Abstract
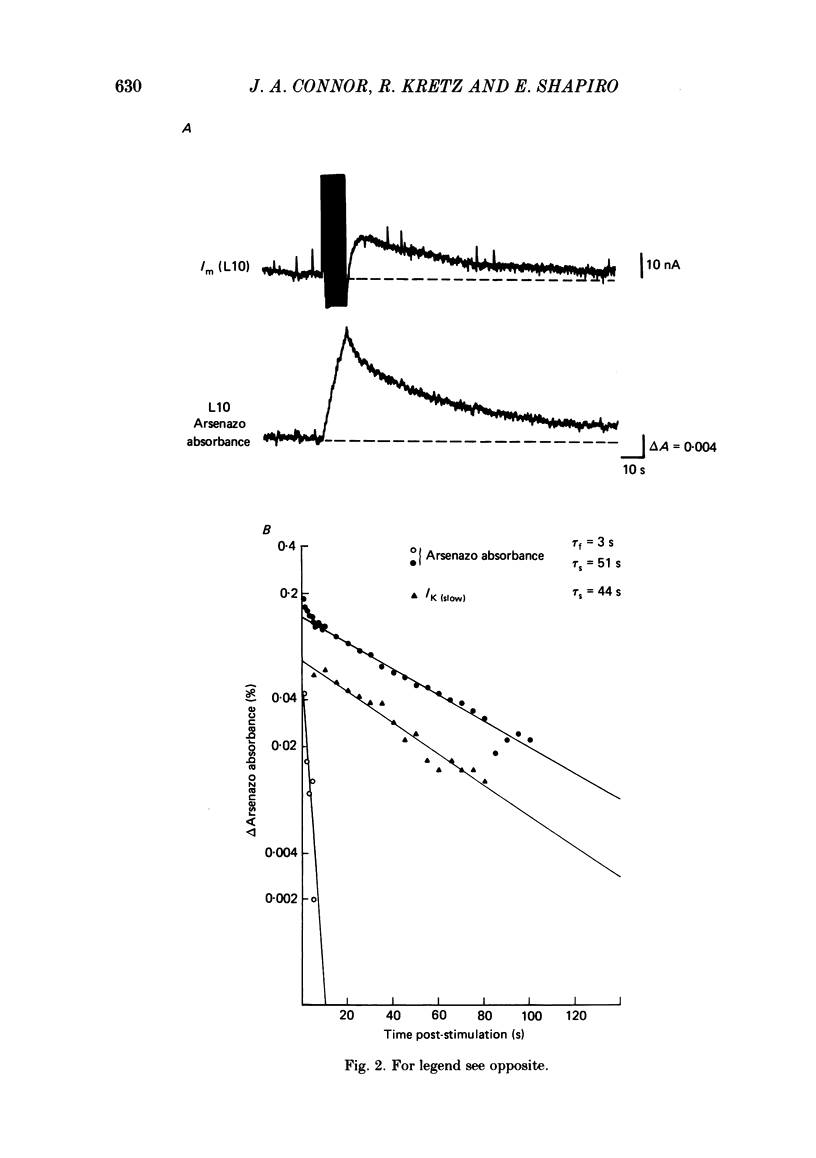
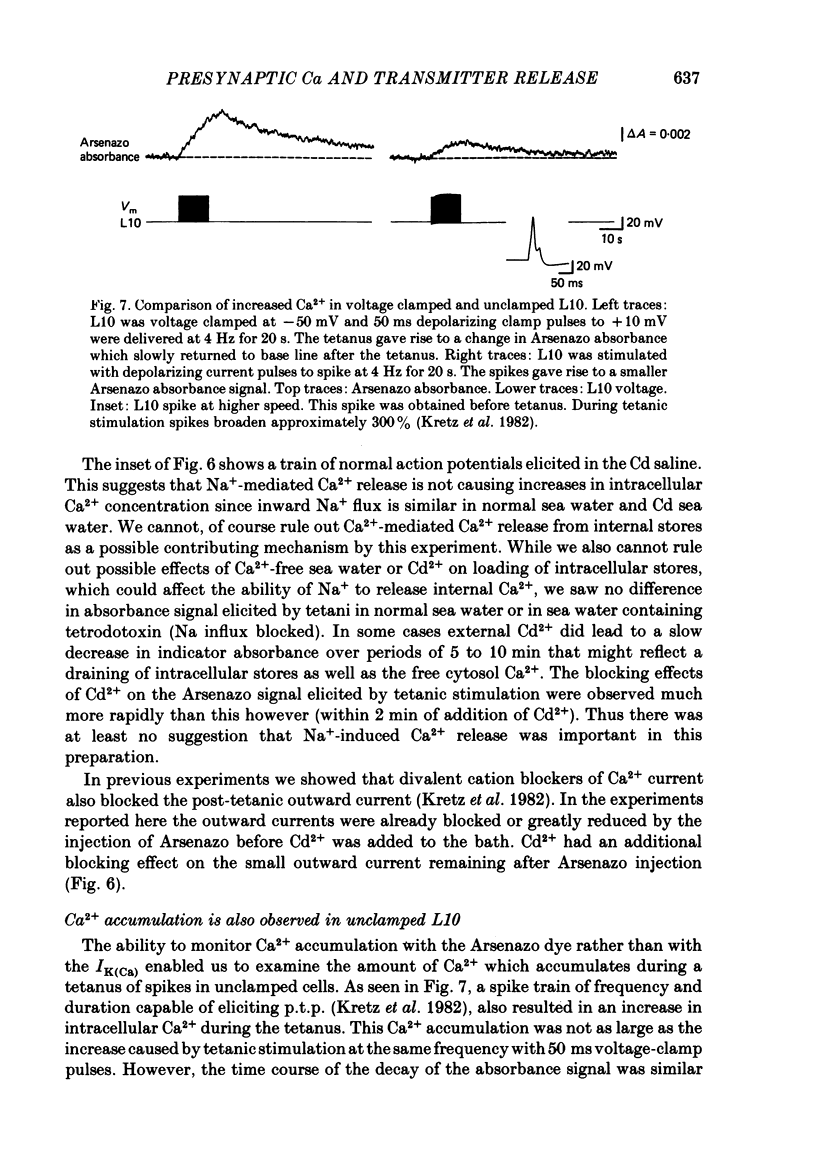
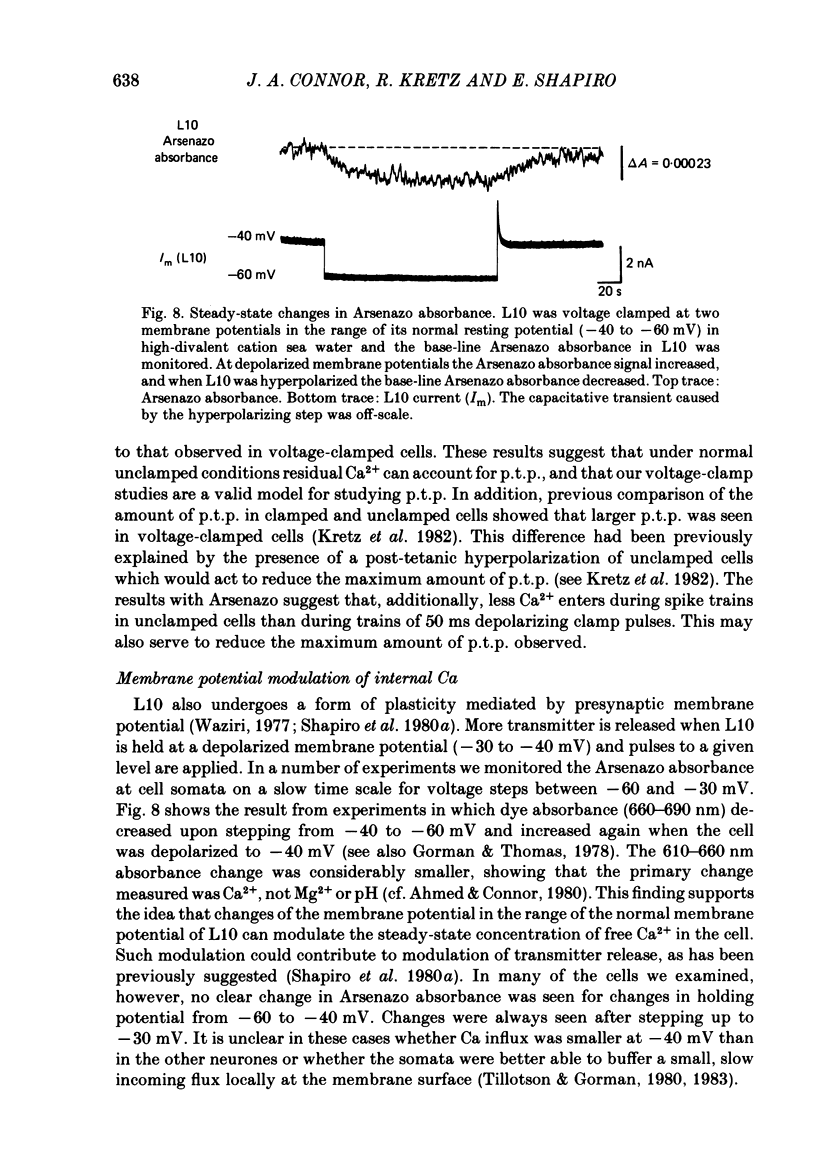
We have utilized the Ca2+ indicator dye, Arsenazo III, to examine the role of presynaptic Ca2+ concentration in two types of synaptic plasticity observed at the synapses of cell L10 in Aplysia californica; post-tetanic potentiation (p.t.p. - the increased transmitter release which follows high frequency stimulation), and resting membrane potential modulation of release. Intracellular Ca2+ was monitored in the cell body and main neurites of L10 injected with Arsenazo III. Tetanic stimulation caused an increase in intracellular Ca2+ concentration that decayed, after tetanus, with fast and slow time constants which paralleled the time course of decay of p.t.p. When the voltage-sensitive Ca2+ current was reduced by removing external Ca2+ (0 mM-Ca2+, 4 mM-EGTA) or by blocking Ca2+ channels with divalent cation channel blocker (4 mM-Cd2+), tetanic stimulation did not cause increases in Arsenazo absorbance even when Na+ currents were not blocked. This finding suggests that Ca2+ entering the cell through voltage-dependent Ca2+ channels was the major source of Ca2+ which accumulated during the tetanus. Transmitter release is increased when L10 is maintained at a depolarized membrane potential, and is decreased when L10 is hyperpolarized. We found that the base-line Arsenazo absorbance signal in L10 increased when L10 was depolarized from -60 to -40 mV and decreased when L10 was hyperpolarized. This finding supports the idea that the steady-state Ca2+ concentration contributes to the membrane-potential modulation of transmitter release. These results support the idea that transmitter release can be modulated by the residual or resting Ca2+ concentration of the presynaptic cell.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. B., Levitan I. B. Voltage and ion dependences of the slow currents which mediate bursting in Aplysia neurone R15. J Physiol. 1985 Mar;360:69–93. doi: 10.1113/jphysiol.1985.sp015604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams W. B. Slow depolarizing and hyperpolarizing currents which mediate bursting in Aplysia neurone R15. J Physiol. 