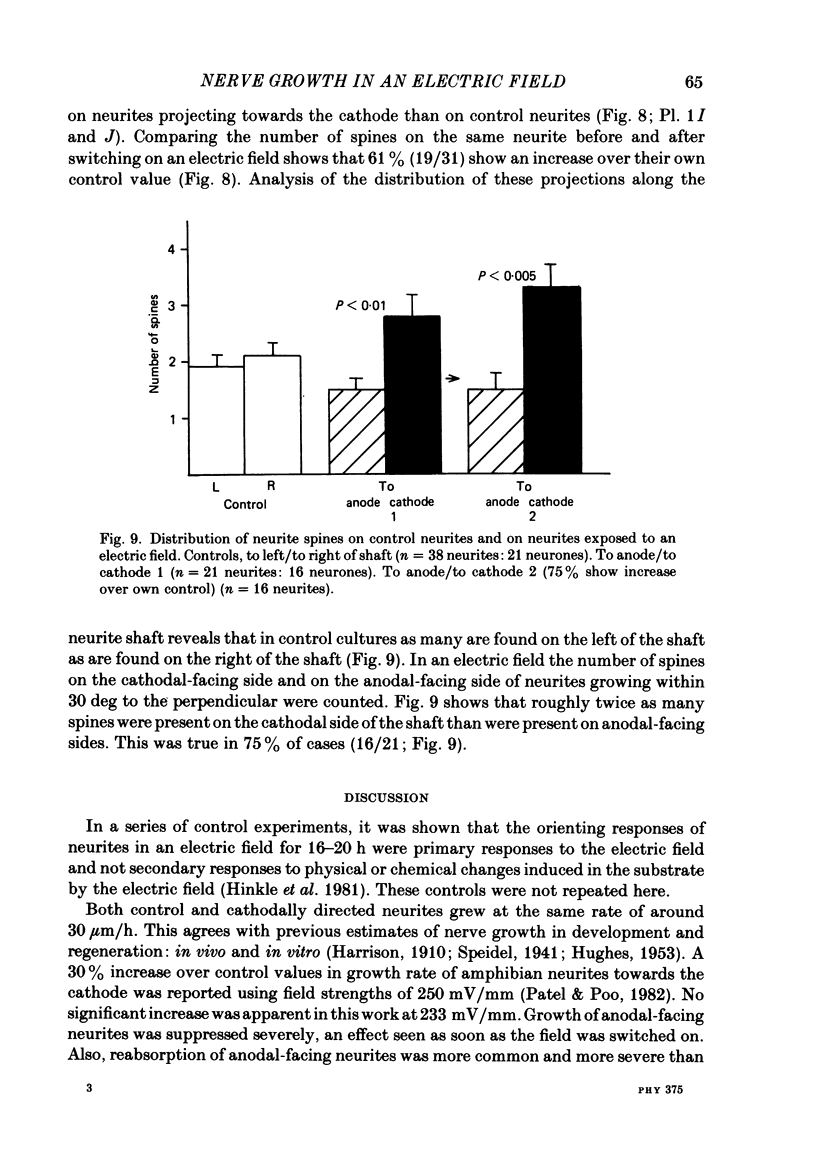
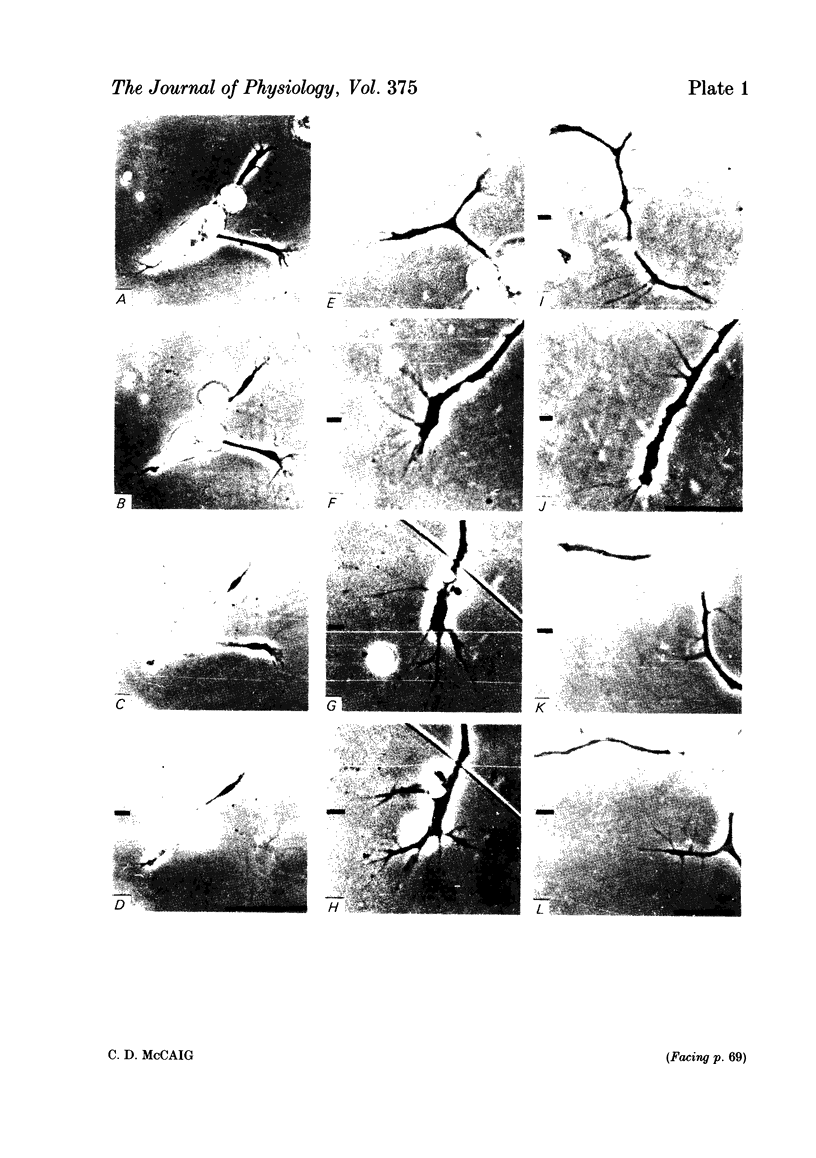
Abstract
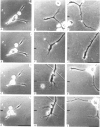
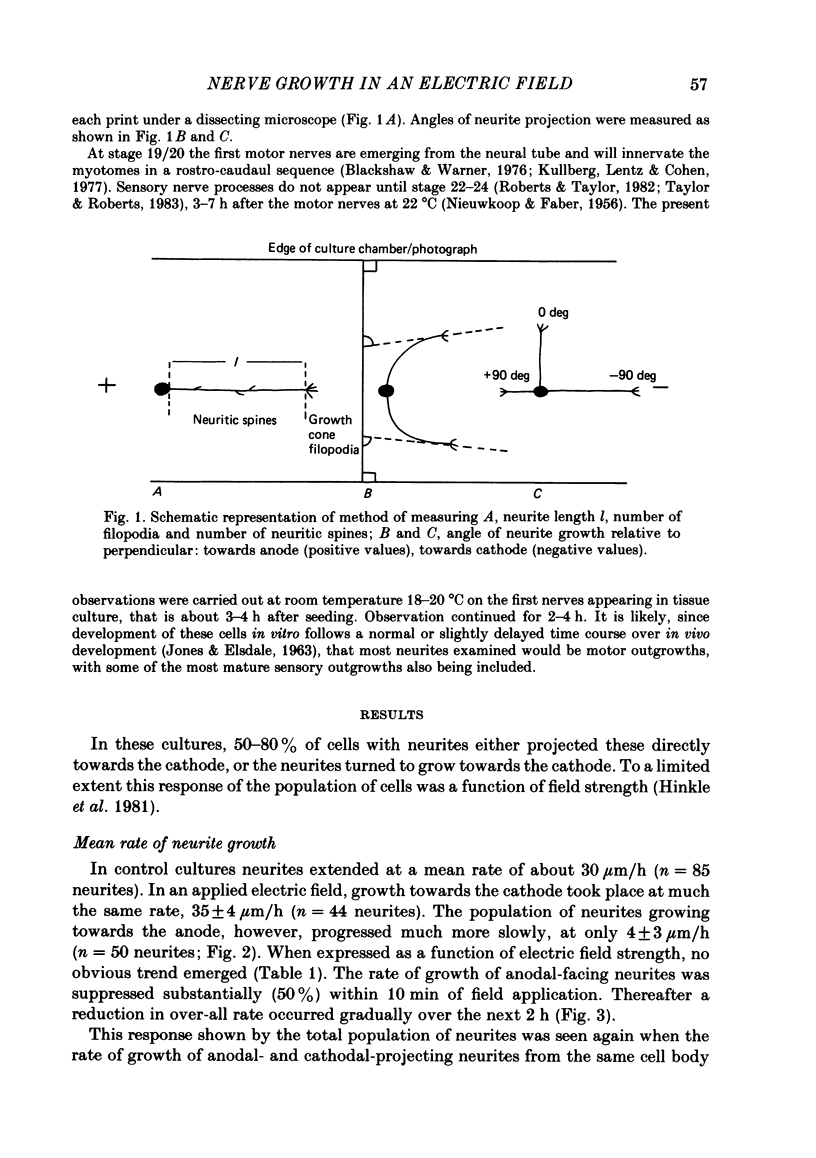
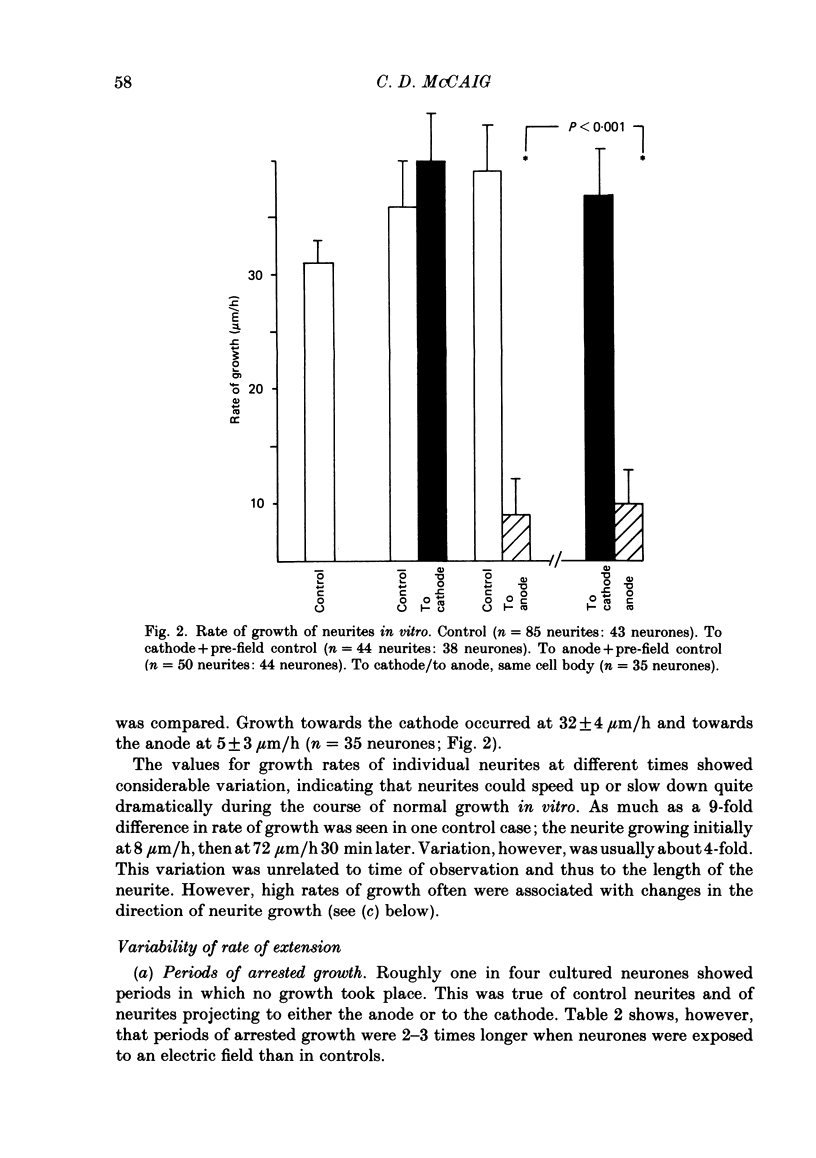
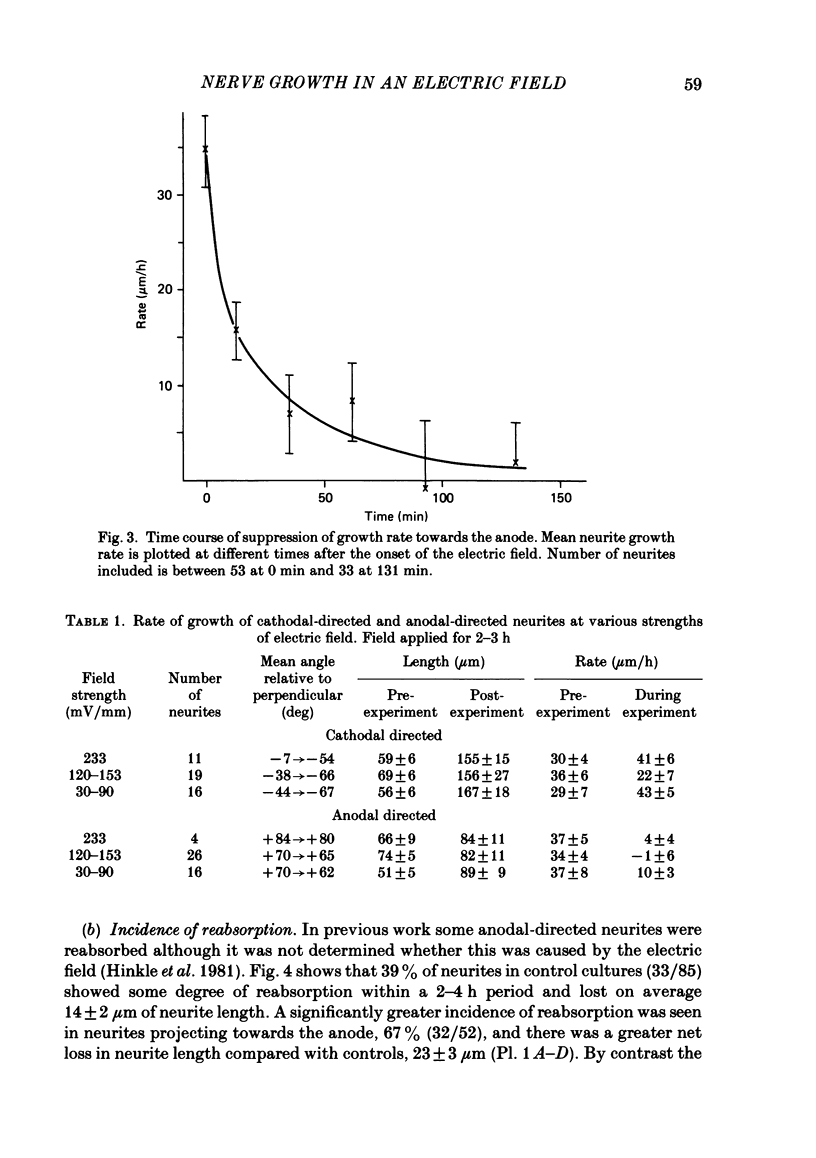
The dynamics of growth of earliest spinal neurites from Xenopus laevis have been studied in vitro in the presence and absence of an applied d.c. electric field. Control and cathode-directed neurites grew at a rate of about 30 micron/h: growth of anodal-facing neurites was 8 times slower. Periods of arrested growth were common in cultured neurones; these lasted 2-3 times longer in an applied electric field. The likelihood and the severity of neurite reabsorption was greatest in neurites directed towards the anode. Many neurites turned to direct their growth towards the cathode. As this happened their rate of growth increased 2-3-fold. The electric field further shaped neurite morphology by increasing the number of filopodia at the growth cone and by increasing the number of cytoplasmic spines along a neurite shaft. The electric field induced an asymmetry in the distribution of these cytoplasmic projections; greater numbers being found on the cathodal-facing than on the anodal-facing side. Implications of these data for nerve growth in