Abstract
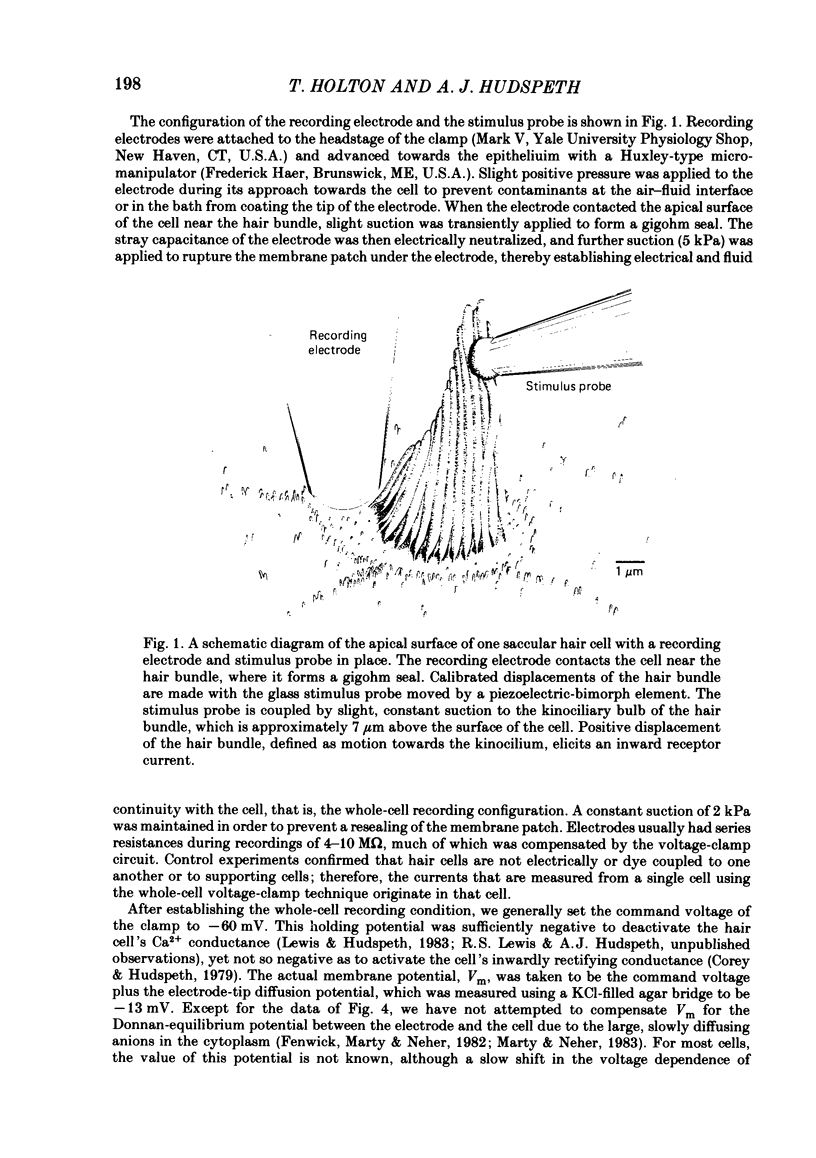
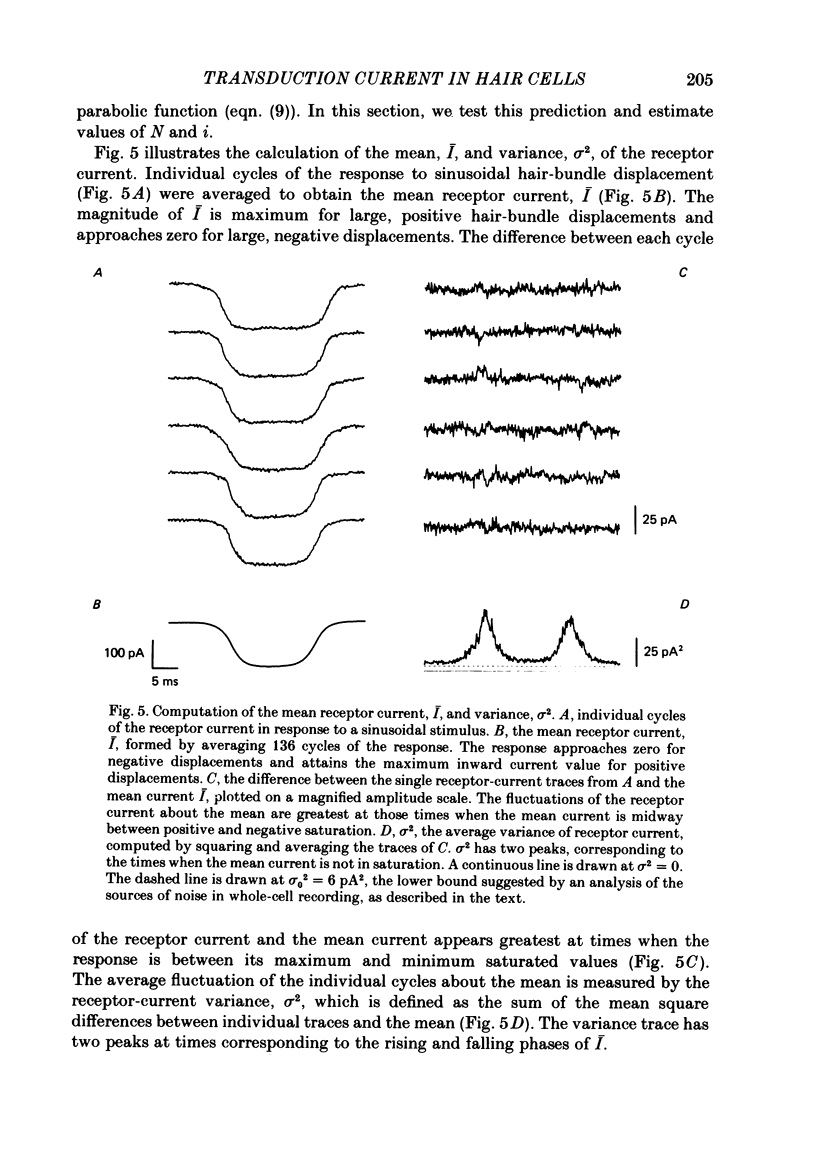
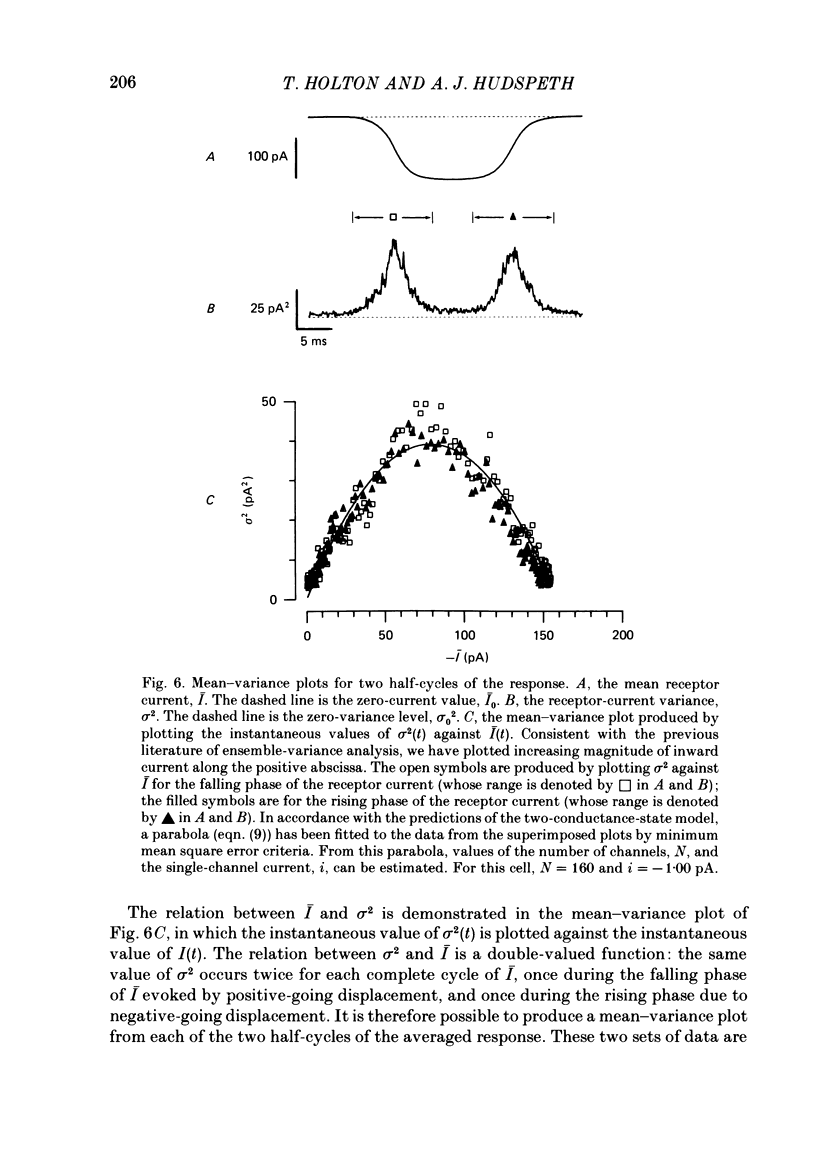
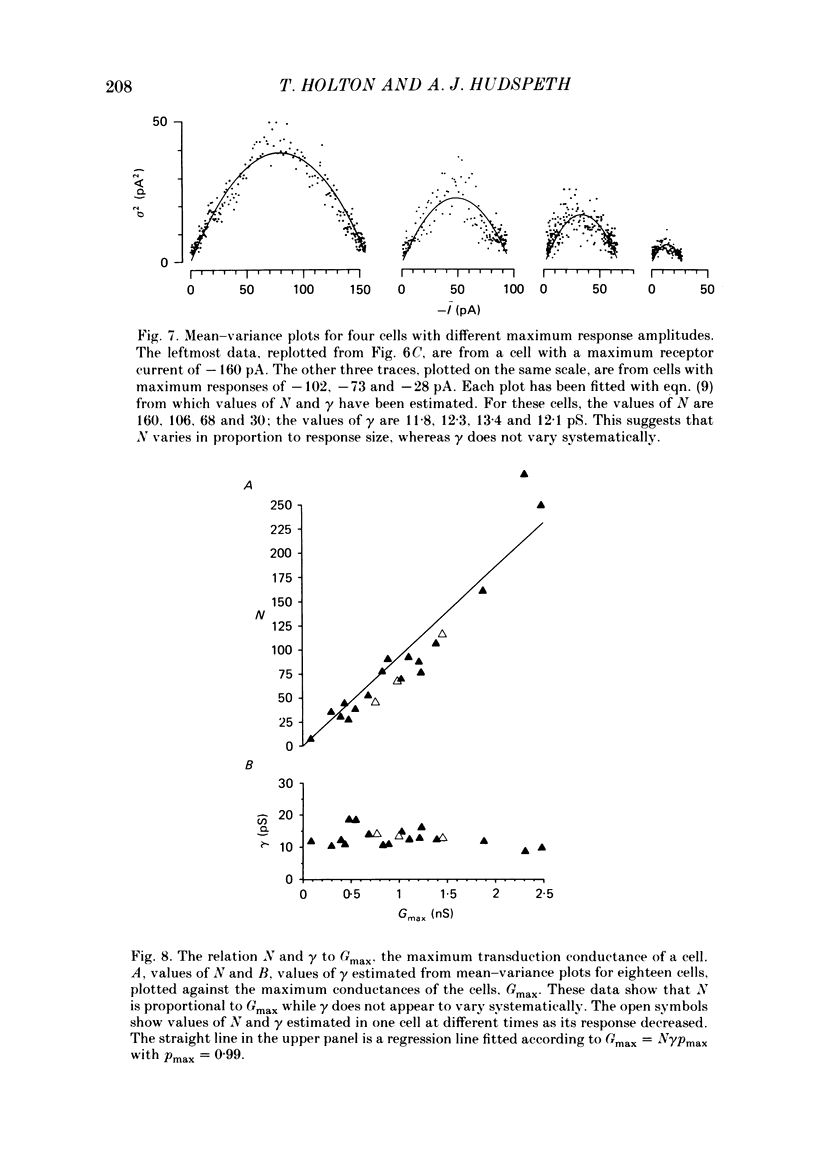
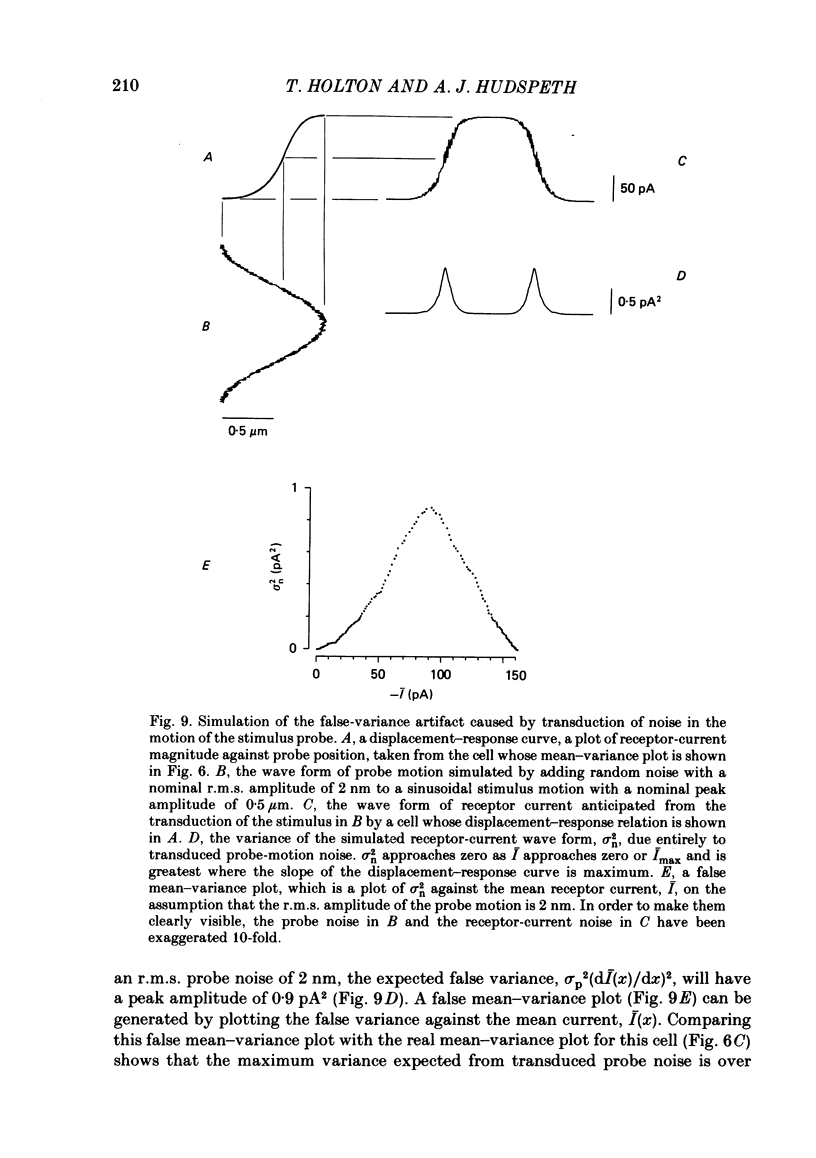
Receptor currents in response to mechanical stimuli were recorded from hair cells in the excised epithelium of the bull-frog sacculus by the whole-cell, gigohm-seal voltage-clamp technique. The stimulus-dependent transduction current was separated from the cell's stimulus-independent K+ and Ca2+ currents; the K+ currents were blocked with an internal solution containing Cs+ while the Ca2+ current was reduced by holding the membrane potential below -70 mV. The temperature of the preparation was maintained at about 10 degrees C to slow the kinetics of the cells' transduction channels. Calibrated displacements of hair bundles of individual hair cells were made with a probe coupled by suction to the kinociliary bulb and moved with a piezoelectricbimorph stimulator. The root mean square noise of probe motion was less than 2 nm. The mean, I, and the variance, sigma 2, of