1985 Mar;360:51–68. doi: 10.1113/jphysiol.1985.sp015603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Connor J. A. Intracellular pH changes induced by calcium influx during electrical activity in molluscan neurons. J Gen Physiol. 1980 Apr;75(4):403–426. doi: 10.1085/jgp.75.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Kragie L., Connor J. A. Stoichiometry and apparent dissociation constant of the calcium-arsenazo III reaction under physiological conditions. Biophys J. 1980 Dec;32(3):907–920. doi: 10.1016/S0006-3495(80)85025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Brown A. M., Dahl G., Higashi H., Isenberg G., Tsuda Y., Yatani A. Voltage-dependent activation of potassium current in Helix neurones by endogenous cellular calcium. J Physiol. 1983 Jan;334:309–324. doi: 10.1113/jphysiol.1983.sp014496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood H. L., Charlton M. P., Thompson C. S. Neuromuscular transmission in crustaceans is enhanced by a sodium ionophore, monensin, and by prolonged stimulation. J Physiol. 1983 Feb;335:179–195. doi: 10.1113/jphysiol.1983.sp014527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P. J. Affinity and stoichiometry of calcium binding by arsenazo III. Anal Biochem. 1981 Jan 1;110(1):61–72. doi: 10.1016/0003-2697(81)90112-3. [DOI] [PubMed] [Google Scholar]
- Brown H. M., Rydqvist B. Arsenazo III-Ca2+. Effect of pH, ionic strength, and arsenazo III concentration on equilibrium binding evaluated with Ca2+ ion-sensitive electrodes and absorbance measurements. Biophys J. 1981 Oct;36(1):117–137. doi: 10.1016/S0006-3495(81)84720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Cohen L. B., De Weer P., Pinto L. H., Ross W. N., Salzberg B. M. Rapid changes in intracellular free calcium concentration. Detection by metallochromic indicator dyes in squid giant axon. Biophys J. 1975 Nov;15(11):1155–1160. doi: 10.1016/S0006-3495(75)85891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. STIMULUS-SECRETION COUPLING IN A NEUROSECRETORY ORGAN: THE ROLE OF CALCIUM IN THE RELEASE OF VASOPRESSIN FROM THE NEUROHYPOPHYSIS. J Physiol. 