Abstract
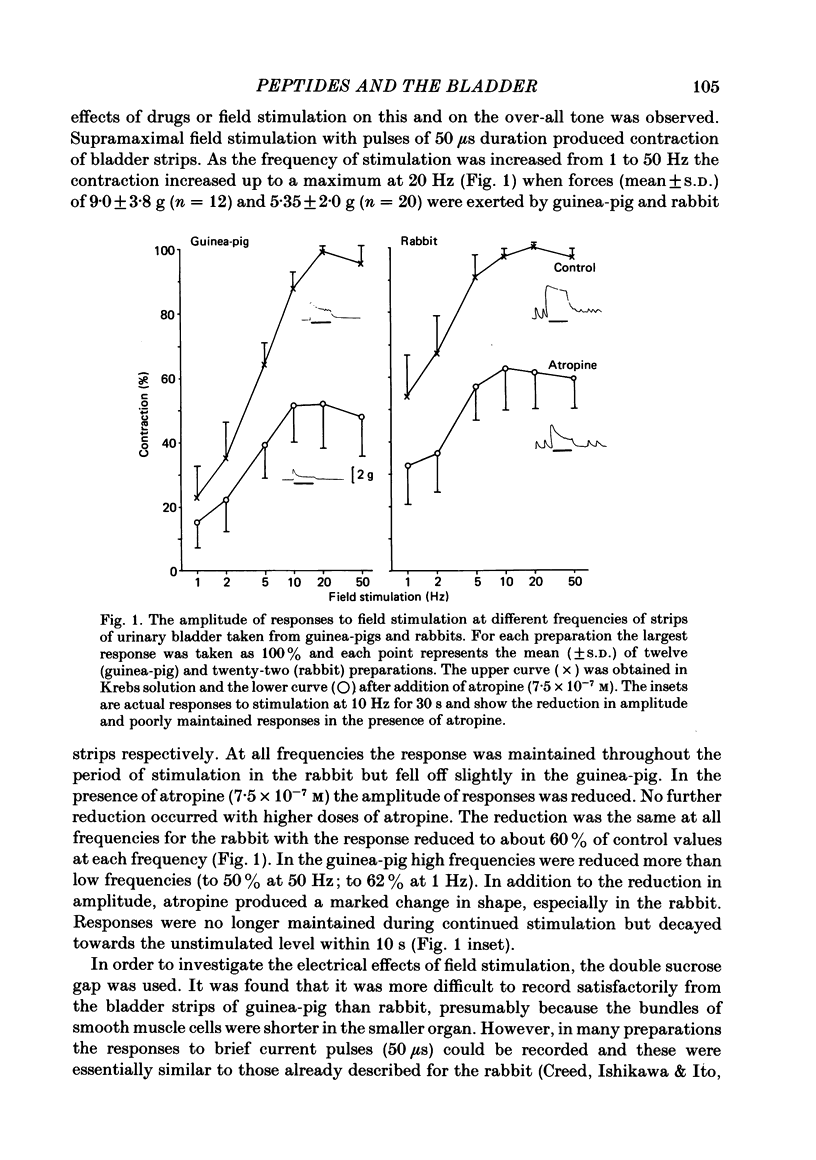
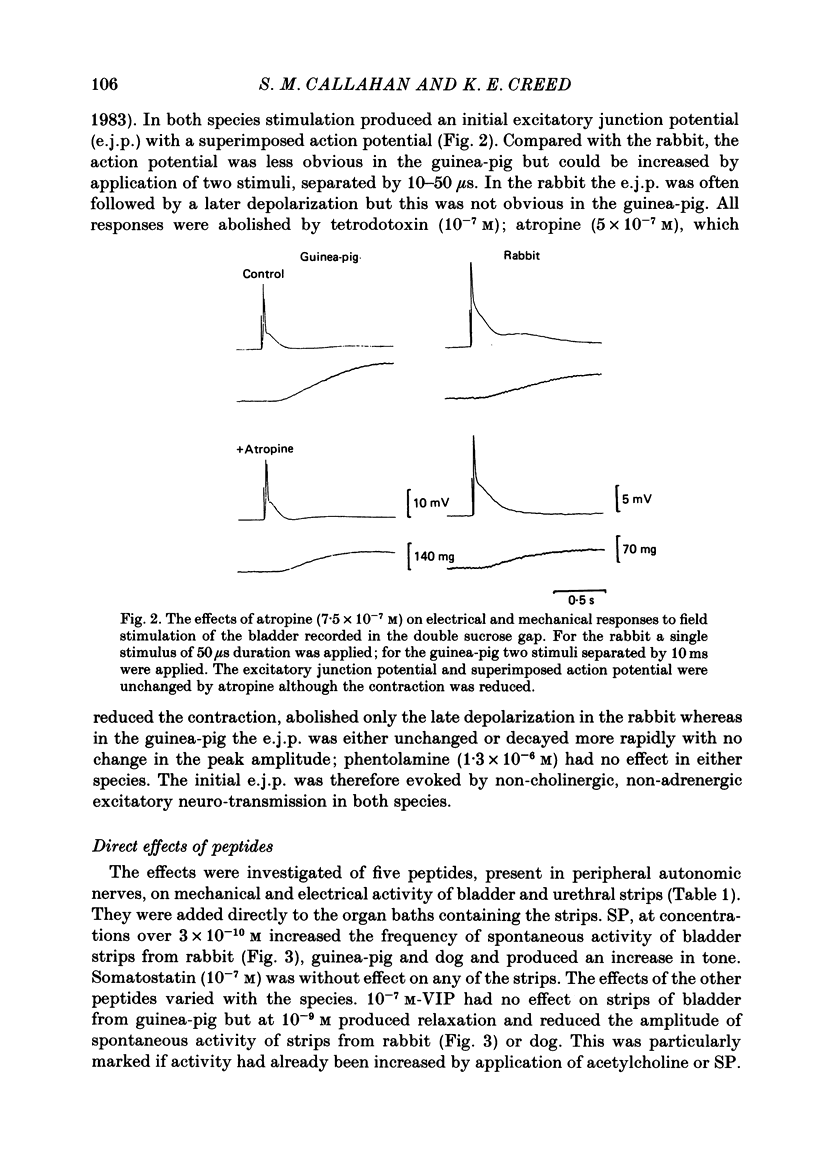
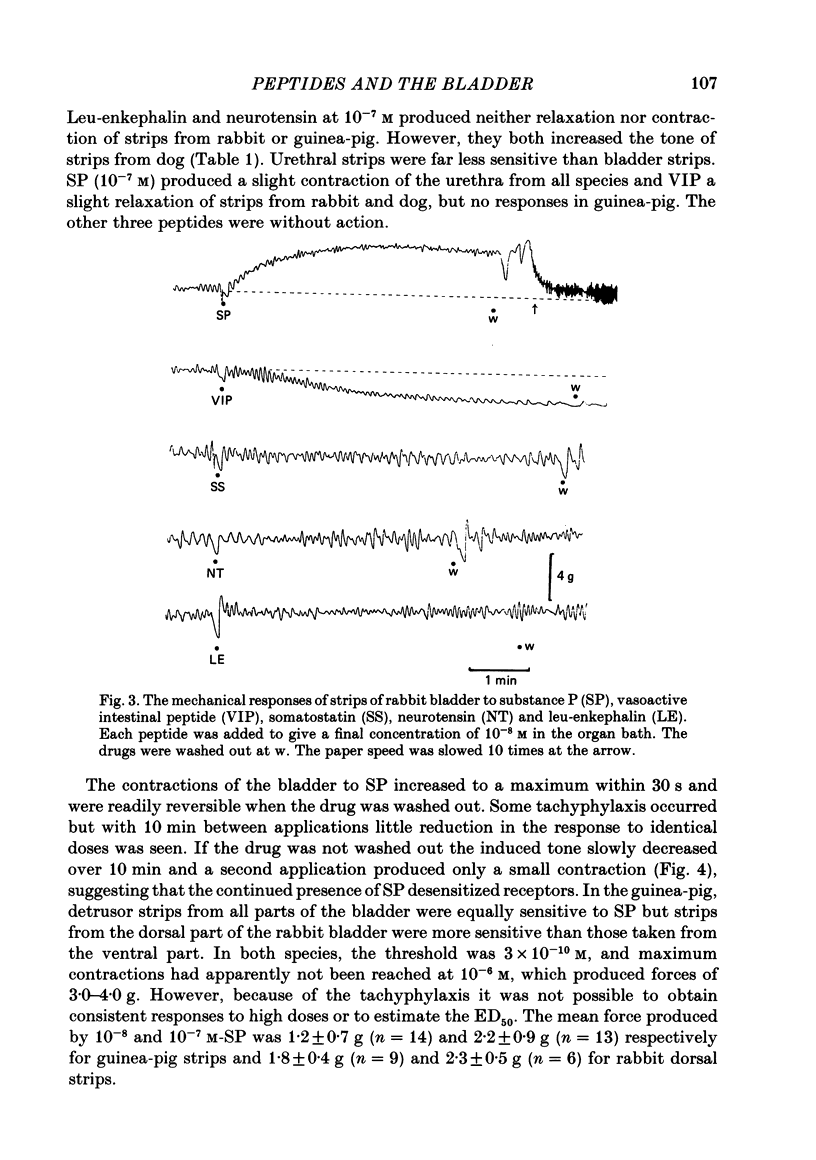
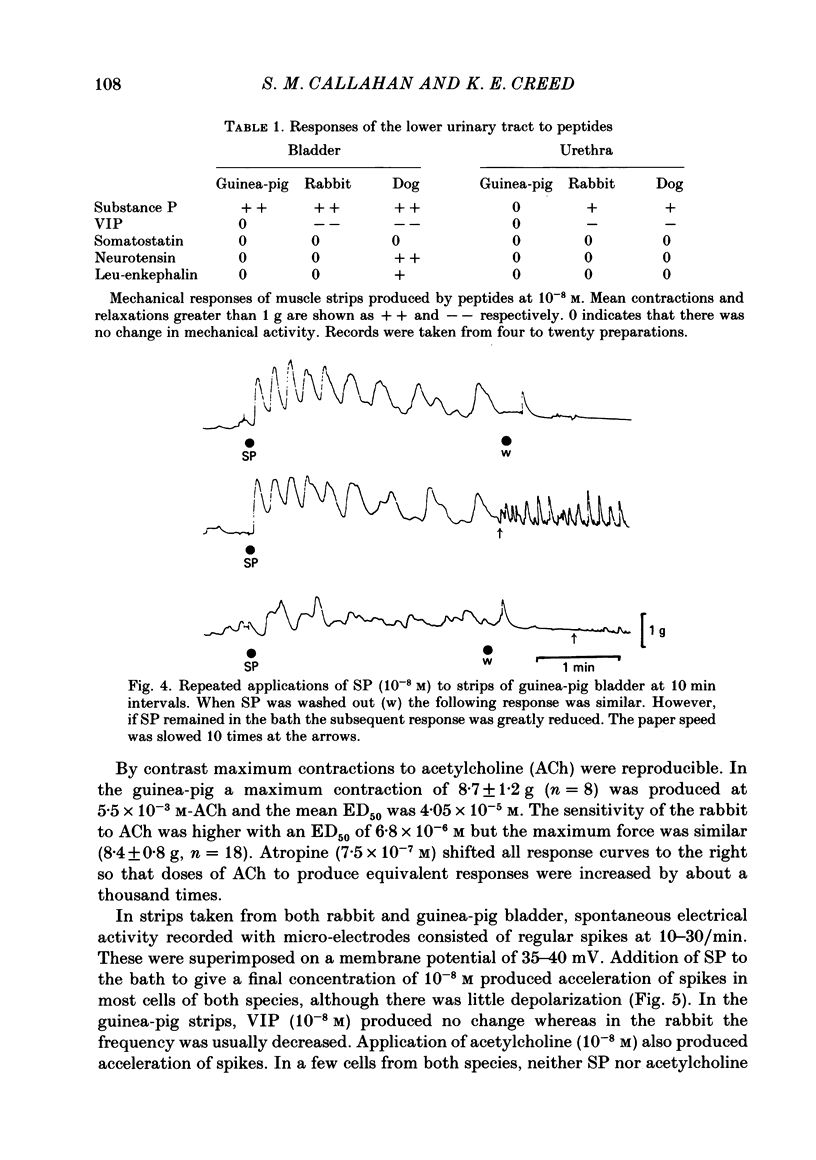
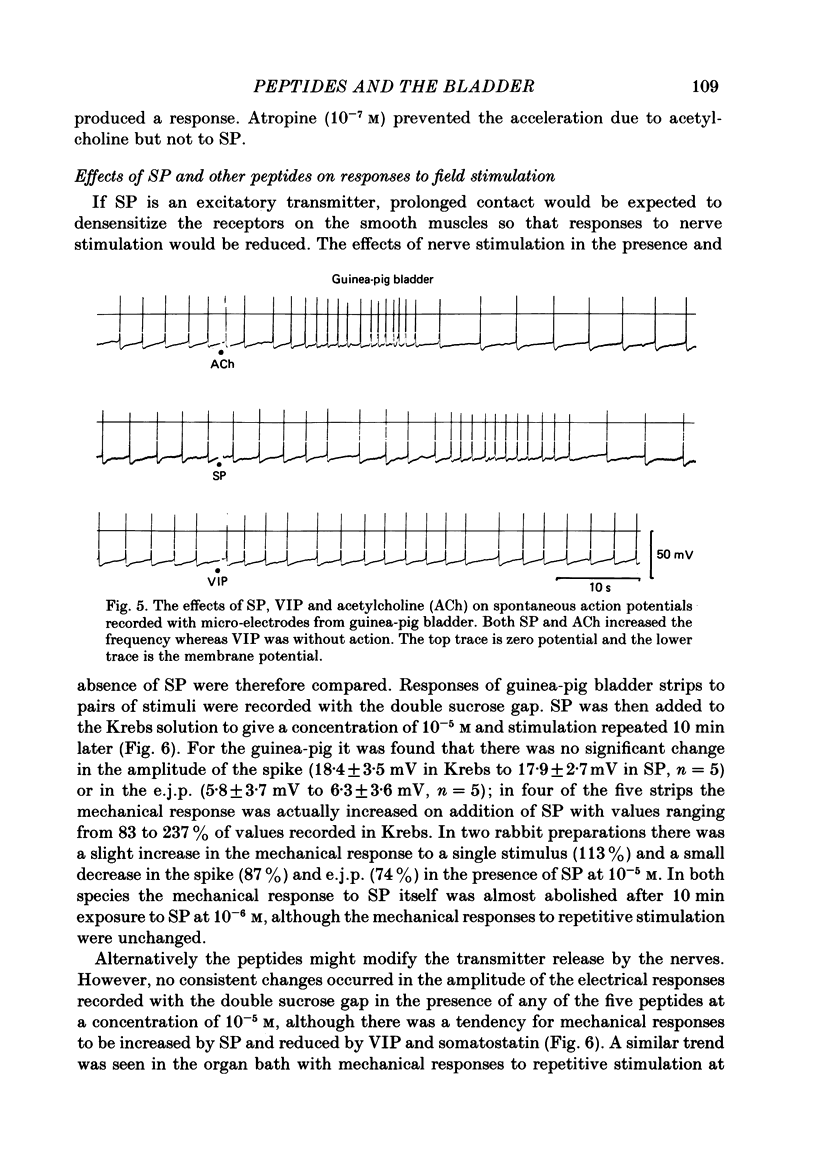
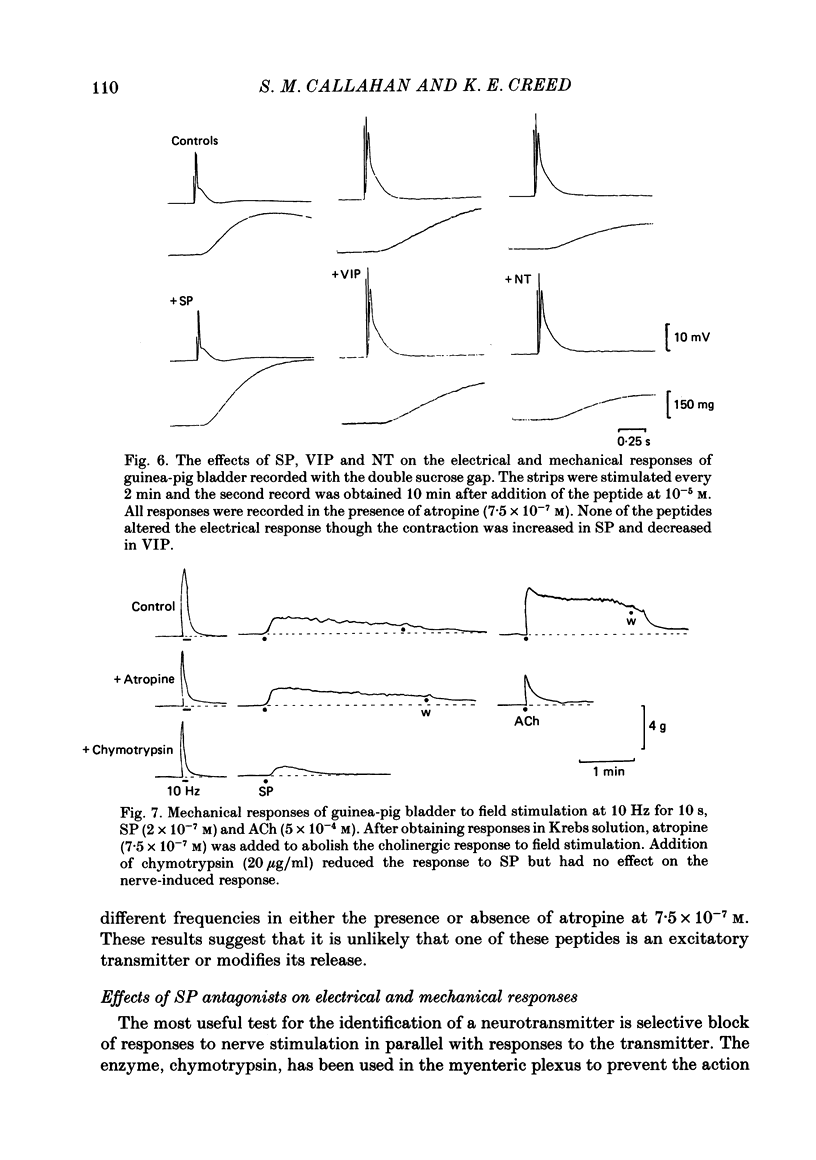
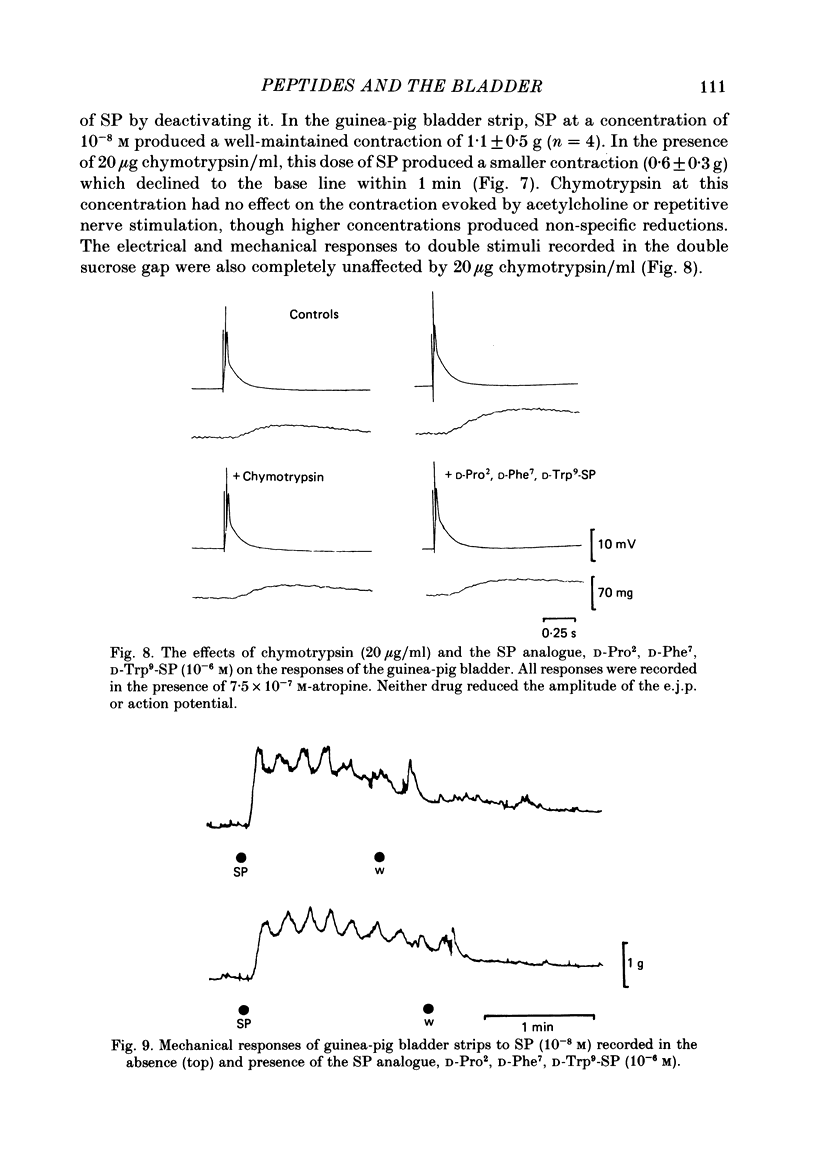
Supramaximal repetitive field stimulation with pulses of 50 microseconds produced contraction of strips of bladder from rabbits and guinea-pigs. Atropine reduced responses at all frequencies to about 60% and the contraction was poorly maintained. With the double sucrose-gap technique large excitatory junction potentials (e.j.p.s) were recorded with superimposed action potentials. These were not reduced by atropine or phentolamine. Substance P (SP) produced contraction and increased the frequency of spontaneous action potentials recorded with micro-electrodes from bladder strips. Vasoactive intestinal peptide (VIP) produced relaxation and slowed action potentials