Abstract
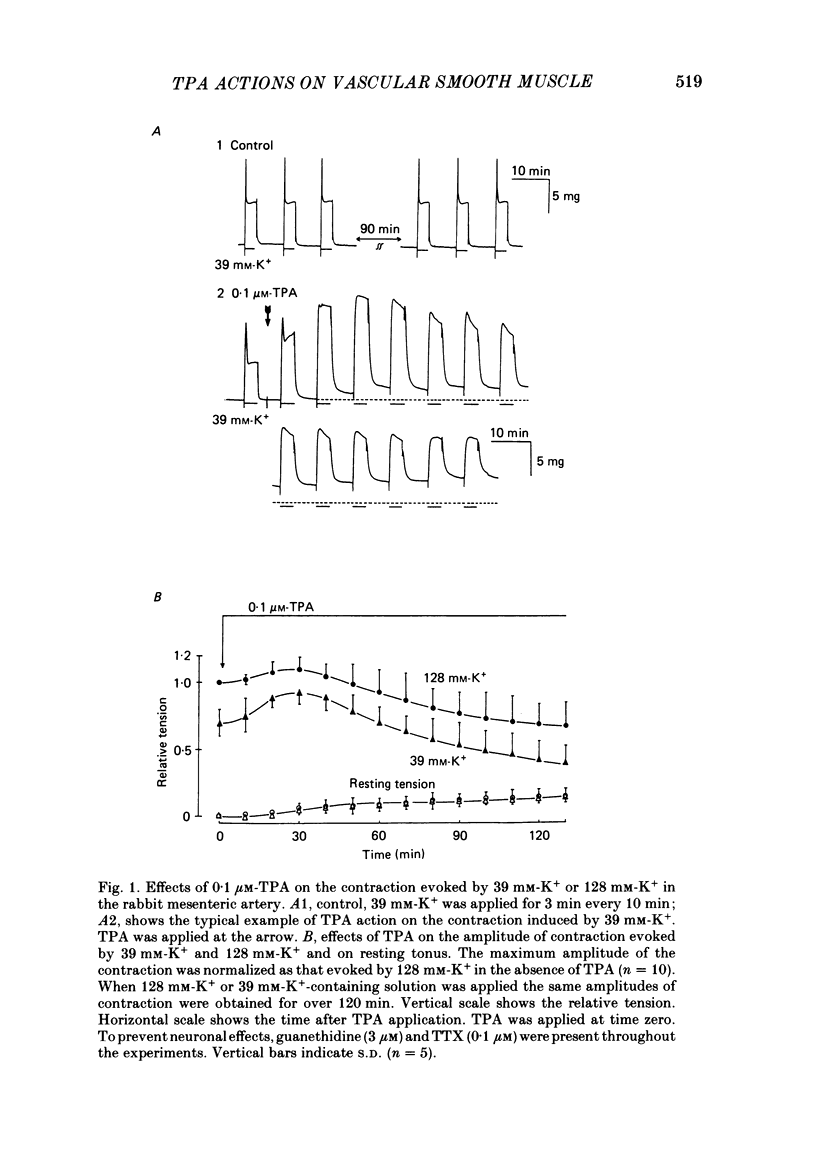
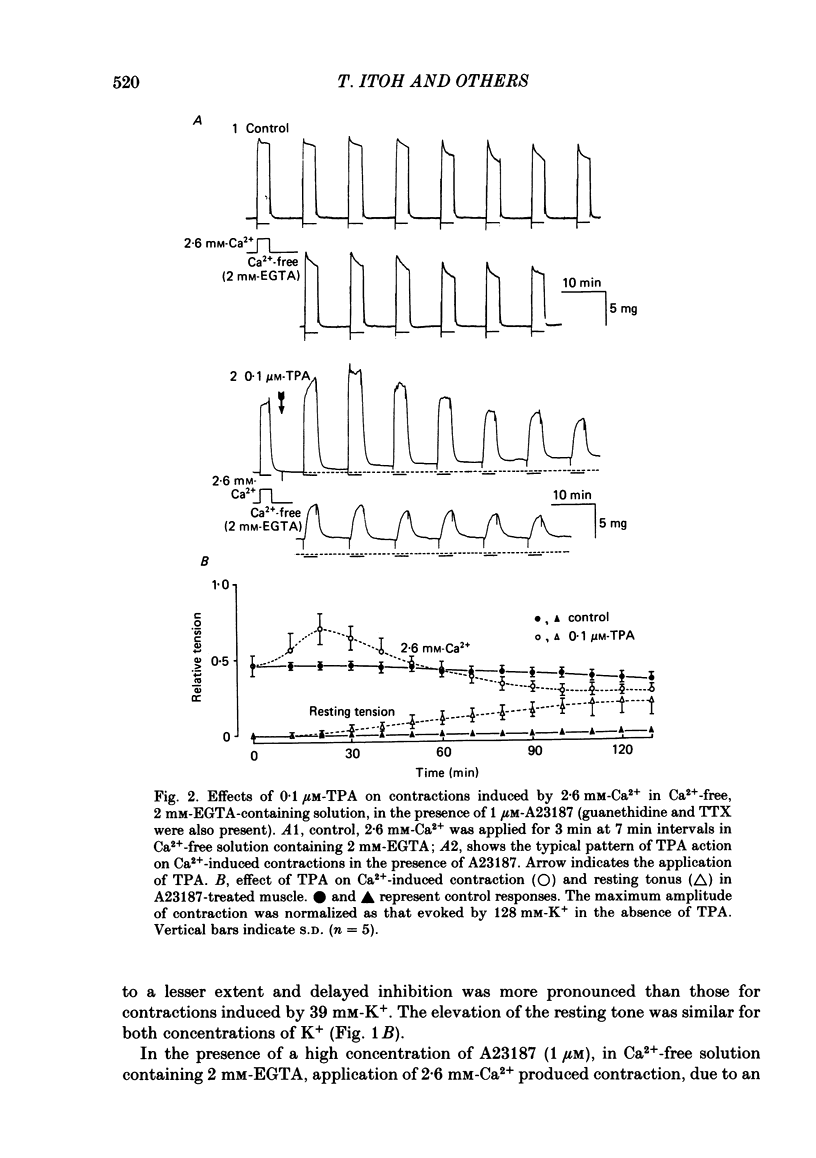
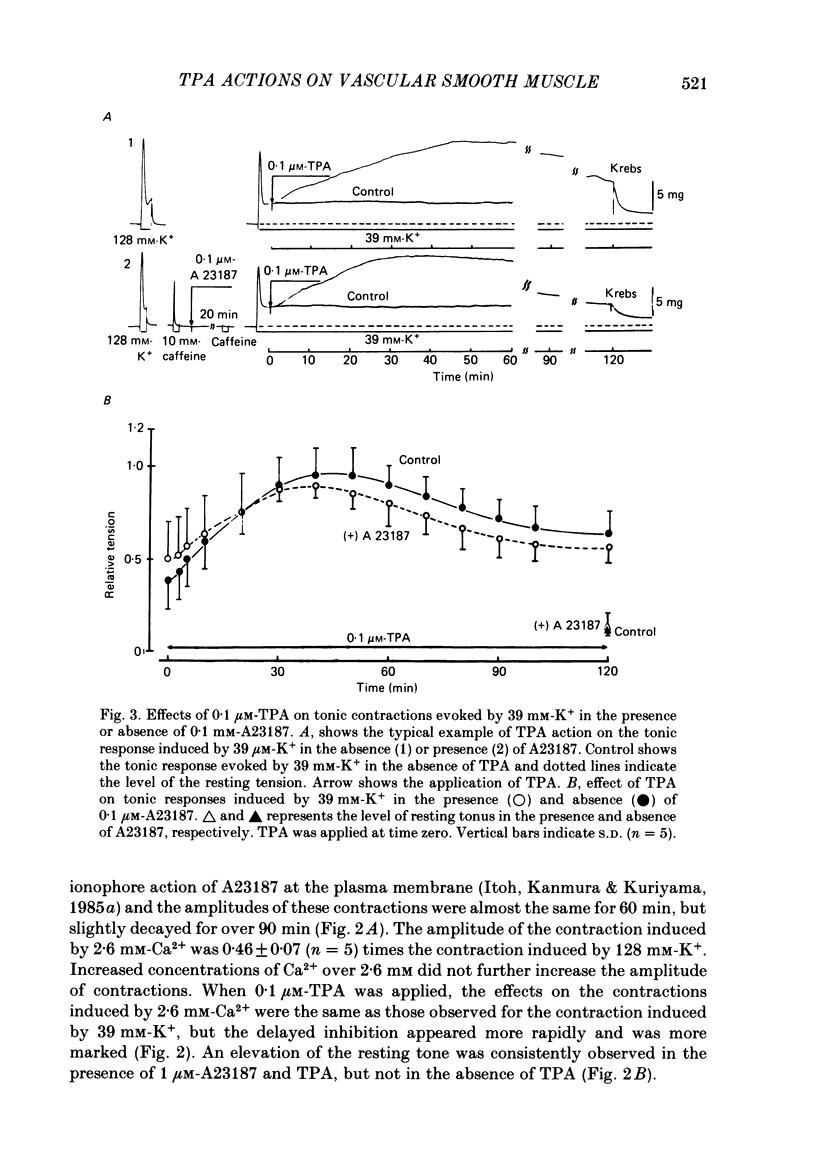
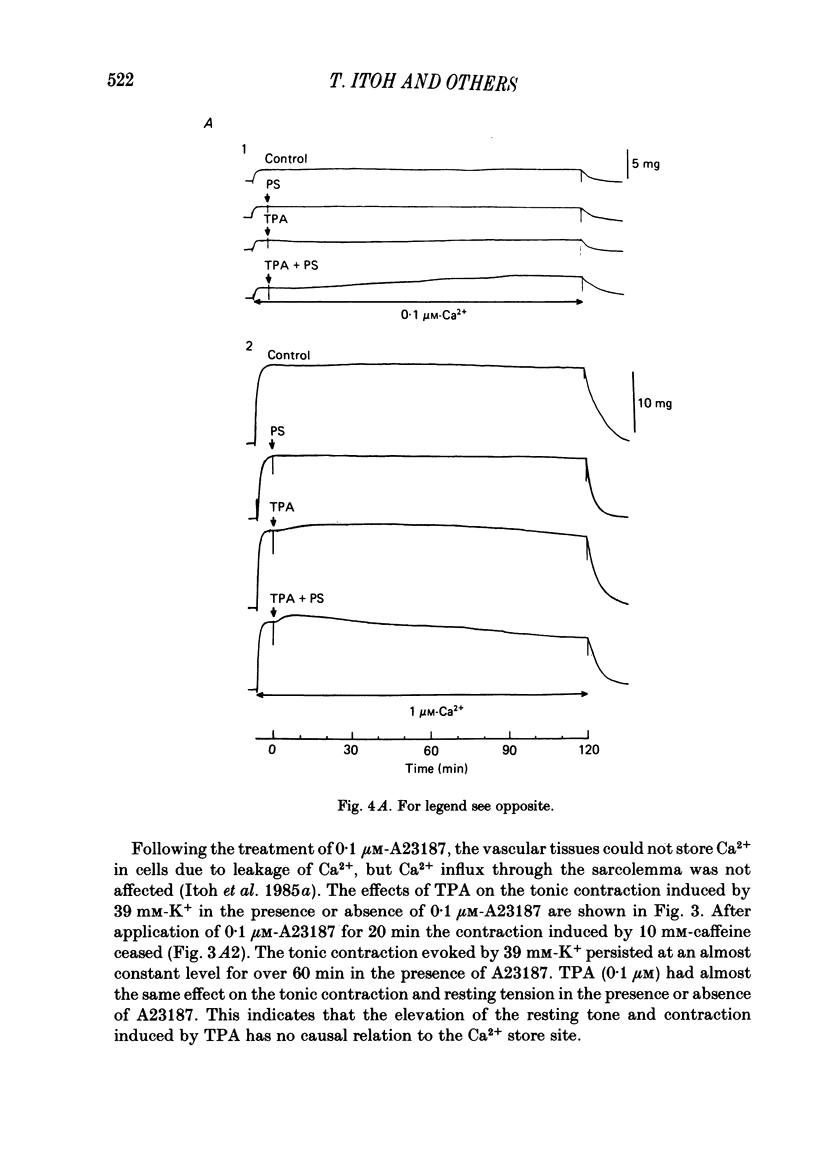
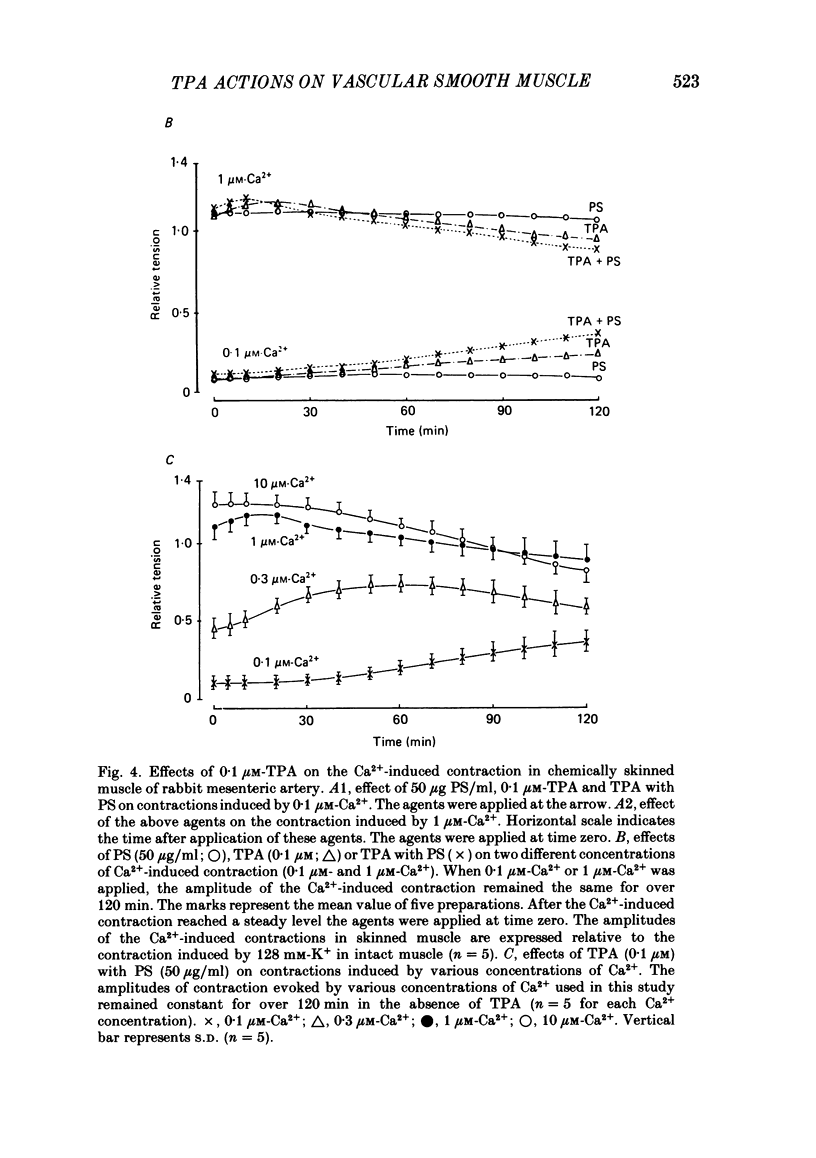
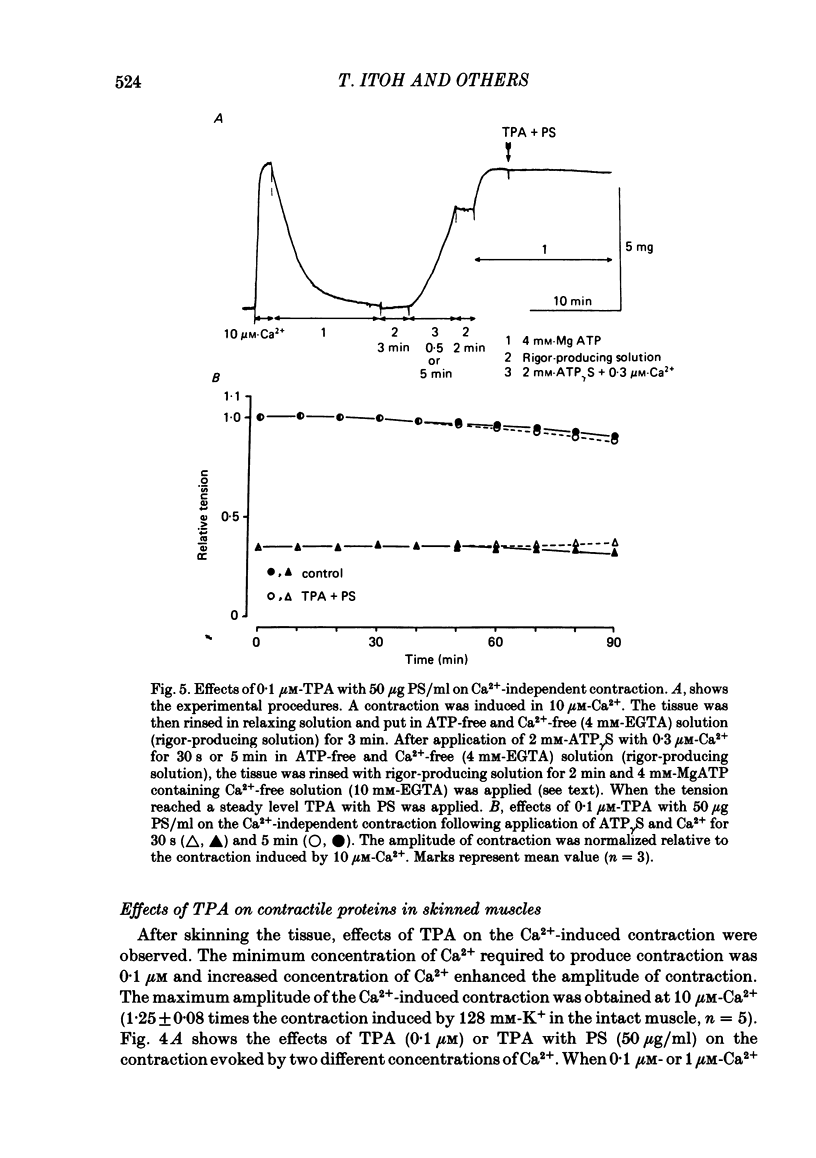
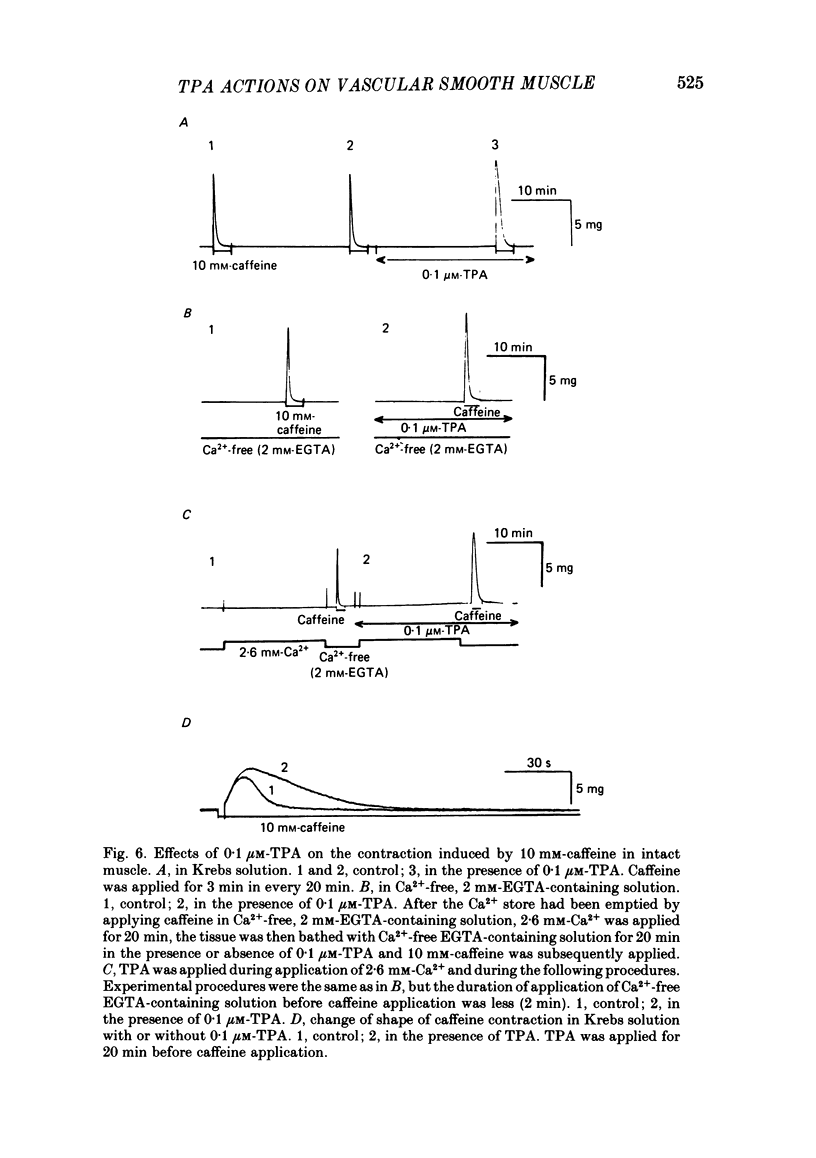
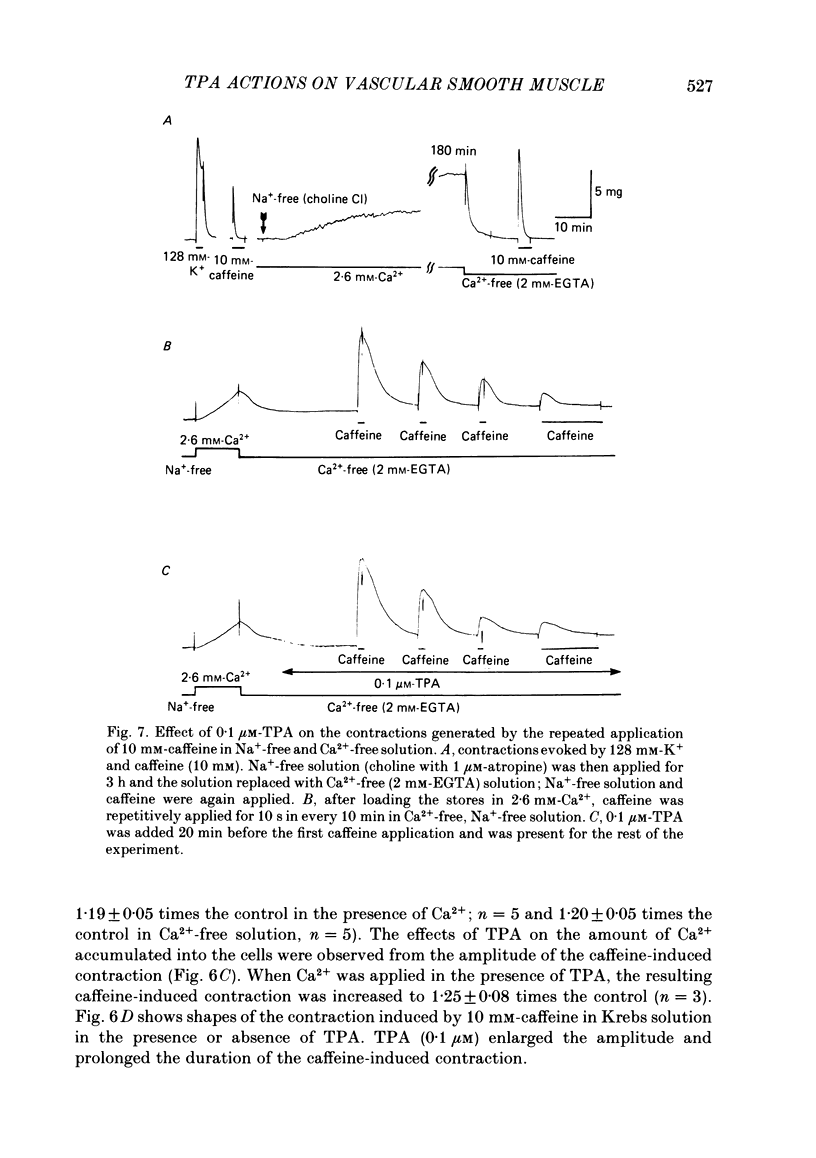
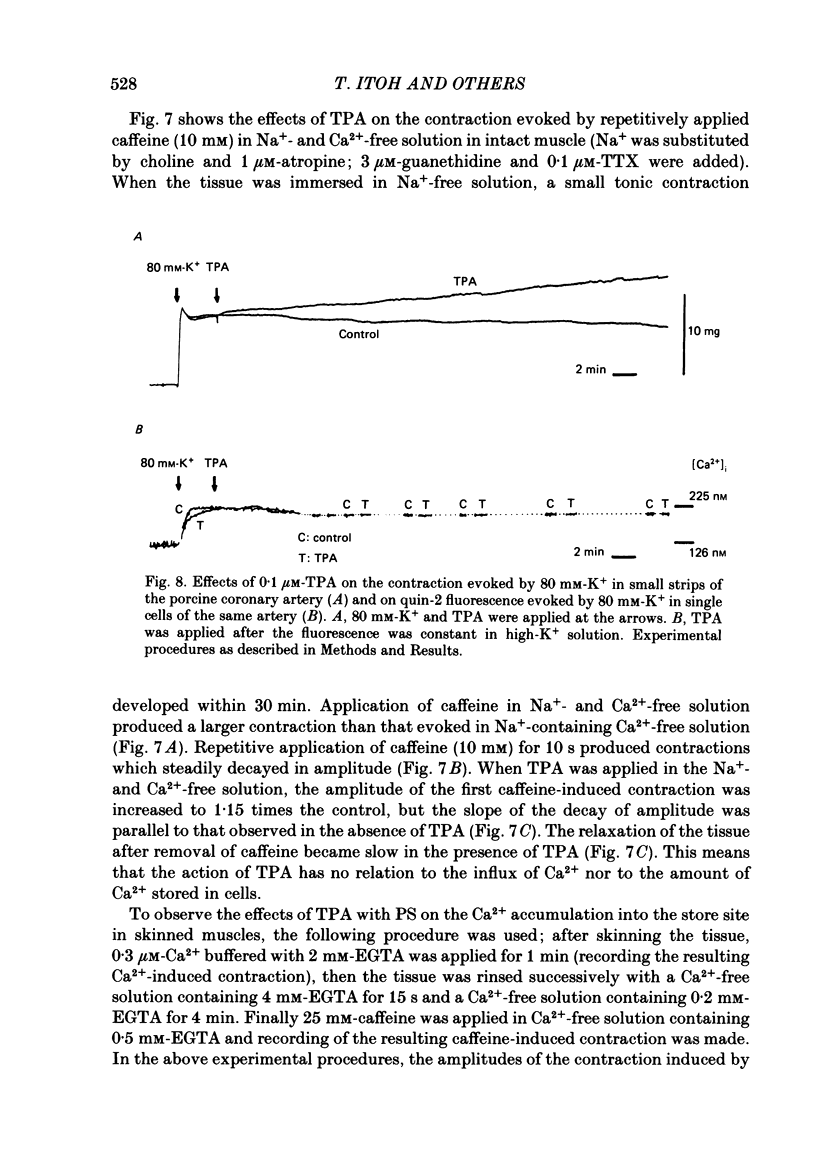
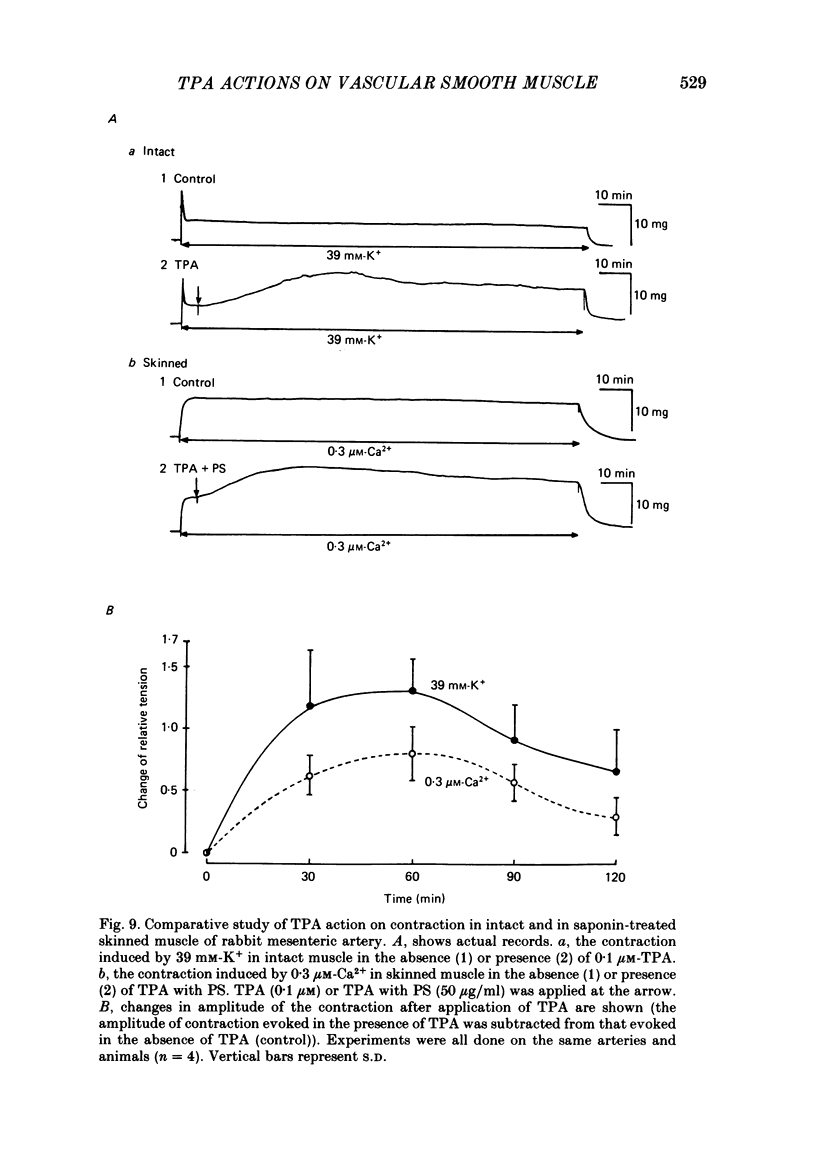
To clarify the role of protein kinase C in the mechanical response, the effects of 12-o-tetradecanoylphorbol-13-acetate (TPA), an activator of protein kinase C, were investigated on intact and skinned smooth muscle preparations of the rabbit mesenteric artery. TPA (0.1 microM) showed dual actions (initial enhancement followed by inhibition during long exposure) on the K+-induced contraction. The