1964 Jul;172:1–18. doi: 10.1113/jphysiol.1964.sp007399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Changes in the intracellular concentration of free calcium ions in a pace-maker neurone, measured with the metallochromic indicator dye arsenazo III. J Physiol. 1978 Feb;275:357–376. doi: 10.1113/jphysiol.1978.sp012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Intracellular calcium accumulation during depolarization in a molluscan neurone. J Physiol. 1980 Nov;308:259–285. doi: 10.1113/jphysiol.1980.sp013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockberger P., Connor J. A. Intracellular calcium measurements with arsenazo III during cyclic AMP injections into molluscan neurons. Science. 1983 Feb 18;219(4586):869–871. doi: 10.1126/science.6297009. [DOI] [PubMed] [Google Scholar]
- Hofmeier G., Lux H. D. The time courses of intracellular free calcium and related electrical effects after injection of CaCl2 into neurons of the snail, Helix pomatia. Pflugers Arch. 1981 Sep;391(3):242–251. doi: 10.1007/BF00596178. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The timing of calcium action during neuromuscular transmission. J Physiol. 1967 Apr;189(3):535–544. doi: 10.1113/jphysiol.1967.sp008183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Shapiro E., Kandel E. R. Synaptic plasticity and the modulation of the Ca2+ current. J Exp Biol. 1980 Dec;89:117–157. doi: 10.1242/jeb.89.1.117. [DOI] [PubMed] [Google Scholar]
- Koester J., Mayeri E., Liebeswar G., Kandel E. R. Neural control of circulation in Aplysia. II. Interneurons. J Neurophysiol. 1974 May;37(3):476–496. doi: 10.1152/jn.1974.37.3.476. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-dependent inward current in Aplysia bursting pace-maker neurones. J Physiol. 1985 May;362:107–130. doi: 10.1113/jphysiol.1985.sp015666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R. H., Zucker R. S. Calcium-induced inactivation of calcium current causes the inter-burst hyperpolarization of Aplysia bursting neurones. J Physiol. 