Abstract
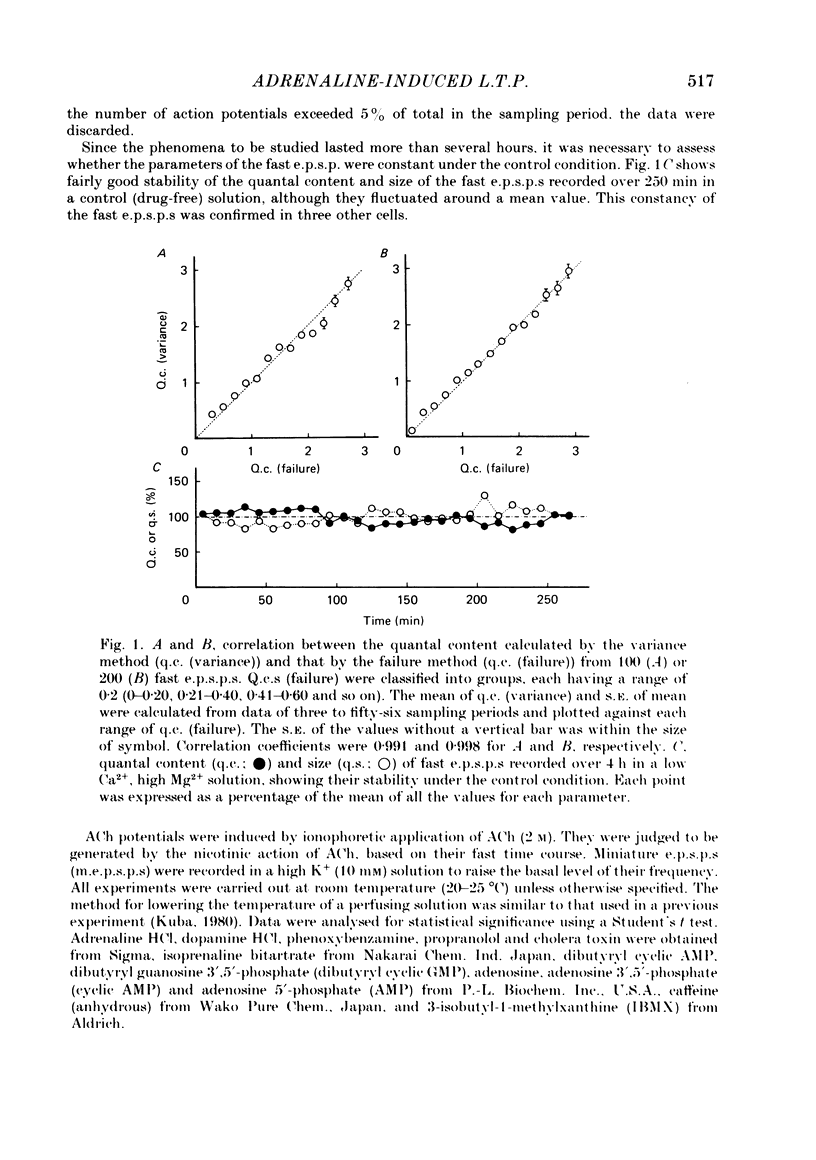
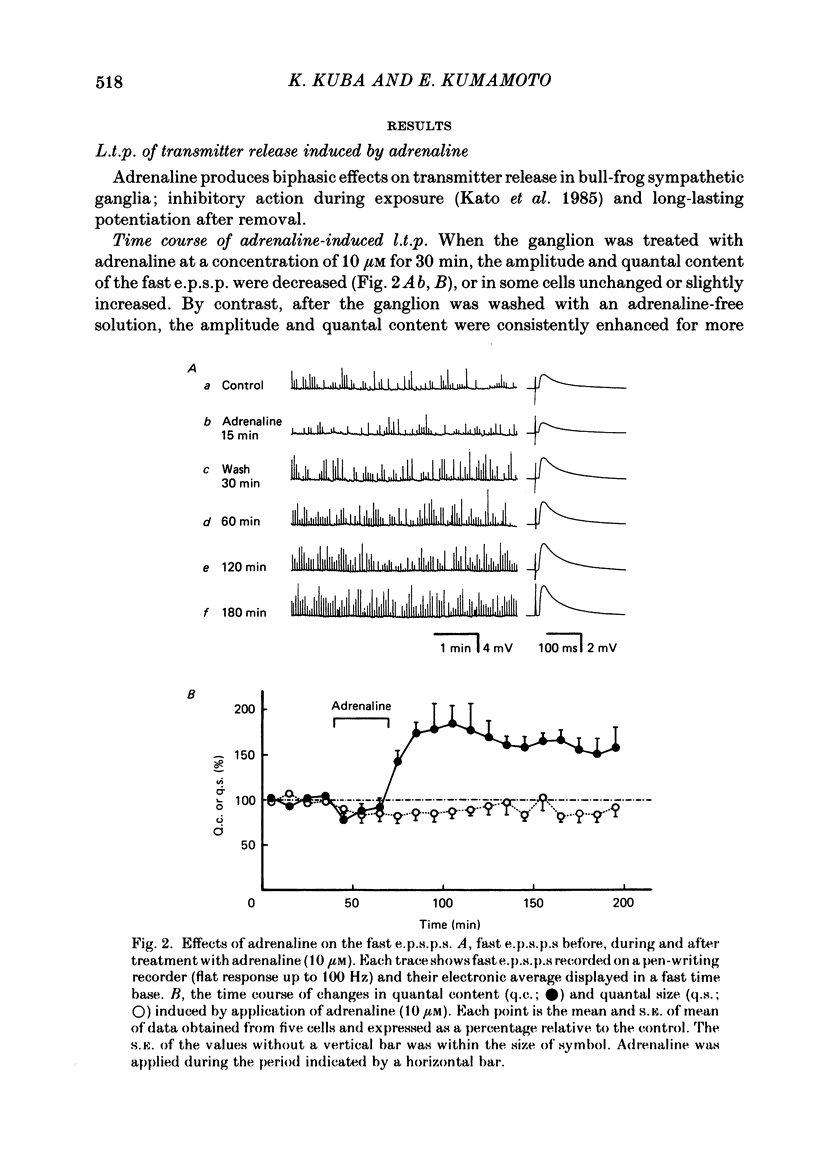
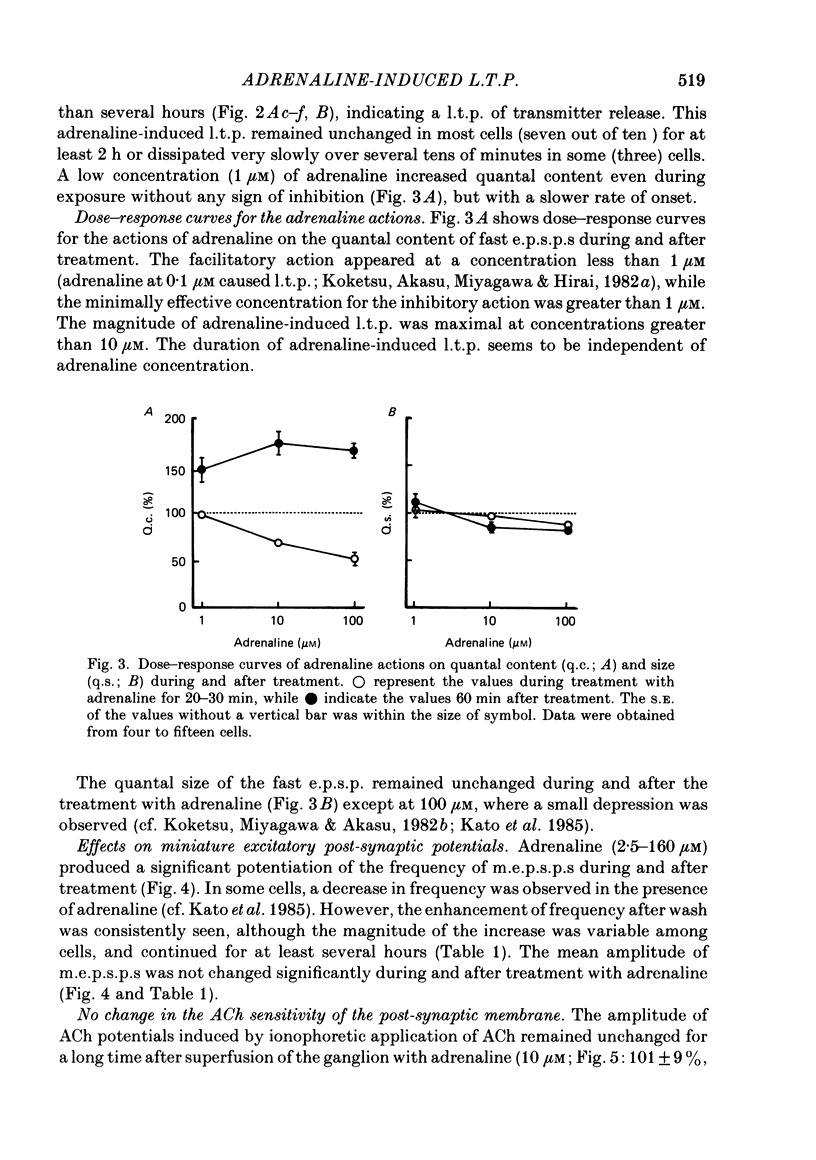
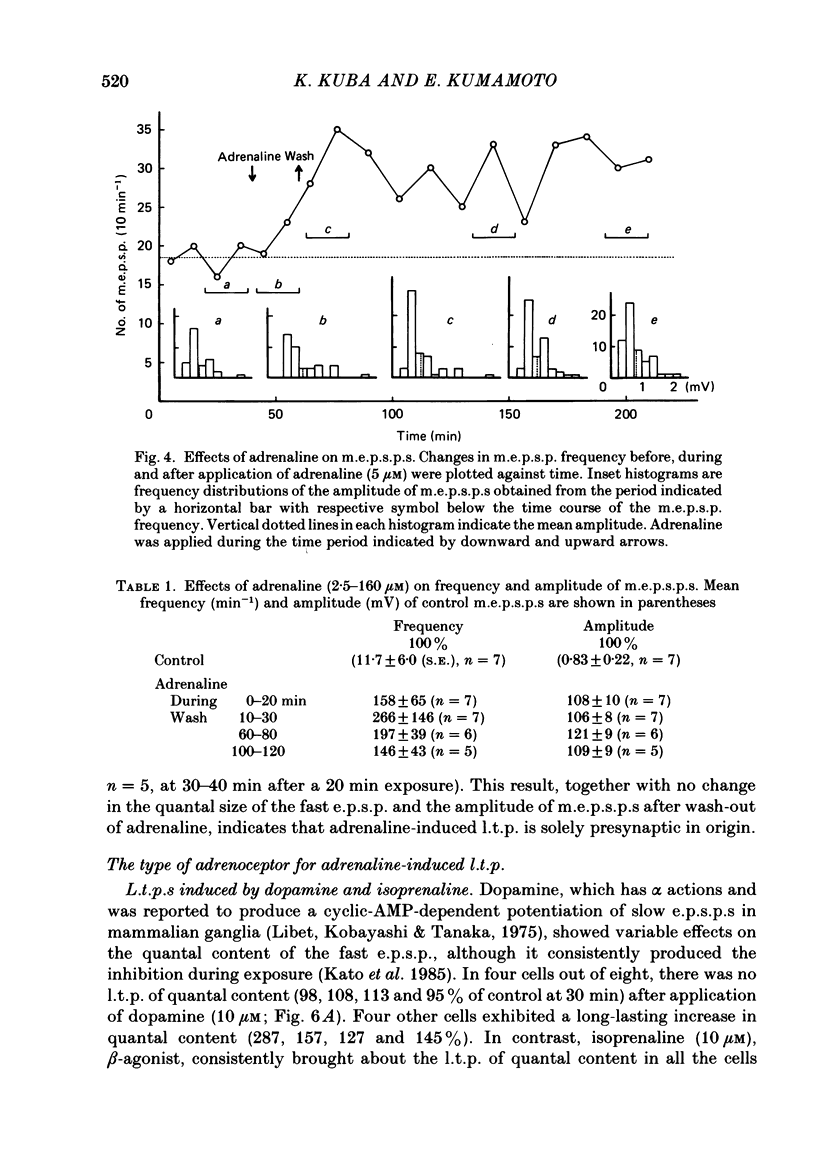
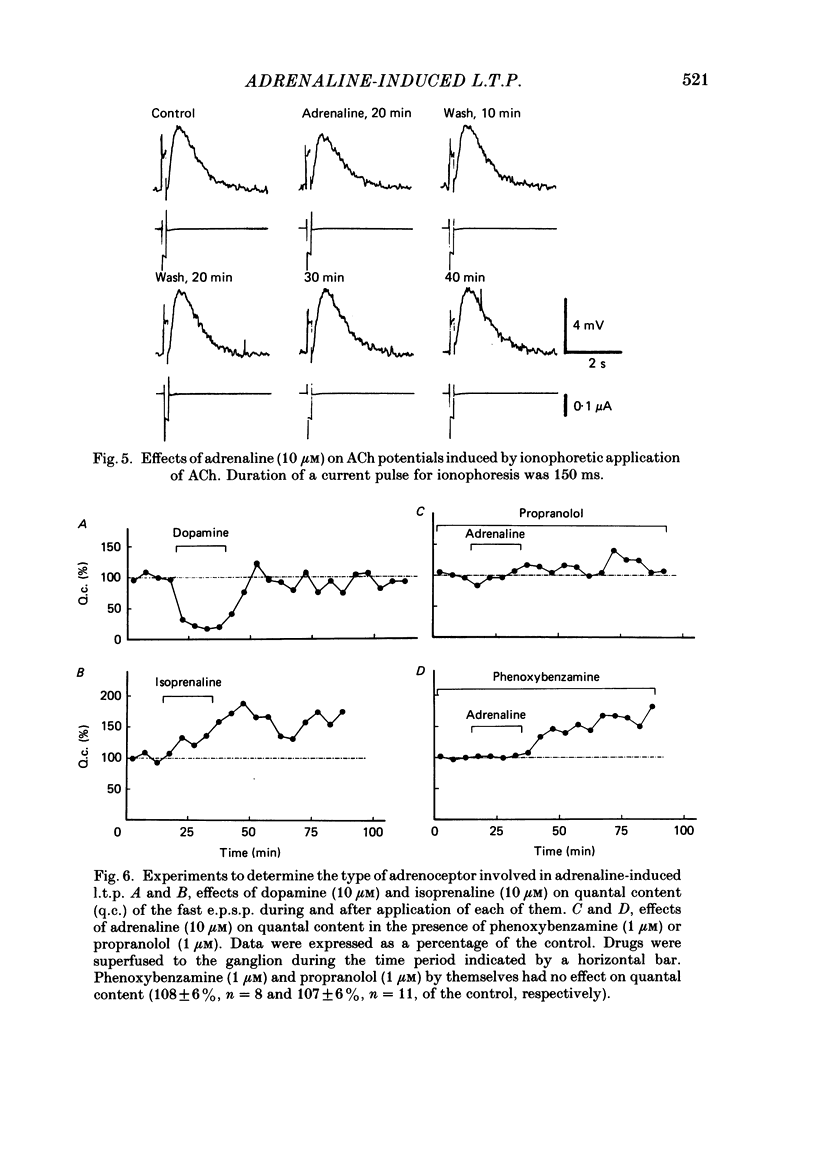
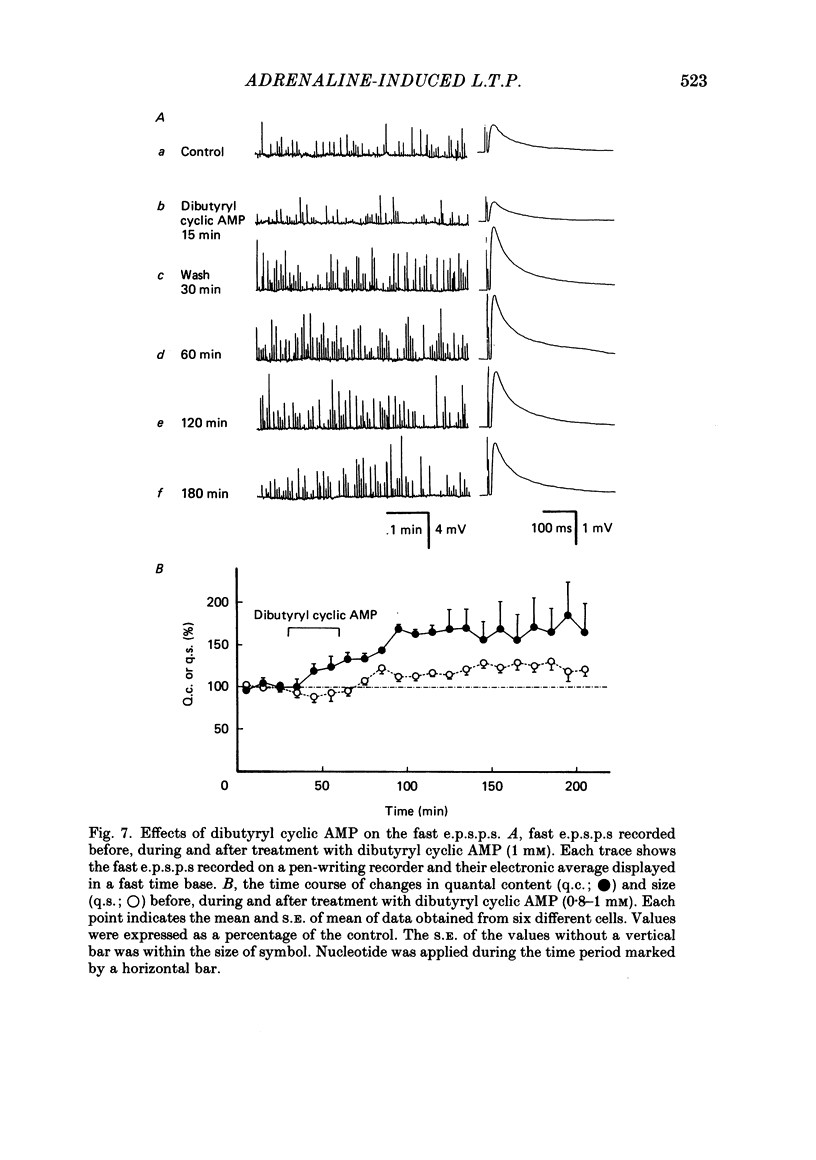
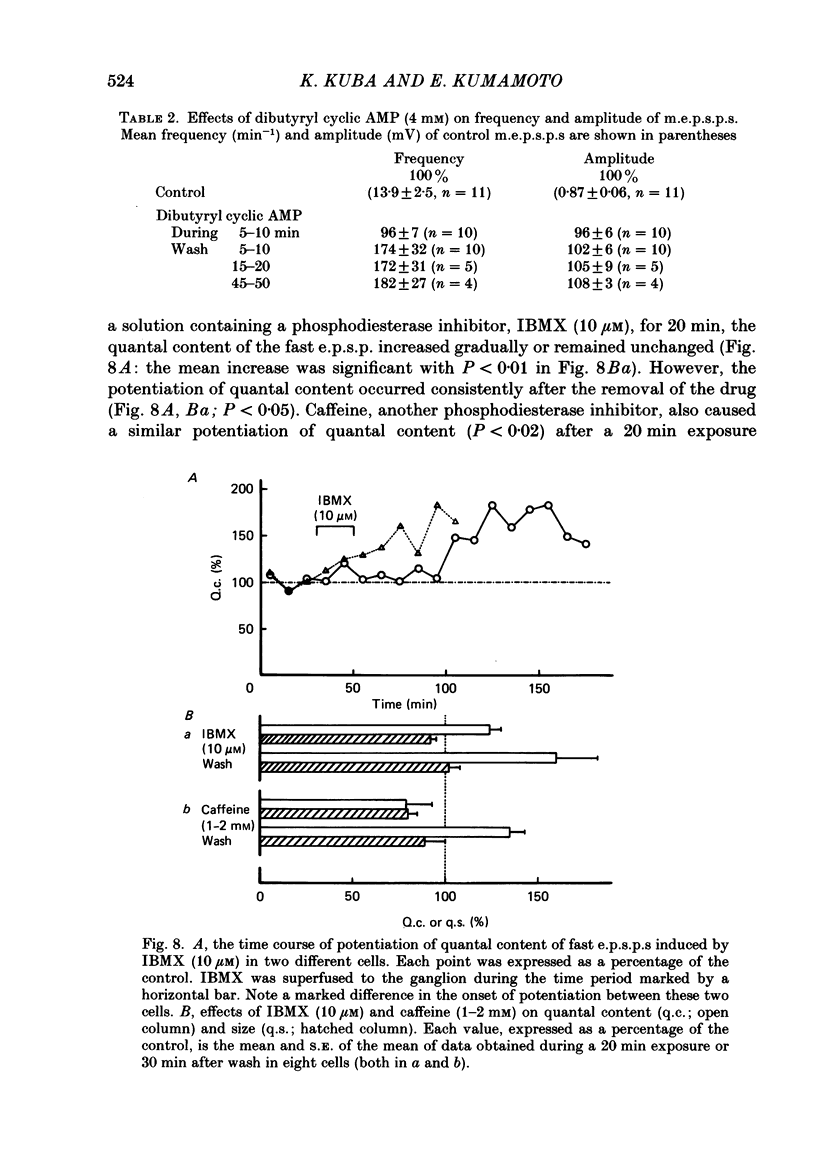
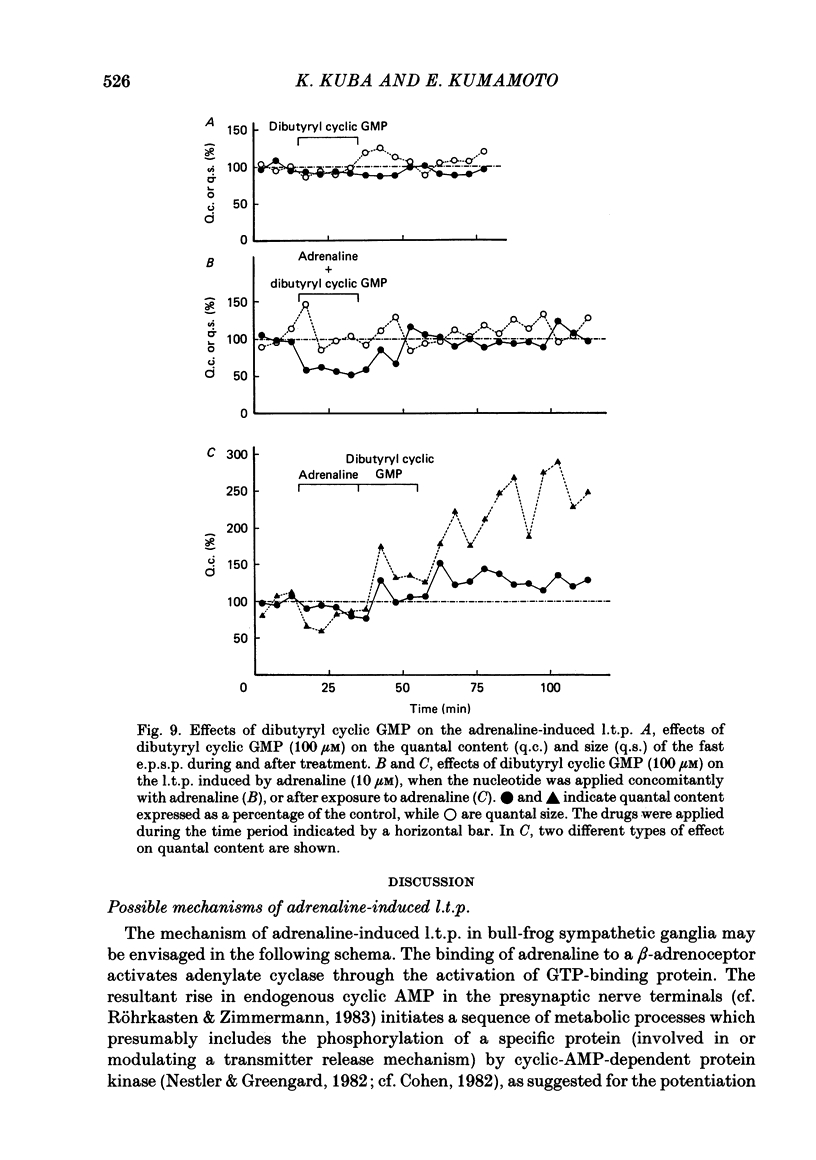
