Abstract
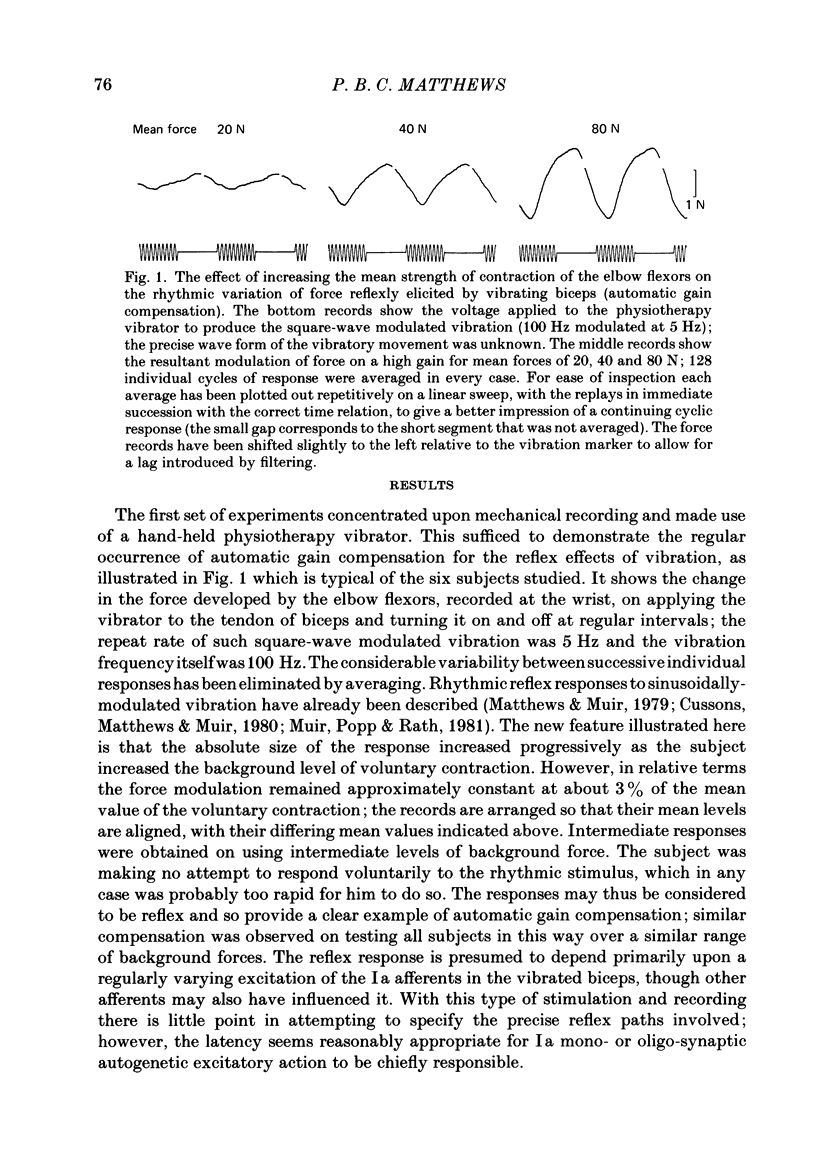
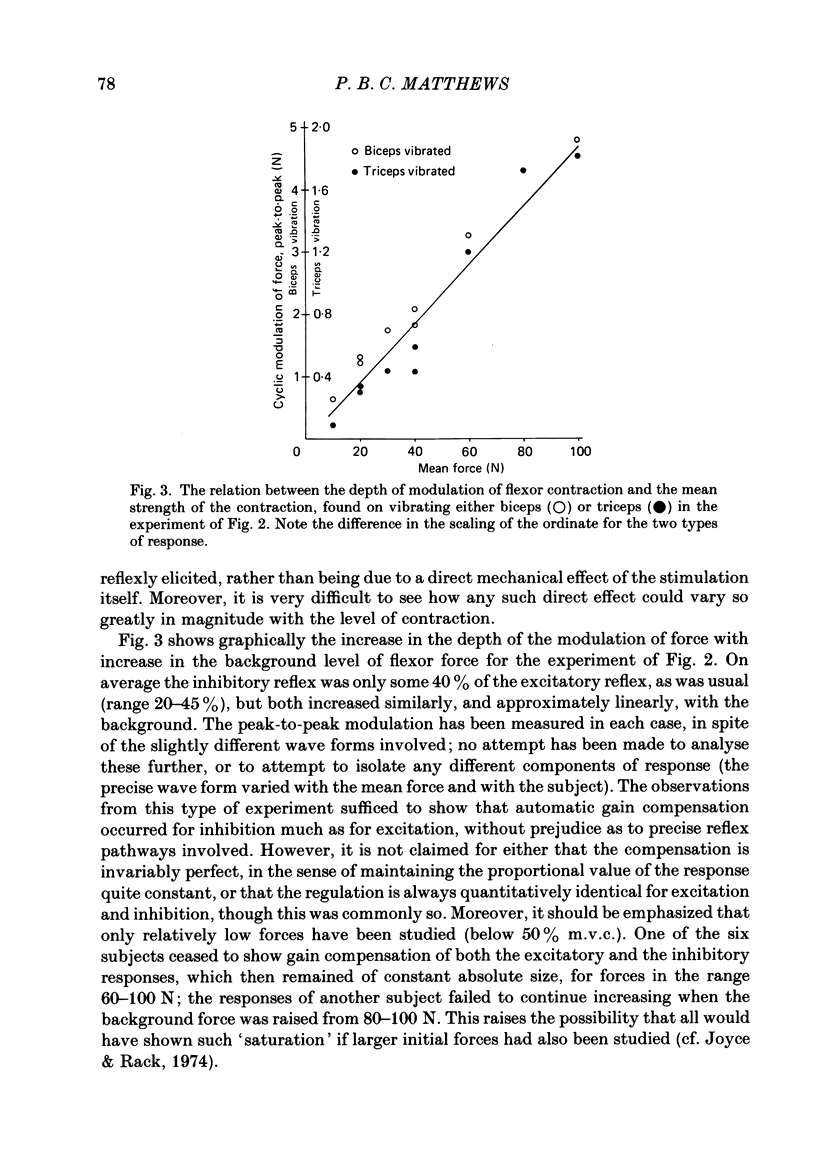
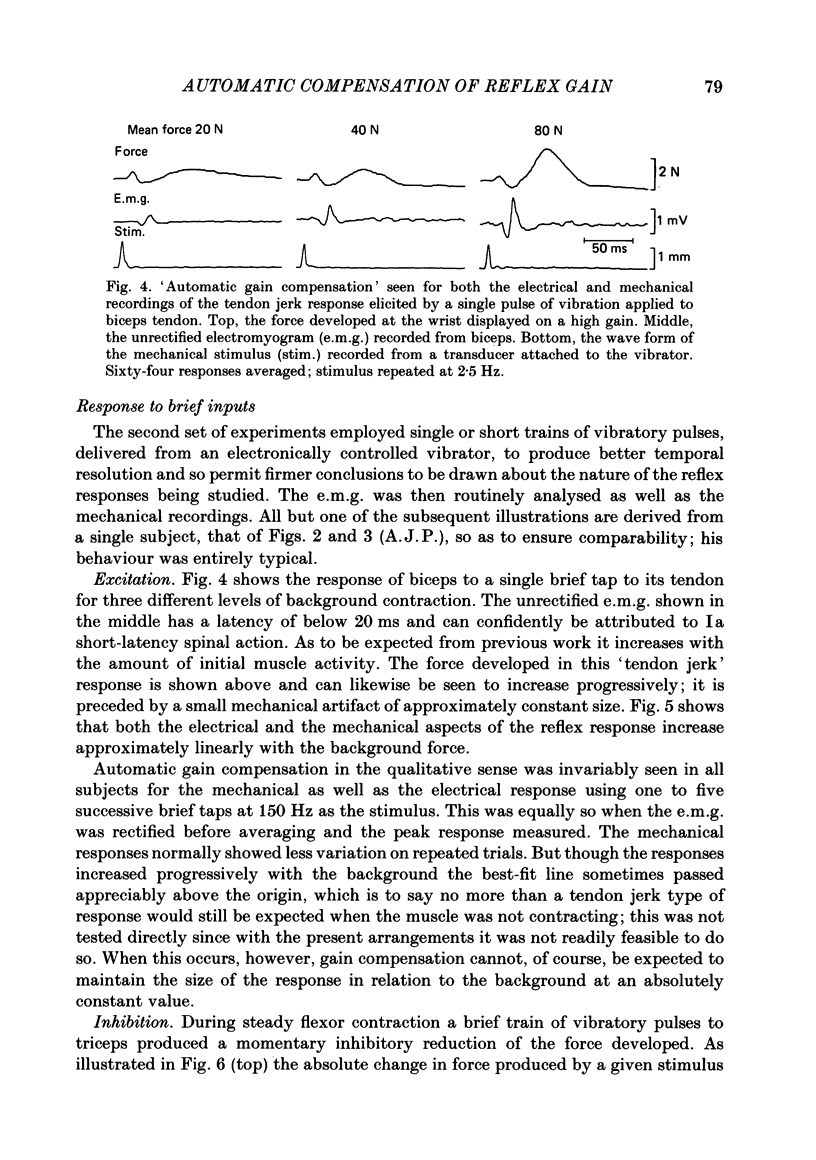
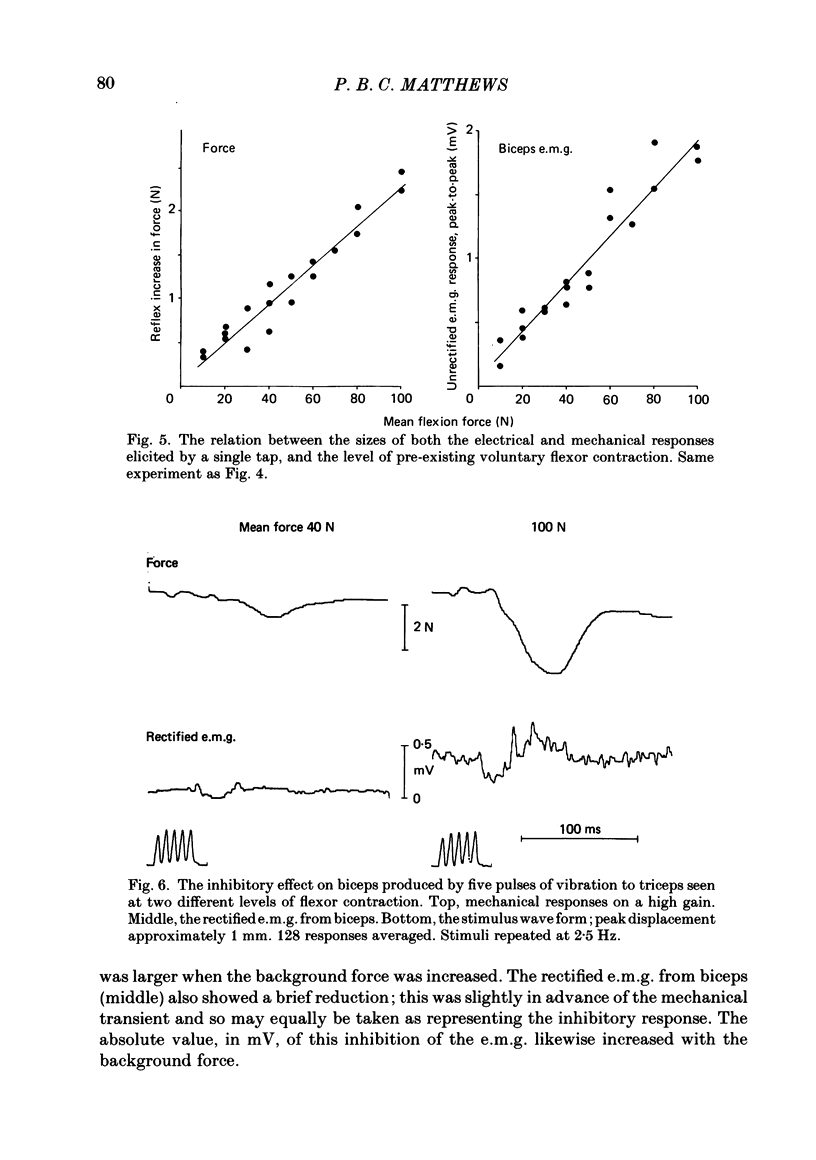
The human stretch reflex is well known to show 'automatic gain compensation'; in other words, the electromyographic (e.m.g.) response evoked by a given disturbance increases progressively with the level of pre-existing voluntary activity, and so remains an approximately constant proportion of the background. Such behaviour has now been observed using vibration as the stimulus to Ia action and recording the reflexly developed force, in addition to the e.m.g. Inhibition was studied as well as excitation by vibrating the antagonist as well as the agonist, and found to be similarly regulated. The experiments were performed on the elbow flexors while they were contracting isometrically under voluntary drive. The vibration was either square-wave modulated at 5 Hz or delivered in bursts of one to five pulses. The latency of the e.m.g. responses produced by the latter was sufficiently short to show that gain compensation was a feature of spinal reflex action. In the Discussion, it is concluded that in principle 'automatic gain compensation' can be readily attributed to the known organization of the motoneurone pool. As the background force increases so does the number of active motoneurones available to be frequency-modulated by a given input, and the larger and stronger will be those motor units which are on the verge of recruitment or de-recruitment.