1985 May;362:131–160. doi: 10.1113/jphysiol.1985.sp015667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz R., Shapiro E., Kandel E. R. Post-tetanic potentiation at an identified synapse in Aplysia is correlated with a Ca2+-activated K+ current in the presynaptic neuron: evidence for Ca2+ accumulation. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5430–5434. doi: 10.1073/pnas.79.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. V. Spike aftercurrents in R15 of Aplysia: their relationship to slow inward current and calcium influx. J Neurophysiol. 1984 Feb;51(2):387–403. doi: 10.1152/jn.1984.51.2.387. [DOI] [PubMed] [Google Scholar]
- Lewis D. V., Wilson W. A. Calcium influx and poststimulus current during early adaptation in Aplysia giant neurons. J Neurophysiol. 1982 Jul;48(1):202–216. doi: 10.1152/jn.1982.48.1.202. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A dual effect of repetitive stimulation on post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):163–182. doi: 10.1113/jphysiol.1975.sp010839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol. 1976 May;257(2):449–470. doi: 10.1113/jphysiol.1976.sp011378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Harafuji H., Kurebayashi N. Comparison of the characteristics of four metallochromic dyes as potential calcium indicators for biological experiments. J Biochem. 1980 May;87(5):1293–1303. doi: 10.1093/oxfordjournals.jbchem.a132867. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Lev-Tov A., Meiri H. Primary and secondary regulation of quantal transmitter release: calcium and sodium. J Exp Biol. 1980 Dec;89:5–18. doi: 10.1242/jeb.89.1.5. [DOI] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic inhibition in Aplysia involves a decrease in the Ca2+ current of the presynaptic neuron. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1185–1189. doi: 10.1073/pnas.77.2.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci U S A. 1980 Jan;77(1):629–633. doi: 10.1073/pnas.77.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Zucker R. S. Aequorin response facilitation and intracellular calcium accumulation in molluscan neurones. J Physiol. 1980 Mar;300:167–196. doi: 10.1113/jphysiol.1980.sp013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Calcium influx in active Aplysia neurones detected by injected aequorin. Nat New Biol. 1973 Mar 28;242(117):113–115. doi: 10.1038/newbio242113b0. [DOI] [PubMed] [Google Scholar]