Long-term potentiation (l.t.p.) of transmitter release induced by adrenaline in bull-frog sympathetic ganglia was studied using intracellular recording techniques. The quantal content of the fast excitatory post-synaptic potentials (fast e.p.s.p.s: evoked by the nicotinic action of acetylcholine) was potentiated for more than several hours after treatment with adrenaline (1-100 microM). A similar l.t.p. of quantal content was produced consistently by isoprenaline (10 microM) and only in a certain fraction of cells by dopamine (10 microM). The l.t.p. induced by adrenaline (10 microM) was blocked by a beta-antagonist, propranolol (1 microM), but not by an alpha-antagonist, phenoxybenzamine (1 microM). Dibutyryl adenosine 3',5'-phosphate (dibutyryl cyclic AMP) (0.8-1.0 mM), adenosine 3',5'-phosphate (cyclic AMP) (4 mM), 3-isobutyl-1-methylxanthine (10 microM), caffeine (1-2 mM), and cholera toxin (2 micrograms ml-1) applied for 20-30 min, all caused the l.t.p. of quantal content. By contrast, adenosine 5'-phosphate (AMP) (4 mM) and adenosine (4 mM) had no potentiating action. Treatment of the ganglion with adrenaline (2.5-160 microM) or dibutyryl cyclic AMP (4 mM) for 15-30 min resulted in the l.t.p. of the frequency of miniature e.p.s.p.s. The l.t.p. of quantal content induced by adrenaline was markedly suppressed by lowering temperature from 20-25 degrees C to 11-13 degrees C, and blocked by dibutyryl guanosine 3',5'-phosphate (dibutyryl cyclic GMP) (100 microM) consistently when applied together, but inconsistently when given after adrenaline. The post-synaptic sensitivity to acetylcholine was unchanged for at least 1 h after exposure to adrenaline (2.5-160 microM) or dibutyryl cyclic AMP (0.8-4 mM). It can be concluded that adrenaline produces l.t.p. of transmitter release by activating a cyclic-AMP-dependent metabolic process through the activation of beta-adrenoceptors, and that this mechanism is presumably regulated by a process involving endogenous guanosine 3',5'-phosphate (cyclic GMP).
