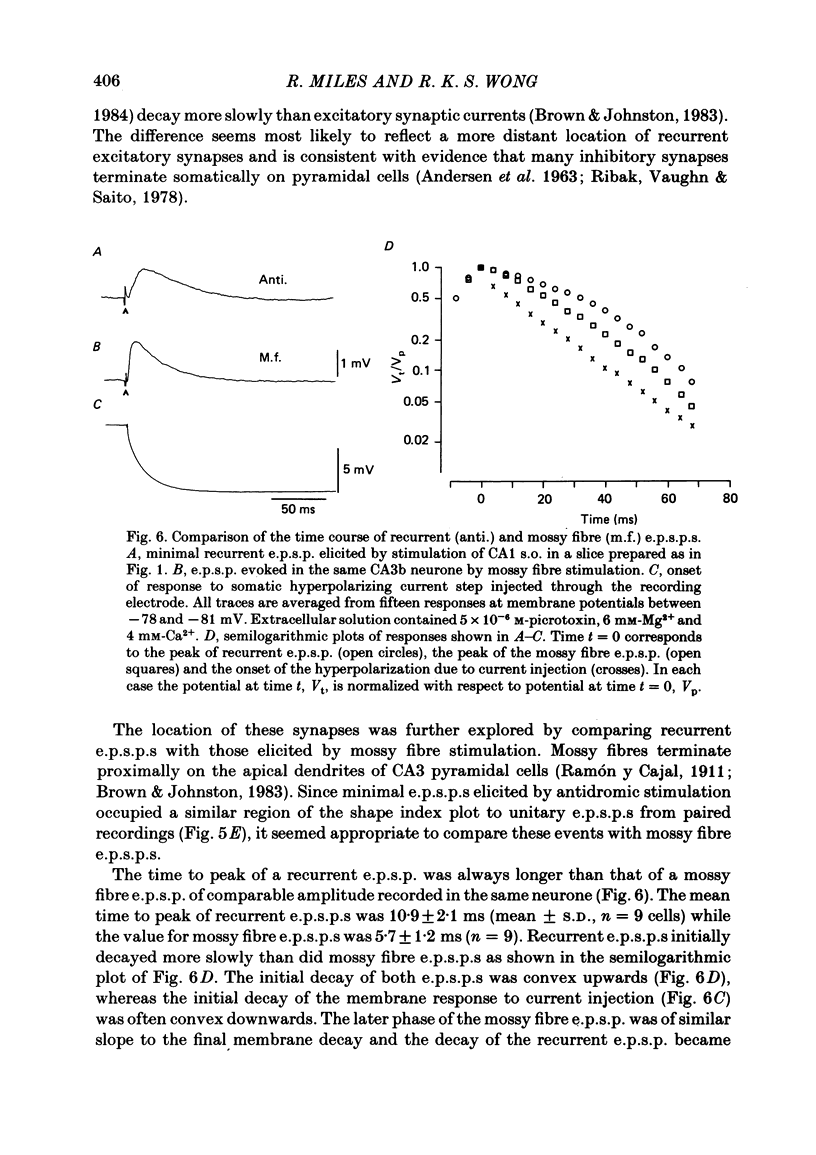
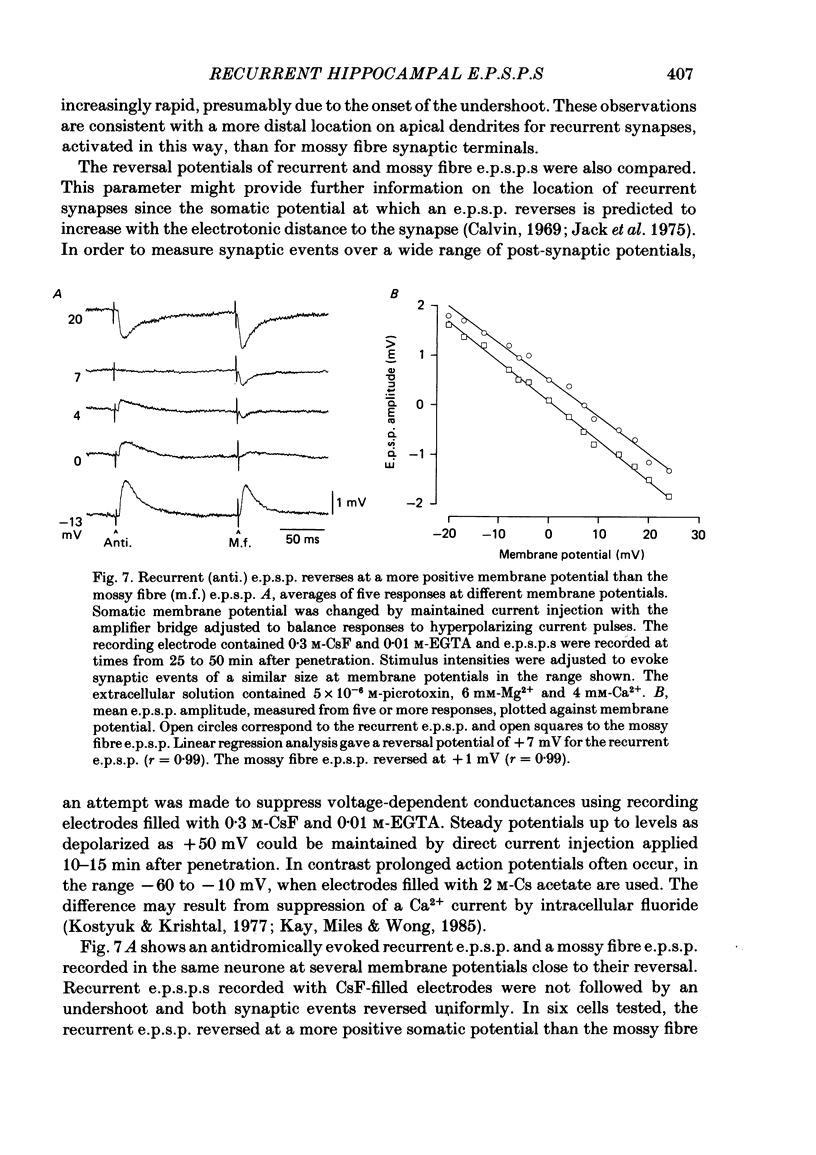
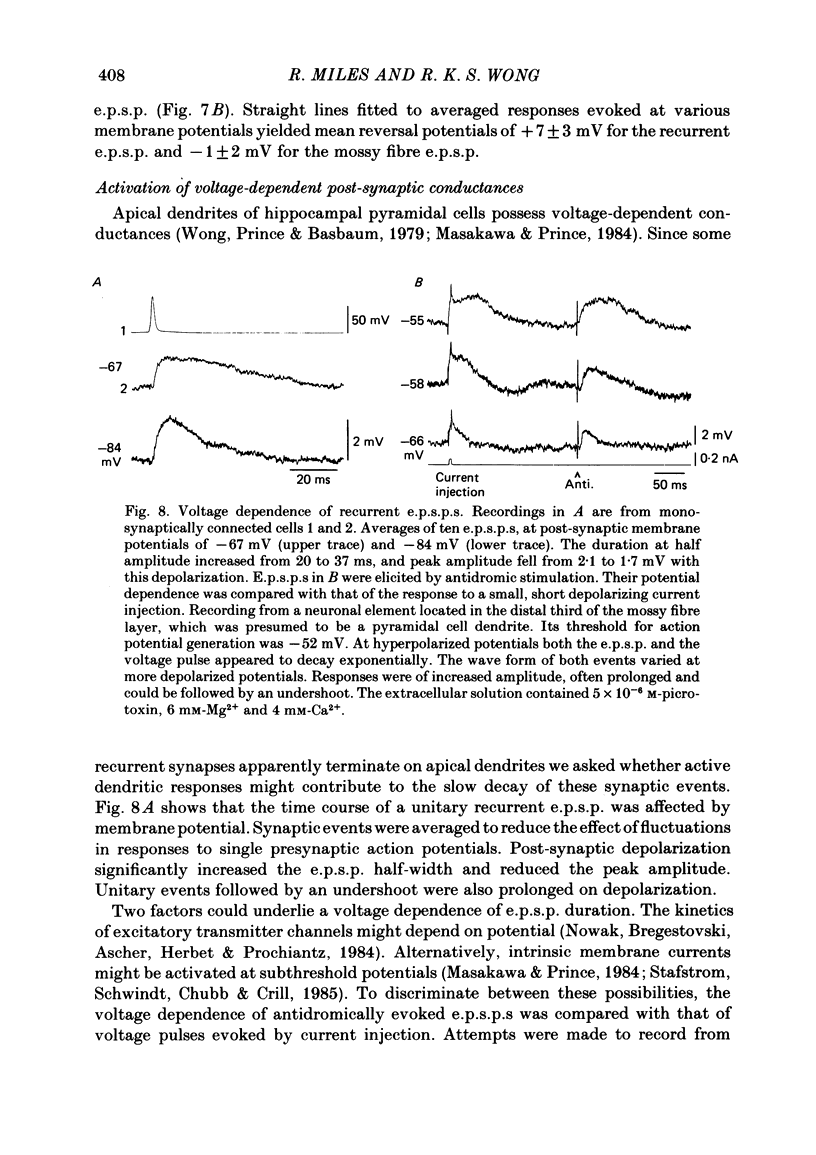
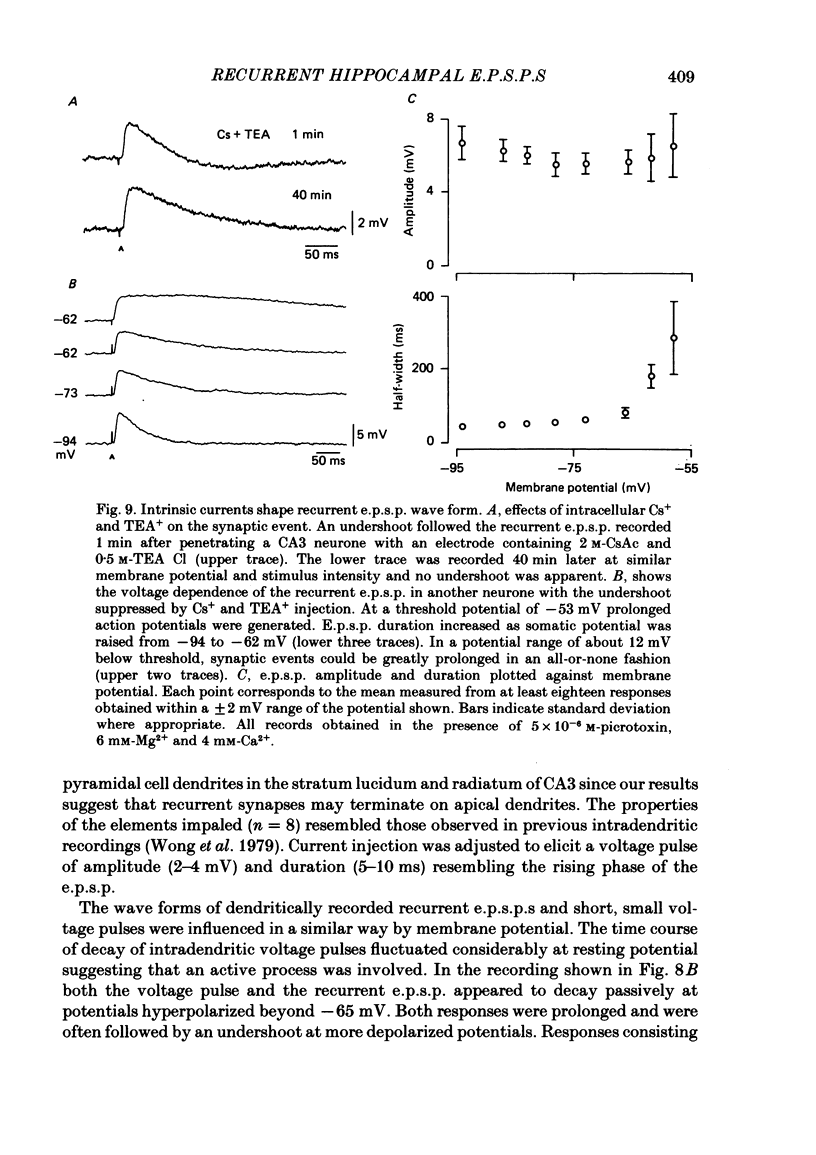
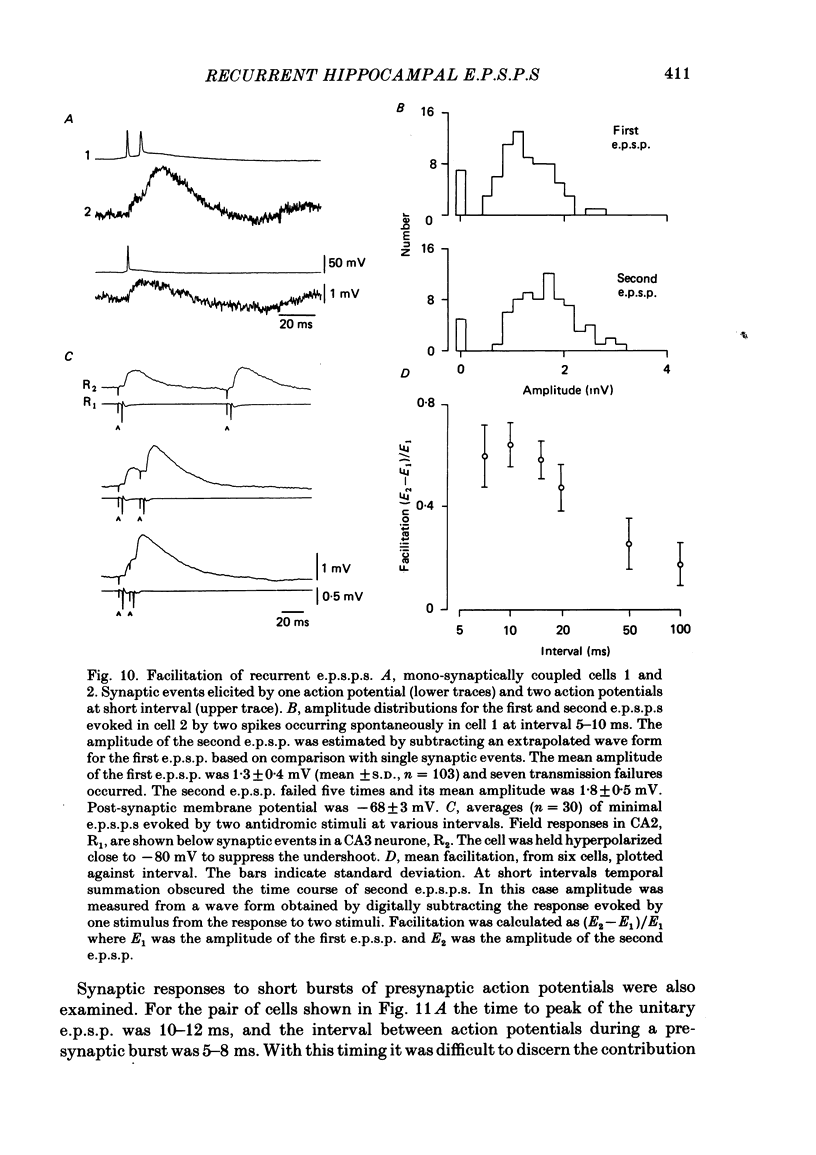
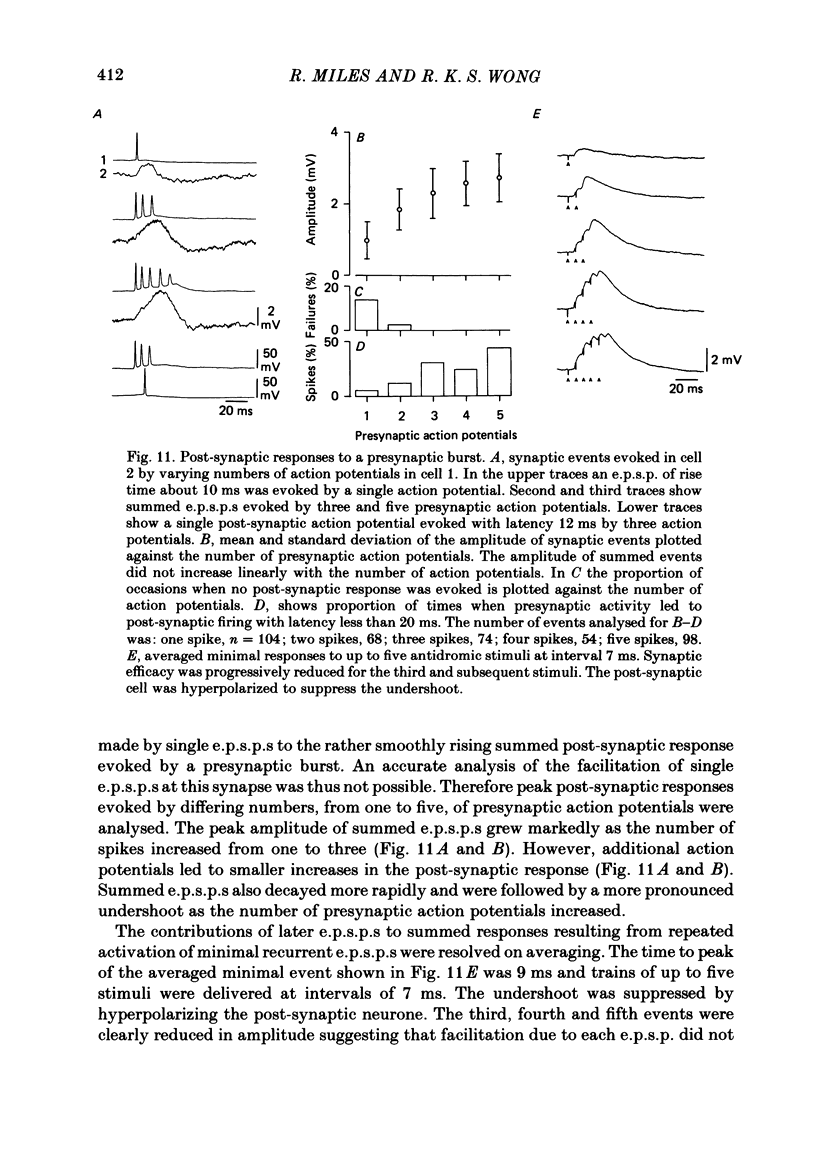
Excitatory synaptic interactions between CA3 neurones in slices from guinea-pig hippocampus were examined. Recurrent excitatory post-synaptic potentials (e.p.s.p.s) were evoked by action potentials in a single presynaptic neurone or by the antidromic activation of part of the CA3 pyramidal cell population. The peak amplitude of unitary e.p.s.p.s was 1-2 mV at potentials between -64 and -70 mV. Their time to peak was 7-12 ms and the initial phase of their decay was slower than that of a somatically injected voltage pulse. Recurrent e.p.s.p.s were often followed by a small (0.3 mV) hyperpolarization, or undershoot. Recurrent e.p.s.p.s were compared with e.p.s.p.s evoked by stimulating mossy fibres, which terminate proximally on apical dendrites of CA3 pyramidal cells. They were of slower time course and reversed at a more positive potential than mossy fibre e.p.s.p.s. Some synaptic terminals made by recurrent axon collaterals apparently terminate at distant locations on apical dendrites. The decay of both recurrent e.p.s.p.s and dendritic voltage pulses was prolonged by membrane depolarization within a 10-15 mV