enhancement was marked in the presence of 39 mM-K+ but inhibition was the predominant effect in the presence of 128 mM-K+. Addition of 2.6 mM-Ca2+ to a Ca2+-free solution containing 2 mM-EGTA following application of A23187 (1 microM), produced contraction. TPA showed the same dual actions on this Ca2+-induced contraction. In chemically skinned muscles, TPA increased the amplitude of Ca2+-induced contractions evoked by low concentrations of Ca2+ (0.1-0.3 microM), but reduced those evoked by high concentrations of Ca2+ (1-10 microM). Both actions of TPA were facilitated in the presence of phosphatidylserine (PS). TPA with PS had no effect on the Ca2+-independent contraction evoked in relaxing solution containing 10 mM-EGTA and 4 mM-Mg ATP following application of adenosine-5-o-3-thiotriphosphate (ATP gamma S) and 0.3 microM-Ca2+. The amount of Ca2+ stored in cells estimated from the amplitude of the caffeine-induced contraction was not modified by application of TPA with PS in skinned or intact muscle tissues. The effects of TPA were investigated on the Ca2+ transient measured from the intensity of fluorescence of quin-2 in dispersed cell suspensions prepared from the porcine coronary artery. TPA had no effect on the Ca2+ transient in high K+ but enhanced the amplitude of the contraction. Amplitudes of the tonic response evoked by 39 mM-K+ in intact muscle tissues and the contraction induced by 0.3 microM-Ca2+ in skinned muscle were much the same. TPA with PS enhanced the amplitudes of both contractions to the same extent. From the above results, we concluded that TPA shows dual actions on the contractile