the receptor current were measured from the response to saturating (+/- 0.5 micron) displacements of the hair bundle. I was corrected for current offsets and sigma 2 for the transduction-independent background variance. The relation between sigma 2 and I is consistent with the predictions of a two-conductance-state model of the transduction channel, a model having only one non-zero conductance state. The relation between sigma 2 and I was fitted by the equation sigma 2 = Ii-I2/N, where N is the number of transduction channels in the cell and i is the current through a single open channel. The conductance of the transduction channel is approximately ohmic with a reversal potential near 0 mV. The estimated conductance of a single transduction channel, gamma, is 12.7 +/- 2.7 pS (mean +/- S.D.; n = 18) at 10 degrees C. gamma is independent of the maximum transduction conductance of the cell, Gmax. The number of transduction channels, N, is proportional to Gmax. N ranges from 7 to 280 in cells with Gmax ranging from 0.08 to 2.48 nS. The largest values of N correspond to a few, perhaps four, active transduction channels per stereocilium. Control experiments show that transduction by the hair cell of two artifactual sources of hair-bundle stimulation, noisy or discontinuous motion of the probe, do not contribute substantially to the measured variance, sigma 2. Displacement-response curves are generally sigmoidal and symmetrical; they reasonably fit the predictions of a two-kinetic-state model, comprising one open state and one closed state. The estimated displacement-sensitive free energy, Z, is 5.7 +/- 1.1 kcal/mol micron (mean +/- S.D., n = 18).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Analysis of the microphonic potential of the bullfrog's sacculus. J Neurosci. 1983 May;3(5):942–961. doi: 10.1523/JNEUROSCI.03-05-00942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983 May;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Mechanical stimulation and micromanipulation with piezoelectric bimorph elements. J Neurosci Methods. 1980 Dec;3(2):183–202. doi: 10.1016/0165-0270(80)90025-4. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Reversal of hair cell responses by current [proceedings]. J Physiol. 1979 Oct;295:66P–66P. [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guharay F., Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984 Jul;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Corey D. P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982 Jan;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Jacobs R. Stereocilia mediate transduction in vertebrate hair cells (auditory system/cilium/vestibular system). Proc Natl Acad Sci U S A. 1979 Mar;76(3):1506–1509. doi: 10.1073/pnas.76.3.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J. Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Annu Rev Neurosci. 1983;6:187–215. doi: 10.1146/annurev.ne.06.030183.001155. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985 Feb;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. Mechanoelectrical transducer has discrete conductances in the chick vestibular hair cell. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1888–1891. doi: 10.1073/pnas.81.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles J. O., Comis S. D., Osborne M. P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984 Aug;15(2):103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Russell I. J. Origin of the receptor potential in inner hair cells of the mammalian cochlea--evidence for Davis' theory. Nature. 1983 Jan 27;301(5898):334–336. doi: 10.1038/301334a0. [DOI] [PubMed] [Google Scholar]
- Seiler N., Schmidt-Glenewinkel T. Regional distribution of putrescine, spermidine and spermine in relation to the distribution of RNA and DNA in the rat nervous system. J Neurochem. 1975 Apr;24(4):791–795. [PubMed] [Google Scholar]
- Shotwell S. L., Jacobs R., Hudspeth A. J. Directional sensitivity of individual vertebrate hair cells to controlled deflection of their hair bundles. Ann N Y Acad Sci. 1981;374:1–10. doi: 10.1111/j.1749-6632.1981.tb30854.x. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersäll J., Björkroth B., Flock A., Lundquist P. G. Experiments on ototoxic effects of antibiotics. Adv Otorhinolaryngol. 1973;20:14–41. doi: 10.1159/000393087. [DOI] [PubMed] [Google Scholar]