development and in regeneration are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argiro V., Bunge M. B., Johnson M. I. Correlation between growth form and movement and their dependence on neuronal age. J Neurosci. 1984 Dec;4(12):3051–3062. doi: 10.1523/JNEUROSCI.04-12-03051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S., Warner A. Onset of acetylcholine sensitivity and endplate activity in developing myotome muscles of Xenopus. Nature. 1976 Jul 15;262(5565):217–218. doi: 10.1038/262217a0. [DOI] [PubMed] [Google Scholar]
- Borgens R. B., Roederer E., Cohen M. J. Enhanced spinal cord regeneration in lamprey by applied electric fields. Science. 1981 Aug 7;213(4508):611–617. doi: 10.1126/science.7256258. [DOI] [PubMed] [Google Scholar]
- Bray D. Mechanical tension produced by nerve cells in tissue culture. J Cell Sci. 1979 Jun;37:391–410. doi: 10.1242/jcs.37.1.391. [DOI] [PubMed] [Google Scholar]
- Bray D., Thomas C., Shaw G. Growth cone formation in cultures of sensory neurons. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5226–5229. doi: 10.1073/pnas.75.10.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. S., Keller R. E. Perpendicular orientation and directional migration of amphibian neural crest cells in dc electrical fields. Proc Natl Acad Sci U S A. 1984 Jan;81(1):160–164. doi: 10.1073/pnas.81.