in rabbit but had no effect in guinea-pig; neurotensin, somatostatin and leu-enkephalin were without action in either species. When the tissue was kept in contact with SP, a second application after 10 min produced only a small contraction suggesting that SP receptors were desensitized. However, the electrical response to field stimulation was unchanged and the mechanical response was increased. Chymotrypsin reduced mechanical responses to SP but had no effect on responses to field stimulation. The SP analogue, D-Pro2, D-Phe7, D-Trp9-SP, had no effect on responses to SP or to field stimulation. It is concluded that the bladder receives excitatory non-cholinergic innervation which is responsible for a large excitatory junction potential and contraction. Although SP can contract the detrusor muscle, it is unlikely that it is an excitatory transmitter or that any of the five peptides act as modulators of transmitter release.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm P., Alumets J., Brodin E., Håkanson R., Nilsson G., Sjöberg N. O., Sundler F. Peptidergic (substance P) nerves in the genito-urinary tract. Neuroscience. 1978;3(4-5):419–425. doi: 10.1016/0306-4522(78)90044-1. [DOI] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol. 1970 Oct;210(3):761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Callahan S. M., Creed K. E. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br J Pharmacol. 1981 Oct;74(2):353–358. doi: 10.1111/j.1476-5381.1981.tb09978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E. Effects of ions and drugs on the smooth muscle cell membrane of the guinea-pig urinary bladder. Pflugers Arch. 1971;326(2):127–141. doi: 10.1007/BF00586905. [DOI] [PubMed] [Google Scholar]
- Creed K. E., Ishikawa S., Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol. 1983 May;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. M., Downie J. W. Contribution of adrenergic and "purinergic" neurotransmission to contraction in rabbit detrusor. J Pharmacol Exp Ther. 1978 Nov;207(2):431–445. [PubMed] [Google Scholar]