Full text
PDF



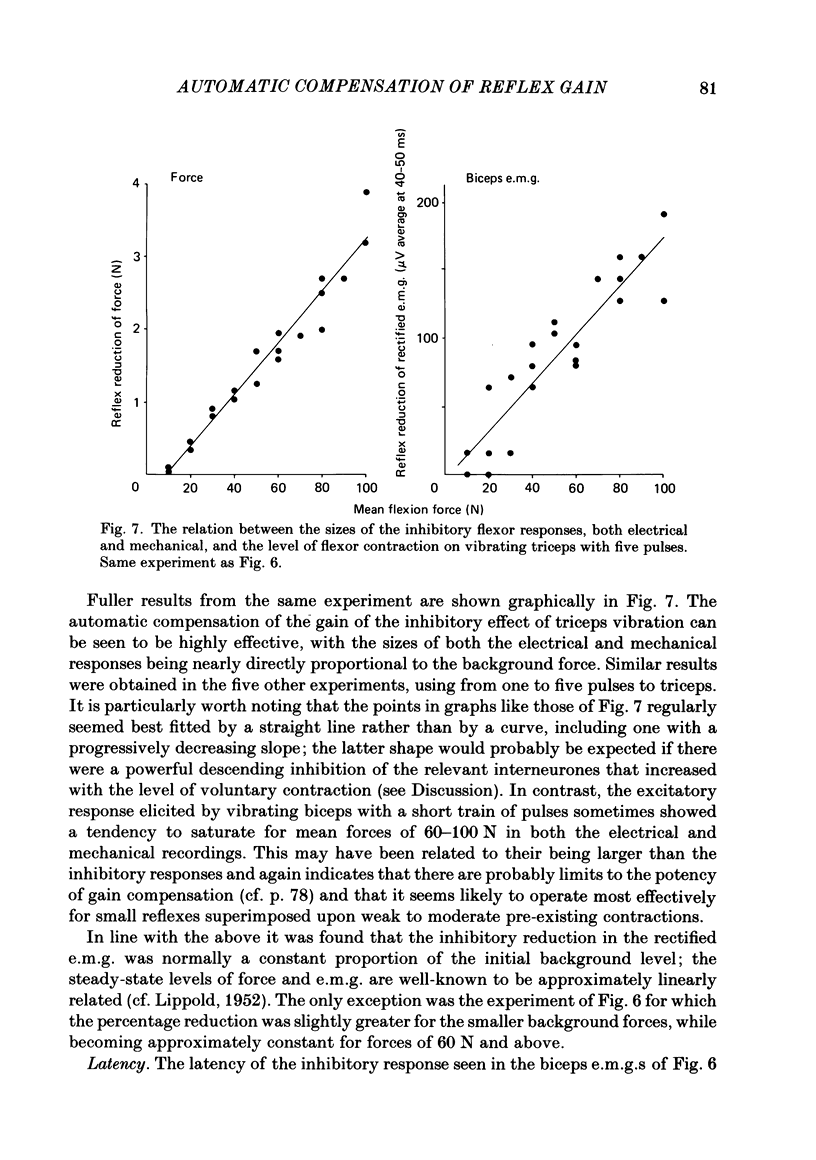
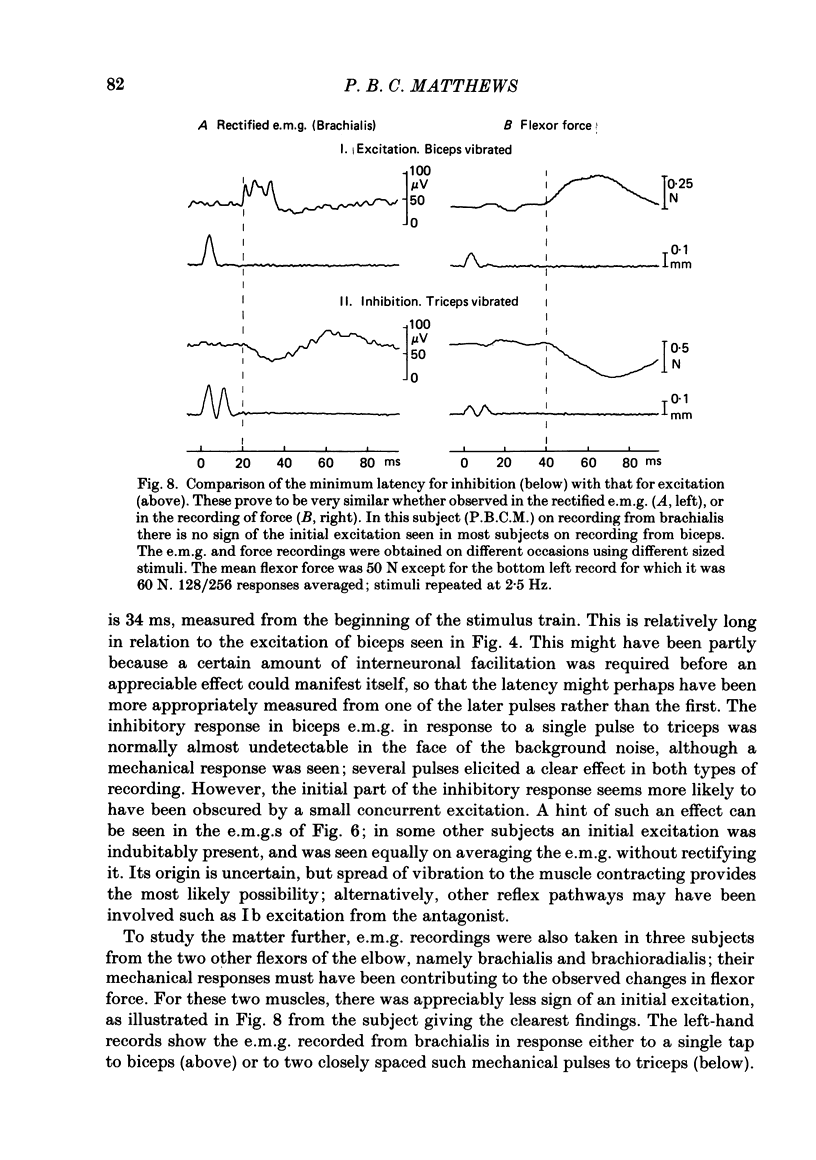








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke D., Hagbarth K. E., Löfstedt L. Muscle spindle responses in man to changes in load during accurate position maintenance. J Physiol. 1978 Mar;276:159–164. doi: 10.1113/jphysiol.1978.sp012225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. The activity of human muscle spindle endings in normal motor behavior. Int Rev Physiol. 1981;25:91–126. [PubMed] [Google Scholar]
- Burke R. E., Jankowska E., ten Bruggencate G. A comparison of peripheral and rubrospinal synaptic input to slow and fast twitch motor units of triceps surae. J Physiol. 1970 May;207(3):709–732. doi: 10.1113/jphysiol.1970.sp009090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Rymer W. Z. Relative strength of synaptic input from short-latency pathways to motor units of defined type in cat medial gastrocnemius. J Neurophysiol. 1976 May;39(3):447–458. doi: 10.1152/jn.1976.39.3.447. [DOI] [PubMed] [Google Scholar]
- Cooke J. D., Eastman M. J. Long-loop reflexes in the tranquilized monkey. Exp Brain Res. 1977 Apr 21;27(5):491–500. doi: 10.1007/BF00239038. [DOI] [PubMed] [Google Scholar]
- Cussons P. D., Matthews P. B., Muir R. B. Enhancement by agonist or antagonist muscle vibration of tremor at the elastically loaded human elbow. J Physiol. 1980 May;302:443–461. doi: 10.1113/jphysiol.1980.sp013255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Stephens J. A. The effects of digital nerve stimulation on the firing of motor units in human first dorsal interosseous muscle. J Physiol. 1981 Sep;318:501–510. doi: 10.1113/jphysiol.1981.sp013880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Marsden C. D., Obeso J. A., Rothwell J. C. Reciprocal inhibition between the muscles of the human forearm. J Physiol. 1984 Apr;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C. J., LeFever R. S., McCue M. P., Xenakis A. P. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol. 1982 Aug;329:113–128. doi: 10.1113/jphysiol.1982.sp014293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Effects of initial conditions on the Hoffman reflex. J Neurol Neurosurg Psychiatry. 1971 Jun;34(3):226–230. doi: 10.1136/jnnp.34.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C., Stark L. Interactions between voluntary and postural mechanisms of thehuman motor system. J Neurophysiol. 1970 May;33(3):365–381. doi: 10.1152/jn.1970.33.3.365. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- Harrison P. J., Taylor A. Individual excitatory post-synaptic potentials due to muscle spindle Ia afferents in cat triceps surae motoneurones. J Physiol. 1981 Mar;312:455–470. doi: 10.1113/jphysiol.1981.sp013638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J. F. Responses in human pretibial muscles to sudden stretch and to nerve stimulation. Exp Brain Res. 1977 Dec 19;30(4):451–470. doi: 10.1007/BF00237637. [DOI] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M., Ross H. F. The forces generated at the human elbow joint in response to imposed sinusoidal movements of the forearm. J Physiol. 1974 Jul;240(2):351–374. doi: 10.1113/jphysiol.1974.sp010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce G. C., Rack P. M. The effects of load and force on tremor at the normal human elbow joint. J Physiol. 1974 Jul;240(2):375–396. doi: 10.1113/jphysiol.1974.sp010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPPOLD O. C. J. The relation between integrated action potentials in a human muscle and its isometric tension. J Physiol. 1952 Aug;117(4):492–499. doi: 10.1113/jphysiol.1952.sp004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in the human thumb. J Physiol. 1976 May;257(1):1–44. doi: 10.1113/jphysiol.1976.sp011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Evidence from the use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984 Mar;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Muir R. B. Comparison of electromyogram spectra with force spectra during human elbow tremor. J Physiol. 