subthreshold potential range. Voltage-dependent inward currents activated by the synaptic depolarization may contribute to the slow initial decay of these synaptic events. The undershoot did not occur when transmission of a unitary e.p.s.p. failed and was of slower time course than the hyperpolarization due to an inhibitory post-synaptic potential (i.p.s.p.). It was suppressed by intracellular application of K+ channel blockers and probably reflects an intrinsic outward current activated as a consequence of the synaptic depolarization. Considerable temporal summation of synaptic potentials occurred when recurrent synapses were activated twice at an interval of 5-10 ms, typical of the spontaneous burst firing pattern of CA3 neurones. The mean facilitation of a second e.p.s.p. at this interval was about 0.6. The efficacy of a third and subsequent e.p.s.p.s at similar interval was reduced. Presynaptic bursts of three to five action potentials evoked summed e.p.s.p.s of amplitude 2-4 mV, with time to peak 20-40 ms and decaying phase of similar duration. Their rising phase was relatively smooth and summed events were succeeded by an undershoot. Presynaptic bursts could cause a post-synaptic neurone to discharge.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. Recurrent inhibition in the hippocampus with identification of the inhibitory cell and its synapses. Nature. 1963 May 11;198:540–542. doi: 10.1038/198540a0. [DOI] [PubMed] [Google Scholar]