- Downie J. W., Dean D. M. The contribution of cholinergic postganglionic neurotransmission to contractions of rabbit detrusor. J Pharmacol Exp Ther. 1977 Nov;203(2):417–425. [PubMed] [Google Scholar]
- Finkbeiner A. E. In vitro effects of vasoactive intestinal polypeptide on guinea pig urinary bladder. Urology. 1983 Sep;22(3):275–277. doi: 10.1016/s0090-4295(83)80013-2. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- Gosling J. A., Dixon J. S. The structure and innervation of smooth muscle in the wall of the bladder neck and proximal urethra. Br J Urol. 1975 Oct;47(5):549–558. doi: 10.1111/j.1464-410x.1975.tb06260.x. [DOI] [PubMed] [Google Scholar]
- Hourani S. M. Desensitization of the guinea-pig urinary bladder by the enantiomers of adenylyl 5'-(beta, gamma-methylene)-diphosphonate and by substance P. Br J Pharmacol. 1984 May;82(1):161–164. doi: 10.1111/j.1476-5381.1984.tb16454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted S., Sjögren C., Andersson K. E. Substance P and somatostatin and excitatory neurotransmission in rabbit urinary bladder. Arch Int Pharmacodyn Ther. 1981 Jul;252(1):72–85. [PubMed] [Google Scholar]
- Hökfelt T., Schultzberg M., Elde R., Nilsson G., Terenius L., Said S., Goldstein M. Peptide neurons in peripheral tissues including the urinary tract: immunohistochemical studies. Acta Pharmacol Toxicol (Copenh) 1978;43 (Suppl 2):79–89. doi: 10.1111/j.1600-0773.1978.tb03224.x. [DOI] [PubMed] [Google Scholar]
- Johns A. The effect of vasoactive intestinal polypeptide on the urinary bladder and taenia coli of the guinea pig. Can J Physiol Pharmacol. 1979 Jan;57(1):106–108. doi: 10.1139/y79-015. [DOI] [PubMed] [Google Scholar]
