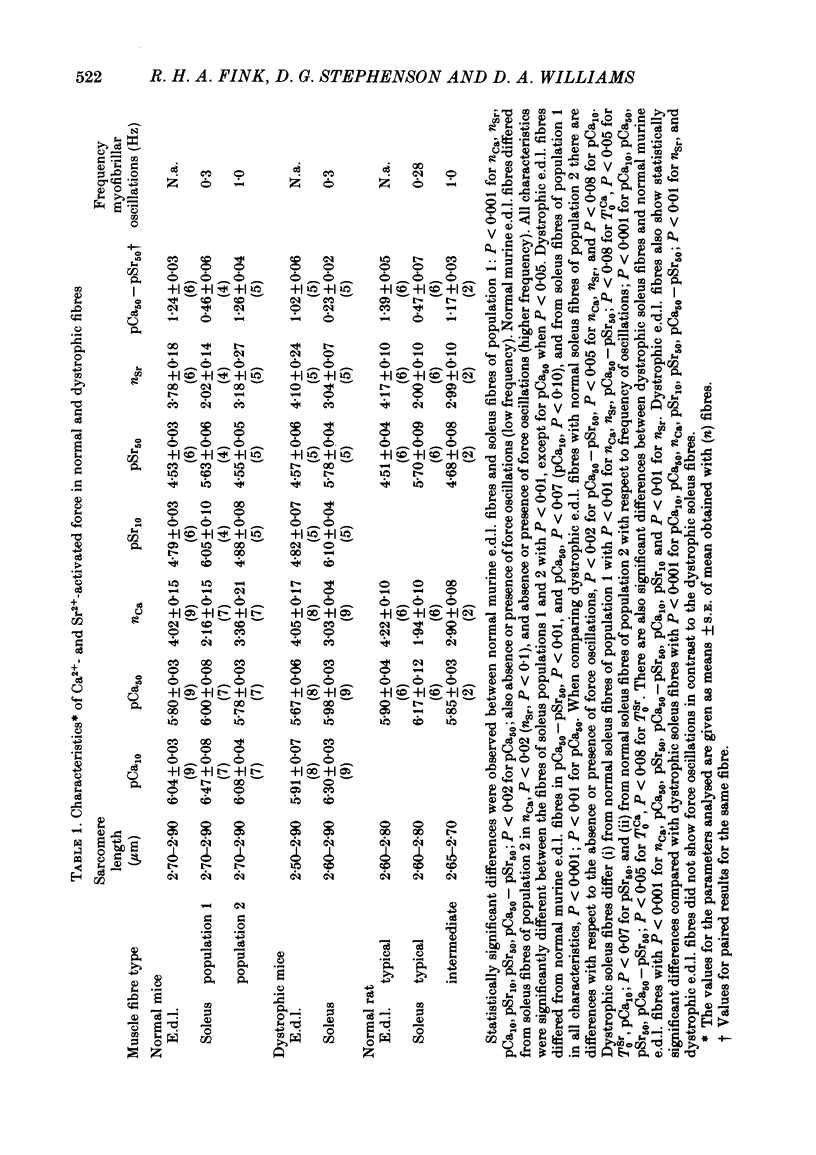
Abstract
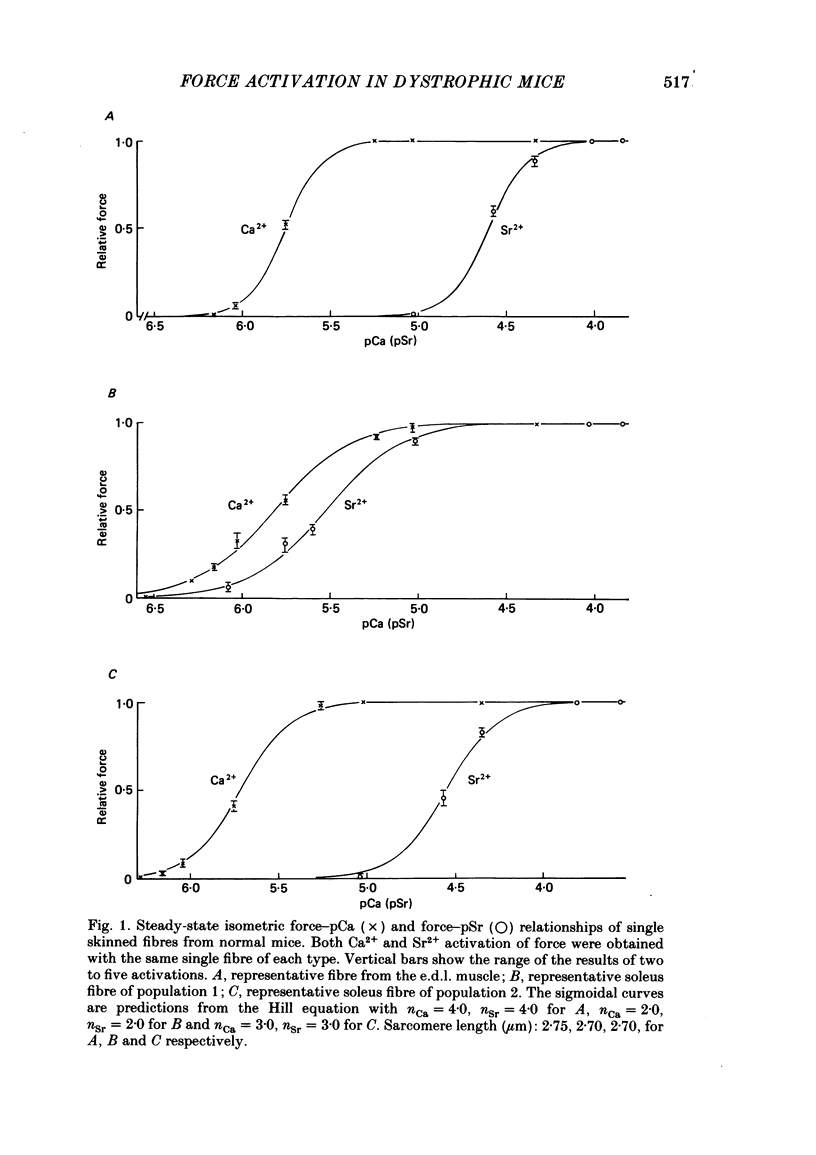
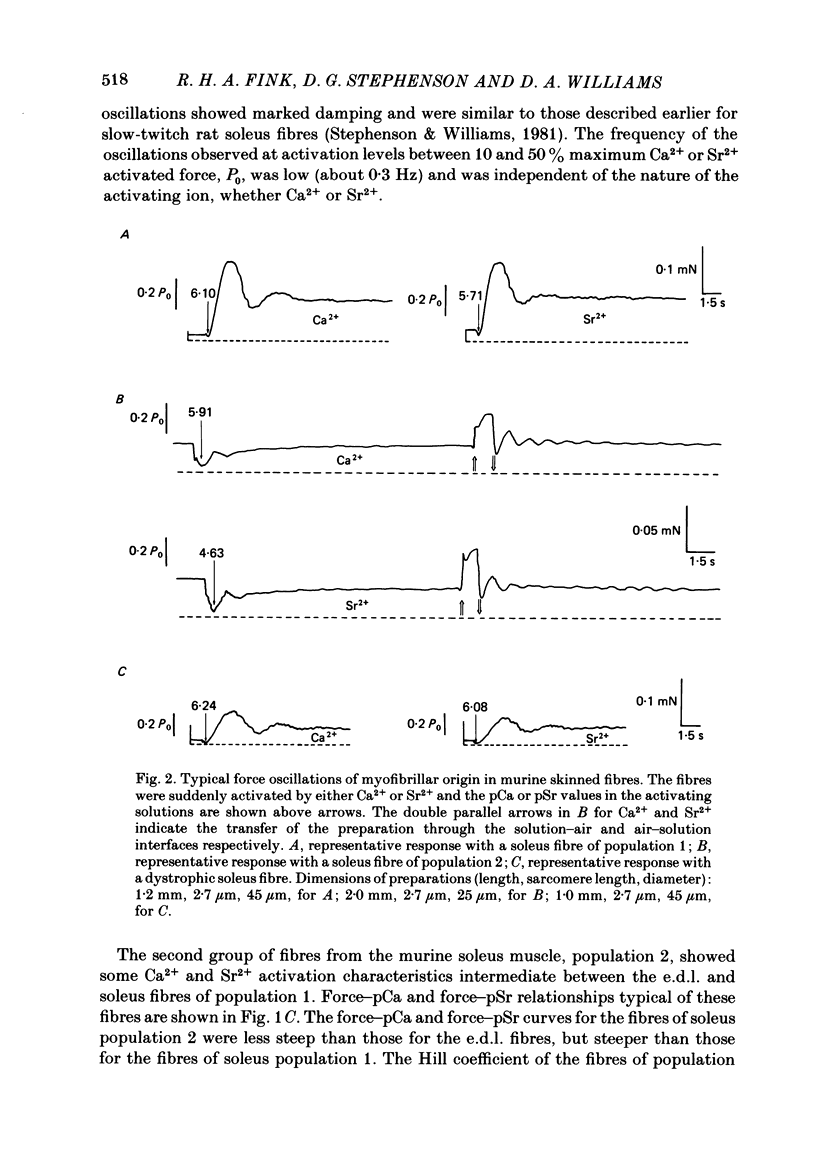
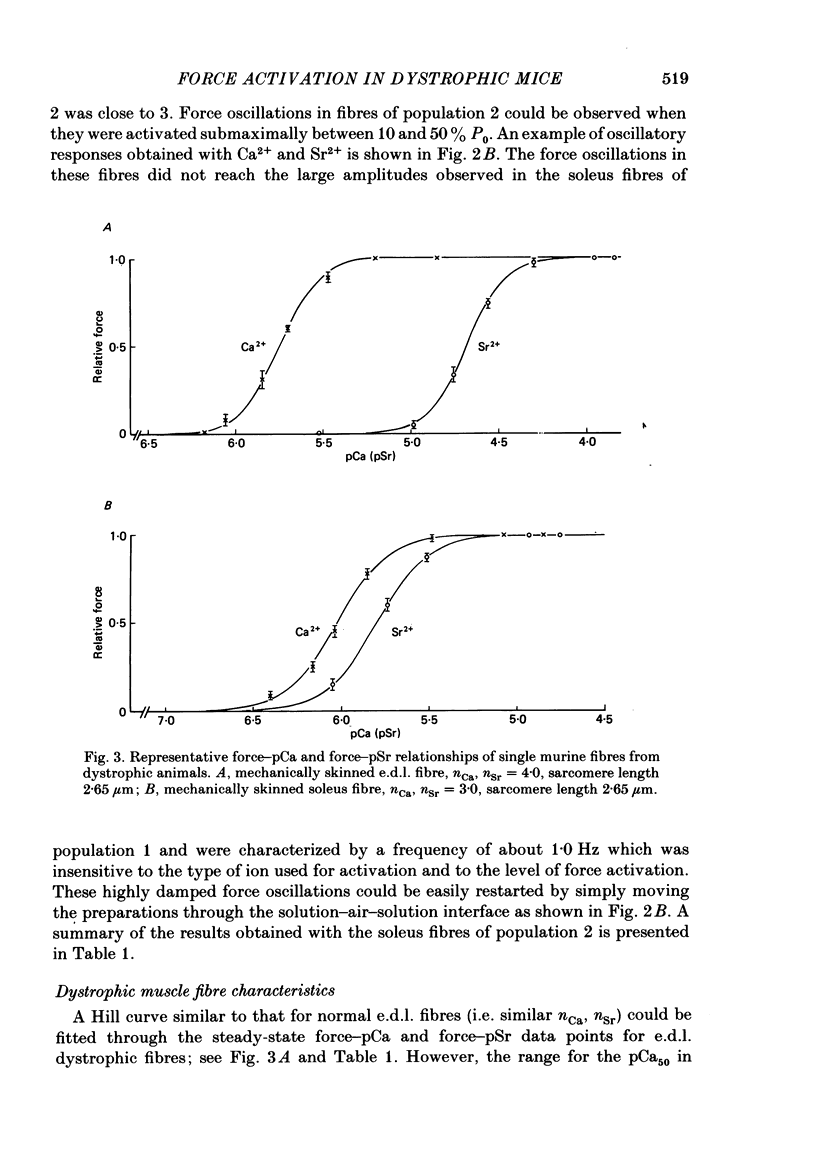
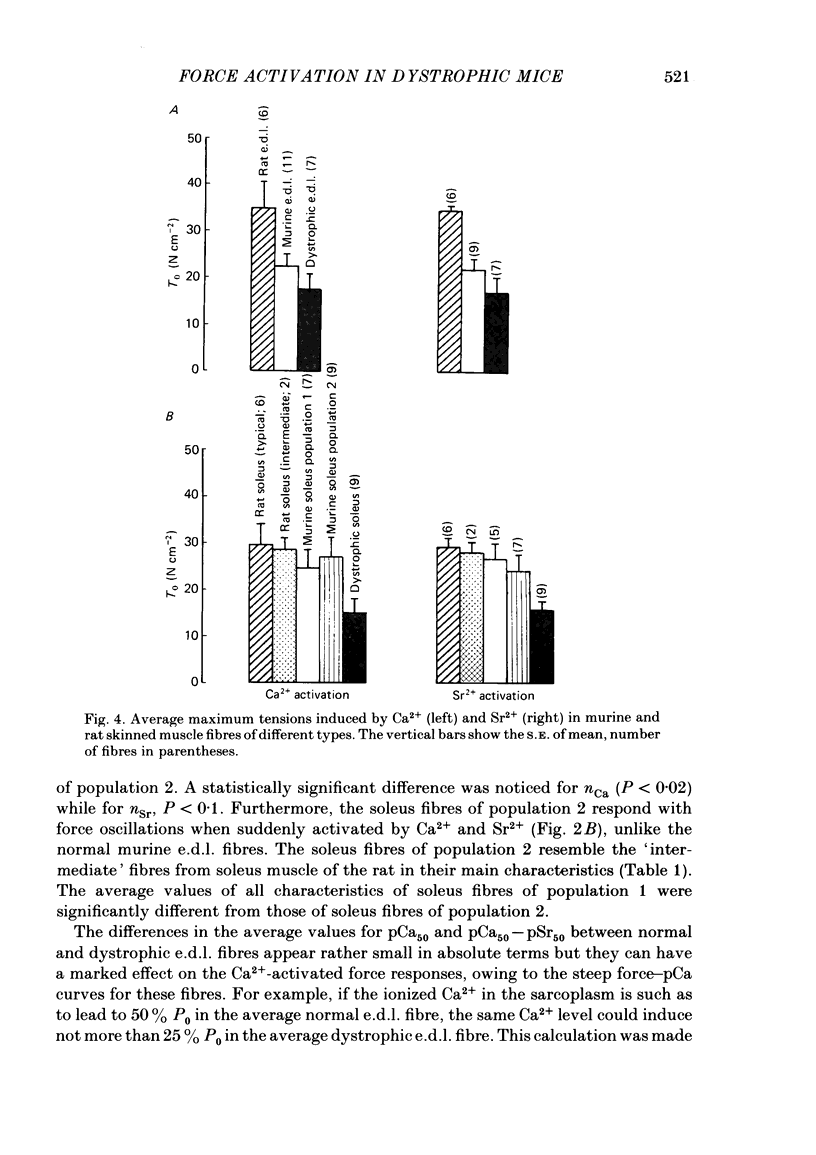
Differences in contractile activation by Ca2+ and Sr2+ between various types of normal and dystrophic murine muscle fibres were investigated using mechanically skinned fibres derived from soleus and extensor digitorum longus (e.d.l.) muscles of normal and dystrophic mice of strain 129ReJ. In terms of contractile activation, the normal e.d.l. muscle was found to consist of one relatively homogeneous population of muscle fibres characterized by steep force-pCa and force-pSr curves, low sensitivity to Ca2+ and very low sensitivity to Sr2+. Normal soleus muscles contained two fibre populations of similar size which could be distinguished on the basis of their contractile activation properties. The first fibre population was characterized mainly by its shallow force-pCa and force-pSr curves, high Ca2+ sensitivity, high Sr2+ sensitivity and the occurrence of large, slow force oscillations of myofibrillar origin. The second fibre population was characterized by force-pCa and force-pSr curves of steepness intermediate between those of normal