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E. Characteristics of a slow hyperpolarizing synaptic potential in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1984 Nov;52(5):892–910. doi: 10.1152/jn.1984.52.5.892. [DOI] [PubMed] [Google Scholar]
- Andersen P., Silfvenius H., Sundberg S. H., Sveen O. A comparison of distal and proximal dendritic synapses on CAi pyramids in guinea-pig hippocampal slices in vitro. J Physiol. 1980 Oct;307:273–299. doi: 10.1113/jphysiol.1980.sp013435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Krnjević K., Reinhardt W., Ropert N. Intracellular observations on the disinhibitory action of acetylcholine in the hippocampus. Neuroscience. 1981;6(12):2475–2484. doi: 10.1016/0306-4522(81)90093-2. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Johnston D. Voltage-clamp analysis of mossy fiber synaptic input to hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):487–507. doi: 10.1152/jn.1983.50.2.487. [DOI] [PubMed] [Google Scholar]
- Calvin W. H. Dendritic synapses and reversal potentials: theoretical implications of the view from the soma. Exp Neurol. 1969 Jun;24(2):248–264. doi: 10.1016/0014-4886(69)90018-1. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984 Sep;46(3):413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Gage P. W., Robertson B. Inhibitory post-synaptic currents in rat hippocampal CA1 neurones. J Physiol. 1984 Nov;356:551–564. doi: 10.1113/jphysiol.1984.sp015482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Forda S., Kelly J. S. The reversal potential of excitatory amino acid action on granule cells of the rat dentate gyrus. J Physiol. 1984 Jun;351:327–342. doi: 10.1113/jphysiol.1984.sp015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M., Spencer W. A. Penicillin-induced interictal discharges from the cat hippocampus. II. Mechanisms underlying origin and restriction. J Neurophysiol. 1969 Sep;32(5):663–687. doi: 10.1152/jn.1969.32.5.663. [DOI] [PubMed] [Google Scholar]
- Finch D. M., Nowlin N. L., Babb T. L. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Res. 1983 Jul 25;271(2):201–216. doi: 10.1016/0006-8993(83)90283-4. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Electrical changes in the membrane in junctional transmission. Biochim Biophys Acta. 1973 Nov 28;300(3):289–317. doi: 10.1016/0304-4157(73)90007-5. [DOI] [PubMed] [Google Scholar]
- Haberly L. B., Bower J. M. Analysis of association fiber system in piriform cortex with intracellular recording and staining techniques. J Neurophysiol. 1984 Jan;51(1):90–112. doi: 10.1152/jn.1984.51.1.90. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A., Schwartzkroin P. A. Anomalous inward rectification in hippocampal neurons. J Neurophysiol. 1979 May;42(3):889–895. doi: 10.1152/jn.1979.42.3.889. [DOI] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. The amplitude, time course and charge of unitary excitatory post-synaptic potentials evoked in spinal motoneurone dendrites. J Physiol. 1973 Nov;234(3):665–688. doi: 10.1113/jphysiol.1973.sp010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Anomalous rectification in the metacerebral giant cells and its consequences for synaptic transmission. J Physiol. 1966 Mar;183(2):287–304. doi: 10.1113/jphysiol.1966.sp007867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Restoration of function in external intercostal motoneurones of the cat following partial central deafferentation. J Physiol. 1984 May;350:225–251. doi: 10.1113/jphysiol.1984.sp015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles W. D., Schwartzkroin P. A. Local circuit synaptic interactions in hippocampal brain slices. J Neurosci. 1981 Mar;1(3):318–322. doi: 10.1523/JNEUROSCI.01-03-00318.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R. M., Dichter M., Spencer W. A. Recurrent excitation in the CA3 region of cat hippocampus. Int J Neurosci. 1971 Aug;2(2):99–107. doi: 10.3109/00207457109146996. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Schubert P. Some aspects of the electroanatomy of dendrites. Adv Neurol. 