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Sundberg S. H., Sveen O., Swann J. W., Wigström H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol. 1980 May;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi A., Fehér O. Synaptic facilitation requires paired activation of convergent pathways in the neocortex. Nature. 1981 Apr 2;290(5805):413–415. doi: 10.1038/290413a0. [DOI] [PubMed] [Google Scholar]
- Bernier L., Castellucci V. F., Kandel E. R., Schwartz J. H. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3':5'-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982 Dec;2(12):1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Goddard G. V., Riives M. Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J Physiol. 1983 Jan;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., McAfee D. A., McCaman R. E. Long-term potentiation of synaptic acetylcholine release in the superior cervical ganglion of the rat. J Physiol. 1985 Jun;363:181–190. doi: 10.1113/jphysiol.1985.sp015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Plasticity of synaptic functions in the exised sympathetic ganglion of the rat. Brain Res. 1968 Aug 26;10(2):271–273. doi: 10.1016/0006-8993(68)90134-0. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Haddox M. K. Cyclic GMP metabolism and involvement in biological regulation. Annu Rev Biochem. 1977;46:823–896. doi: 10.1146/annurev.bi.46.070177.004135. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kato E., Koketsu K., Kuba K., Kumamoto E. The mechanism of the inhibitory action of adrenaline on transmitter release in bullfrog sympathetic ganglia: independence of cyclic AMP and calcium ions. Br J Pharmacol. 1985 Feb;84(2):435–443. doi: 10.1111/j.1476-5381.1985.tb12928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K., Akasu T., Miyagawa M., Hirai K. Modulation of nicotinic transmission by biogenic amines in bullfrog sympathetic ganglia. J Auton Nerv Syst. 1982 Jul;6(1):47–53. doi: 10.1016/0165-1838(82)90021-2. [DOI] [PubMed] [Google Scholar]
- Koketsu K., Yamada M. Presynaptic muscarinic receptors inhibiting active acetylcholine release in the bullfrog sympathetic ganglion. Br J Pharmacol. 1982 Sep;77(1):75–82. doi: 10.1111/j.1476-5381.1982.tb09271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyano K., Kuba K., Minota S. Long-term potentiation of transmitter release induced by repetitive presynaptic activities in bull-frog sympathetic ganglia. J Physiol. 1985 Feb;359:219–233. doi: 10.1113/jphysiol.1985.sp015582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Kato E., Kumamoto E., Koketsu K., Hirai K. Sustained potentiation of transmitter release by adrenaline and dibutyryl cyclic AMP in sympathetic ganglia. Nature. 1981 Jun 25;291(5817):654–656. doi: 10.1038/291654a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto E., Kuba K. Independence of presynaptic bimodal actions of adrenaline in sympathetic ganglia. Brain Res. 1983 Apr 18;265(2):344–347. doi: 10.1016/0006-8993(83)90354-2. [DOI] [PubMed] [Google Scholar]
- Kumamoto E., Kuba K. Sustained rise in ACh sensitivity of a sympathetic ganglion cell induced by postsynaptic electrical activities. Nature. 1983 Sep 8;305(5930):145–146. doi: 10.1038/305145a0. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Libet B., Kobayashi H., Tanaka T. Synaptic coupling into the production and storage of a neuronal memory trace. Nature. 1975 Nov 13;258(5531):155–157. doi: 10.1038/258155a0. [DOI] [PubMed] [Google Scholar]
- Michaelson D. M., Avissar S., Kloog Y., Sokolovsky M. Mechanism of acetylcholine release: possible involvement of presynaptic muscarinic receptors in regulation of acetylcholine release and protein phosphorylation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6336–6340. doi: 10.1073/pnas.76.12.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Greengard P. Nerve impulses increase the phosphorylation state of protein I in rabbit superior cervical ganglion. Nature. 1982 Apr 1;296(5856):452–454. doi: 10.1038/296452a0. [DOI] [PubMed] [Google Scholar]
- Röhrkasten A., Zimmermann H. Enrichment of cyclic adenosine 3',5'-monophosphate and cyclic guanosine 3',5'-monophosphate in cholinergic axon terminals of the Torpedo electric organ. Neurosci Lett. 1983 Dec 2;42(2):201–206. doi: 10.1016/0304-3940(83)90407-x. [DOI] [PubMed] [Google Scholar]
- Suetake K., Kojima H., Inanaga K., Koketsu K. Catecholamine is released from non-synaptic cell-soma membrane: histochemical evidence in bullfrog sympathetic ganglion cells. Brain Res. 1981 Feb 2;205(2):436–440. doi: 10.1016/0006-8993(81)90357-7. [DOI] [PubMed] [Google Scholar]