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C. A., Nuccitelli R. Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J Cell Biol. 1984 Jan;98(1):296–307. doi: 10.1083/jcb.98.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. S., Newby B. J. Neurofilament disguise, destruction and discipline. Nature. 1975 Aug 14;256(5518):586–589. doi: 10.1038/256586a0. [DOI] [PubMed] [Google Scholar]
- HUGHES A. The growth of embryonic neurites; a study of cultures of chick neural tissues. J Anat. 1953 Apr;87(2):150–162. [PMC free article] [PubMed] [Google Scholar]
- Hinkle L., McCaig C. D., Robinson K. R. The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J Physiol. 1981 May;314:121–135. doi: 10.1113/jphysiol.1981.sp013695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Poo M. M. Neurites grow faster towards the cathode than the anode in a steady field. J Exp Zool. 1979 Jul;209(1):115–128. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Stern C. D. Strong electrical currents leave the primitive streak of chick embryos. Science. 1979 Nov 2;206(4418):569–571. doi: 10.1126/science.573921. [DOI] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Poo M. M. The role of electro-osmosis in the electric-field-induced movement of charged macromolecules on the surfaces of cells. Biophys J. 1981 Apr;34(1):85–93. doi: 10.1016/S0006-3495(81)84838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. B., Poo M. M. Perturbation of the direction of neurite growth by pulsed and focal electric fields. J Neurosci. 1984 Dec;4(12):2939–2947. doi: 10.1523/JNEUROSCI.04-12-02939.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Poo M. M. Orientation of neurite growth by extracellular electric fields. J Neurosci. 1982 Apr;2(4):483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. In situ electrophoresis of membrane components. Annu Rev Biophys Bioeng. 1981;10:245–276. doi: 10.1146/annurev.bb.10.060181.001333. [DOI] [PubMed] [Google Scholar]
- Poo M., Robinson K. R. Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature. 1977 Feb 17;265(5595):602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- Purves D., Hadley R. D. Changes in the dendritic branching of adult mammalian neurones revealed by repeated imaging in situ. 1985 May 30-Jun 5Nature. 315(6018):404–406. doi: 10.1038/315404a0. [DOI] [PubMed] [Google Scholar]
- Rees R. P., Reese T. S. New structural features of freeze-substituted neuritic growth cones. Neuroscience. 1981;6(2):247–254. doi: 10.1016/0306-4522(81)90060-9. [DOI] [PubMed] [Google Scholar]