e.d.l. and those of the first fibre population of normal soleus, by faster myofibrillar force oscillations and by low sensitivity to Ca2+ and Sr2+. The dystrophic e.d.l. fibre population had contractile characteristics which were distinct from those of the three types of normal fibre populations. However, some characteristics of the dystrophic e.d.l. fibres were very similar to those of the normal e.d.l. fibre population. Of all the fibre types investigated, dystrophic e.d.l. fibres were the least sensitive to Ca2+. Dystrophic soleus muscle contained a single homogeneous population of fibres which shared some common contractile activation characteristics with both of the fibre populations present in normal soleus muscle. However, of all fibre types investigated, the dystrophic soleus fibres were the most sensitive to Ca2+. Because of this characteristic, these fibres formed a distinct population. The maximum tensions induced by Ca2+ and Sr2+ were usually smaller in dystrophic fibres than in normal fibres obtained equivalent muscles. In conclusion, various normal murine muscle fibre types can be identified on the basis of differences in the mechanism of force activation by Ca2+ and Sr2+. Furthermore, it is possible to detect significant physiological differences in the mechanism of force activation brought about by murine muscular dystrophy.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Moisescu D. G. Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils. J Physiol. 1977 Sep;270(3):627–652. doi: 10.1113/jphysiol.1977.sp011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust M. Relative resistance to dystrophy of slow skeletal muscle of the mouse. Am J Physiol. 1966 Mar;210(3):445–451. doi: 10.1152/ajplegacy.1966.210.3.445. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Douglas W. B., Jr, Baskin R. J. Contractile properties of developing mouse dystrophic muscle. Am J Physiol. 1971 May;220(5):1344–1354. doi: 10.1152/ajplegacy.1971.220.5.1344. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells. J Gen Physiol. 1978 Nov;72(5):667–699. doi: 10.1085/jgp.72.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R. H., Stephenson D. G., Williams D. A. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. J Physiol. 1986 Jan;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons R. B., Hoh J. F. Myosin isoenzymes in fast-twitch and slow-twitch muscles of normal and dystrophic mice. J Physiol. 