machinery and may act on the regulatory systems of contractile proteins. Both excitatory and inhibitory actions of TPA depended on the concentration of Ca2+. However, the physiological action of protein kinase C as estimated from the action of TPA seems to be related to an excitatory action on the contractile machinery.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron C. B., Cunningham M., Strauss J. F., 3rd, Coburn R. F. Pharmacomechanical coupling in smooth muscle may involve phosphatidylinositol metabolism. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6899–6903. doi: 10.1073/pnas.81.21.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., May W. S., Jr, LeVine H., 3rd, Cragoe E. J., Jr, Cuatrecasas P. Amiloride inhibits phorbol ester-stimulated Na+/H+ exchange and protein kinase C. An amiloride analog selectively inhibits Na+/H+ exchange. J Biol Chem. 1985 Jan 25;260(2):1155–1159. [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. Phosphorylation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase regulates myosin light chain kinase. Fed Proc. 1980 Apr;39(5):1569–1573. [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Mrwa U. Regulation of smooth muscle actomyosin. Blood Vessels. 1982;19(1):1–18. doi: 10.1159/000158369. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Ito Y. A role for inositol 1,4,5-trisphosphate in the initiation of agonist-induced contractions of dog tracheal smooth muscle. Br J Pharmacol. 1985 Sep;86(1):191–199. doi: 10.1111/j.1476-5381.1985.tb09449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H. A23187 increases calcium permeability of store sites more than of surface membranes in the rabbit mesenteric artery. J Physiol. 