- Robinson K. R., Stump R. F. Self-generated electrical currents through Xenopus neurulae. J Physiol. 1984 Jul;352:339–352. doi: 10.1113/jphysiol.1984.sp015295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer E., Goldberg N. H., Cohen M. J. Modification of retrograde degeneration in transected spinal axons of the lamprey by applied DC current. J Neurosci. 1983 Jan;3(1):153–160. doi: 10.1523/JNEUROSCI.03-01-00153.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer W. W. Calcium-induced degeneration of axoplasm in isolated segments of rat peripheral nerve. Brain Res. 1974 Apr 5;69(2):203–215. doi: 10.1016/0006-8993(74)90002-x. [DOI] [PubMed] [Google Scholar]
- Selzer M. E. Mechanisms of functional recovery and regeneration after spinal cord transection in larval sea lamprey. J Physiol. 1978 Apr;277:395–408. doi: 10.1113/jphysiol.1978.sp012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. S., Roberts A. The early development of the primary sensory neurones in an amphibian embryo: a scanning electron microscope study. J Embryol Exp Morphol. 1983 Jun;75:49–66. [PubMed] [Google Scholar]
- Wilson L., Bryan J., Ruby A., Mazia D. Precipitation of proteins by vinblastine and calcium ions. Proc Natl Acad Sci U S A. 1970 Jul;66(3):807–814. doi: 10.1073/pnas.66.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]