- Klarskov P., Gerstenberg T., Hald T. Vasoactive intestinal polypeptide influence on lower urinary tract smooth muscle from human and pig. J Urol. 1984 May;131(5):1000–1004. doi: 10.1016/s0022-5347(17)50748-x. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Wein A. J. Effect of vasoactive intestinal peptide on the contractility of the rabbit urinary bladder. Urol Res. 1981;9(5):217–218. doi: 10.1007/BF00256889. [DOI] [PubMed] [Google Scholar]
- Longhurst P. A., Belis J. A., O'Donnell J. P., Galie J. R., Westfall D. P. A study of the atropine-resistant component of the neurogenic response of the rabbit urinary bladder. Eur J Pharmacol. 1984 Apr 6;99(4):295–302. doi: 10.1016/0014-2999(84)90136-5. [DOI] [PubMed] [Google Scholar]
- Mackenzie I., Burnstock G. Neuropeptide action on the guinea-pig bladder; a comparison with the effects of field stimulation and ATP. Eur J Pharmacol. 1984 Oct 1;105(1-2):85–94. doi: 10.1016/0014-2999(84)90651-4. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Meli A. The effects of topical capsaicin on rat urinary bladder motility in vivo. Eur J Pharmacol. 1984 Aug 3;103(1-2):41–50. doi: 10.1016/0014-2999(84)90187-0. [DOI] [PubMed] [Google Scholar]
- North R. A. Electrophysiology of the enteric nervous system. Neuroscience. 1982 Feb;7(2):315–325. doi: 10.1016/0306-4522(82)90269-x. [DOI] [PubMed] [Google Scholar]
- Sibley G. N. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984 Sep;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren C., Andersson K. E., Husted S. Contractile effects of some polypeptides on the isolated urinary bladder of guinea-pig, rabbit, and rat. Acta Pharmacol Toxicol (Copenh) 1982 Mar;50(3):175–184. doi: 10.1111/j.1600-0773.1982.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Sjögren C., Andersson K. E., Husted S., Mattiasson A., Moller-Madsen B. Atropine resistance of transmurally stimulated isolated human bladder muscle. J Urol. 1982 Dec;128(6):1368–1371. doi: 10.1016/s0022-5347(17)53509-0. [DOI] [PubMed] [Google Scholar]