1983 Oct;343:539–550. doi: 10.1113/jphysiol.1983.sp014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Wilson P. Mechanical properties of dystrophic mouse muscle. J Neurol Neurosurg Psychiatry. 1971 Oct;34(5):512–520. doi: 10.1136/jnnp.34.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John H. A. Myofibrillar proteins of developing and dystrophic skeletal muscle. FEBS Lett. 1976 Apr 15;64(1):116–121. doi: 10.1016/0014-5793(76)80263-3. [DOI] [PubMed] [Google Scholar]
- John H. A. The myosin of developing and dystrophic skeletal muscle. FEBS Lett. 1974 Mar 1;39(3):278–282. doi: 10.1016/0014-5793(74)80130-4. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick W. G., Secrist D., Coby R., Lucas S. Development of difference between red and white muscles in sensitivity to Ca2+ in the rabbit from embryo to adult. Nature. 1976 Apr 1;260(5550):440–441. doi: 10.1038/260440a0. [DOI] [PubMed] [Google Scholar]
- Luff A. R. Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol. 1981;313:161–171. doi: 10.1113/jphysiol.1981.sp013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier H., Southard J. L. Muscular dystrophy in the mouse caused by an allele at the dy-locus. Life Sci. 1970 Feb 8;9(3):137–144. doi: 10.1016/0024-3205(70)90306-1. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Russell E. S., Harman P. J. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. J., Smith G. L. EGTA purity and the buffering of calcium ions in physiological solutions. Am J Physiol. 1984 Jan;246(1 Pt 1):C160–C166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G., Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978 Feb;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDOW A., BRUST M. Contractility of dystrophic mouse muscle. Am J Physiol. 1958 Sep;194(3):557–563. doi: 10.1152/ajplegacy.1958.194.3.557. [DOI] [PubMed] [Google Scholar]
- SANDOW A., BRUST M. Effects of activity on contractions of normal and dystrophic mouse muscles. Am J Physiol. 1962 May;202:815–820. doi: 10.1152/ajplegacy.1962.202.5.815. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Forrest Q. G. Different isometric force - [Ca2+] relationships in slow and fast twitch skinned muscle fibres of the rat. Biochim Biophys Acta. 1980 Feb 8;589(2):358–362. doi: 10.1016/0005-2728(80)90052-3. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol. 1982 Dec;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A., Endo M. Guinea pig soleus and extensor digitorum longus: a study on single-skimmed fibers. Exp Neurol. 1977 Apr;55(1):95–101. doi: 10.1016/0014-4886(77)90161-3. [DOI] [PubMed] [Google Scholar]