1975;12:29–44. [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar B. A., Dudek F. E. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980 Feb 17;184(1):220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Masukawa L. M., Prince D. A. Synaptic control of excitability in isolated dendrites of hippocampal neurons. J Neurosci. 1984 Jan;4(1):217–227. doi: 10.1523/JNEUROSCI.04-01-00217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983 Nov 24;306(5941):371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Miles R., Wong R. K. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984 Nov;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981 May 22;212(4497):957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Redman S., Walmsley B. The time course of synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983 Oct;343:117–133. doi: 10.1113/jphysiol.1983.sp014884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak C. E., Vaughn J. E., Saito K. Immunocytochemical localization of glutamic acid decarboxylase in neuronal somata following colchicine inhibition of axonal transport. Brain Res. 1978 Jan 27;140(2):315–332. doi: 10.1016/0006-8993(78)90463-8. [DOI] [PubMed] [Google Scholar]
- STEFANIS C., JASPER H. INTRACELLULAR MICROELECTRODE STUDIES OF ANTIDROMIC RESPONSES IN CORTICAL PYRAMIDAL TRACT NEURONS. J Neurophysiol. 1964 Sep;27:828–854. doi: 10.1152/jn.1964.27.5.828. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Chubb M. C., Crill W. E. Properties of persistent sodium conductance and calcium conductance of layer V neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1985 Jan;53(1):153–170. doi: 10.1152/jn.1985.53.1.153. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Wyss J. M., Cowan W. M. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978 Oct 15;181(4):681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosaka T., Tasaka J., Miyazaki T., Libet B. Hyperpolarization following activation of K+ channels by excitatory postsynaptic potentials. Nature. 1983 Sep 8;305(5930):148–150. doi: 10.1038/305148a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982 May 14;216(4547):745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Winfield D. A., Brooke R. N., Sloper J. J., Powell T. P. A combined Golgi-electron microscopic study of the synapses made by the proximal axon and recurrent collaterals of a pyramidal cell in the somatic sensory cortex of the monkey. Neuroscience. 1981;6(7):1217–1230. doi: 10.1016/0306-4522(81)90183-4. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A., Basbaum A. I. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci U S A. 1979 Feb;76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]