1980 May;302:427–441. doi: 10.1113/jphysiol.1980.sp013254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. Observations on the time course of the electromyographic response reflexly elicited by muscle vibration in man. J Physiol. 1984 Aug;353:447–461. doi: 10.1113/jphysiol.1984.sp015346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Watson J. D. Effect of vibrating agonist or antagonist muscle of the reflex response to sinusoidal displacement of the human forearm. J Physiol. 1981 Dec;321:297–316. doi: 10.1113/jphysiol.1981.sp013985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton P. A., Hill D. K., Morton H. B., Marsden C. D. Scope of a technique for electrical stimulation of human brain, spinal cord, and muscle. Lancet. 1982 Sep 11;2(8298):597–600. doi: 10.1016/s0140-6736(82)90670-5. [DOI] [PubMed] [Google Scholar]
- Nemeth P., Pette D. Succinate dehydrogenase activity in fibres classified by myosin ATPase in three hind limb muscles of rat. J Physiol. 1981 Nov;320:73–80. doi: 10.1113/jphysiol.1981.sp013935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter M. J., Curtis R. L., Hosko M. J. Voltage threshold and excitability among variously sized cat hindlimb motoneurons. J Neurophysiol. 1983 Sep;50(3):644–657. doi: 10.1152/jn.1983.50.3.644. [DOI] [PubMed] [Google Scholar]
- Shindo M., Harayama H., Kondo K., Yanagisawa N., Tanaka R. Changes in reciprocal Ia inhibition during voluntary contraction in man. Exp Brain Res. 1984;53(2):400–408. doi: 10.1007/BF00238170. [DOI] [PubMed] [Google Scholar]
- Stein R. B. Peripheral control of movement. Physiol Rev. 1974 Jan;54(1):215–243. doi: 10.1152/physrev.1974.54.1.215. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res. 1974;21(5):529–540. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]
- Traub M. M., Rothwell J. C., Marsden C. D. A grab reflex in the human hand. Brain. 1980 Dec;103(4):869–884. doi: 10.1093/brain/103.4.869. [DOI] [PubMed] [Google Scholar]