- Stockbridge N., Ross W. N. Localized Ca2+ and calcium-activated potassium conductances in terminals of a barnacle photoreceptor. Nature. 1984 May 17;309(5965):266–268. doi: 10.1038/309266a0. [DOI] [PubMed] [Google Scholar]
- Thomas M. V. Arsenazo III forms 2:1 complexes with Ca and 1:1 complexes with Mg under physiological conditions. Estimates of the apparent dissociation constants. Biophys J. 1979 Mar;25(3):541–548. doi: 10.1016/S0006-3495(79)85322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. L., Gorman A. L. Localization of neuronal Ca2+ buffering near plasma membrane studied with different divalent cations. Cell Mol Neurobiol. 1983 Dec;3(4):297–310. doi: 10.1007/BF00734712. [DOI] [PubMed] [Google Scholar]
- Tillotson D., Gorman A. L. Non-uniform Ca2+ buffer distribution in a nerve cell body. Nature. 1980 Aug 21;286(5775):816–817. doi: 10.1038/286816a0. [DOI] [PubMed] [Google Scholar]
- Waziri R., Kandel E. R., Frazier W. T. Organization of inhibition in abdominal ganglion of Aplysia. II. Posttetanic potentiation, heterosynaptic depression, and increments in frequency of inhibitory postsynaptic potentials. J Neurophysiol. 1969 Jul;32(4):509–519. doi: 10.1152/jn.1969.32.4.509. [DOI] [PubMed] [Google Scholar]
- Waziri R. Presynaptic electrical coupling in Aplysia: effects on postsynaptic chemical transmission. Science. 1977 Feb 25;195(4280):790–792. doi: 10.1126/science.189390. [DOI] [PubMed] [Google Scholar]
- Winlow W., Kandel E. R. The morphology of identified neurons in the abdominal ganglion of Aplysia californica. Brain Res. 1976 Aug 13;112(2):221–249. doi: 10.1016/0006-8993(76)90282-1. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Characteristics of crayfish neuromuscular facilitation and their calcium dependence. J Physiol. 1974 Aug;241(1):91–110. doi: 10.1113/jphysiol.1974.sp010642. [DOI] [PMC free article] [PubMed] [Google Scholar]