1985 Feb;359:467–484. doi: 10.1113/jphysiol.1985.sp015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kanmura Y., Kuriyama H., Sasaguri T. Nitroglycerine- and isoprenaline-induced vasodilatation: assessment from the actions of cyclic nucleotides. Br J Pharmacol. 1985 Feb;84(2):393–406. doi: 10.1111/j.1476-5381.1985.tb12923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa Y., Hosey M. M. Phosphorylation of cardiac sarcolemma proteins by the calcium-activated phospholipid-dependent protein kinase. J Biol Chem. 1984 Jan 10;259(1):534–540. [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Two rigor states in skinned crayfish single muscle fibers. J Gen Physiol. 1976 Sep;68(3):267–280. doi: 10.1085/jgp.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Lagast H., Pozzan T., Waldvogel F. A., Lew P. D. Phorbol myristate acetate stimulates ATP-dependent calcium transport by the plasma membrane of neutrophils. J Clin Invest. 1984 Mar;73(3):878–883. doi: 10.1172/JCI111284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Movsesian M. A., Nishikawa M., Adelstein R. S. Phosphorylation of phospholamban by calcium-activated, phospholipid-dependent protein kinase. Stimulation of cardiac sarcoplasmic reticulum calcium uptake. J Biol Chem. 1984 Jul 10;259(13):8029–8032. [PubMed] [Google Scholar]
- Naka M., Nishikawa M., Adelstein R. S., Hidaka H. Phorbol ester-induced activation of human platelets is associated with protein kinase C phosphorylation of myosin light chains. Nature. 1983 Dec 1;306(5942):490–492. doi: 10.1038/306490a0. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Hidaka H., Adelstein R. S. Phosphorylation of smooth muscle heavy meromyosin by calcium-activated, phospholipid-dependent protein kinase. The effect on actin-activated MgATPase activity. J Biol Chem. 1983 Dec 10;258(23):14069–14072. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pfitzer G., Hofmann F., DiSalvo J., Rüegg J. C. cGMP and cAMP inhibit tension development in skinned coronary arteries. Pflugers Arch. 1984 Jul;401(3):277–280. doi: 10.1007/BF00582596. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H. Calcium mobilization in enzymically isolated single intact and skinned muscle cells of the porcine coronary artery. J Physiol. 1985 Jun;363:103–117. doi: 10.1113/jphysiol.1985.sp015698. [DOI] [PMC free article] [PubMed] [Google Scholar]


