Abstract
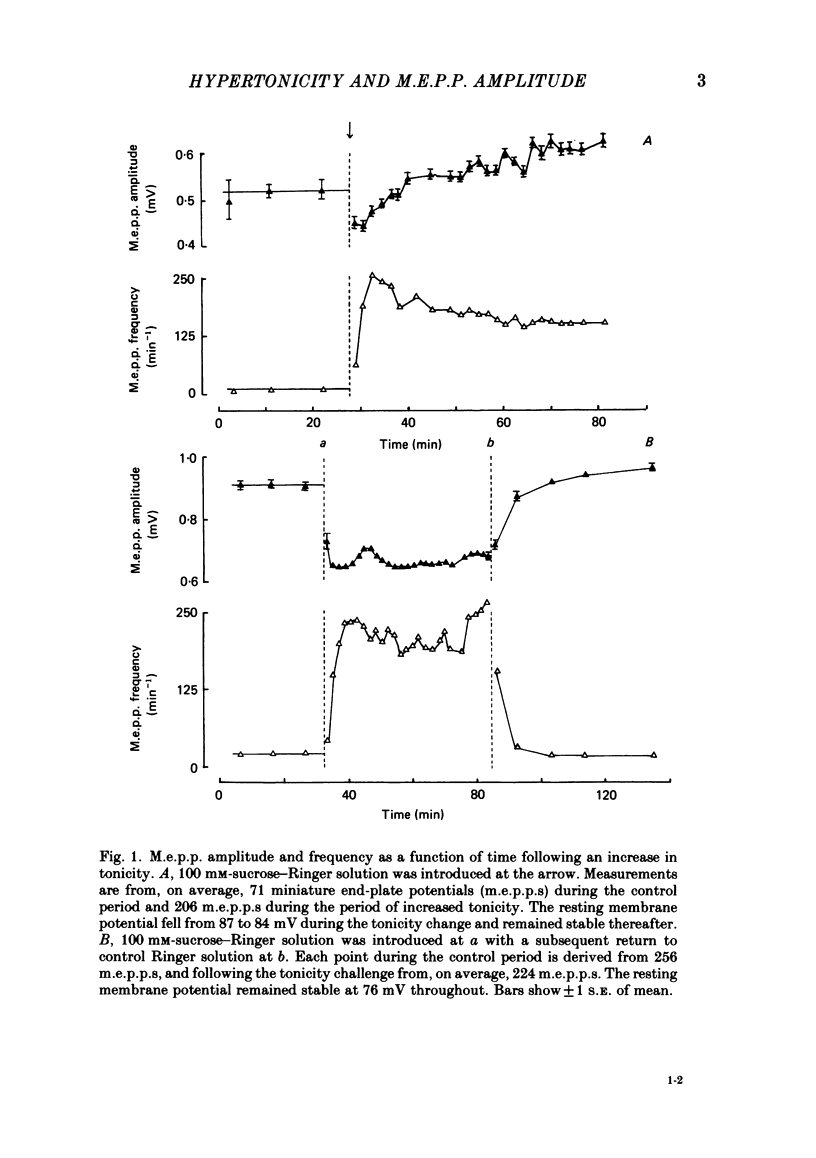
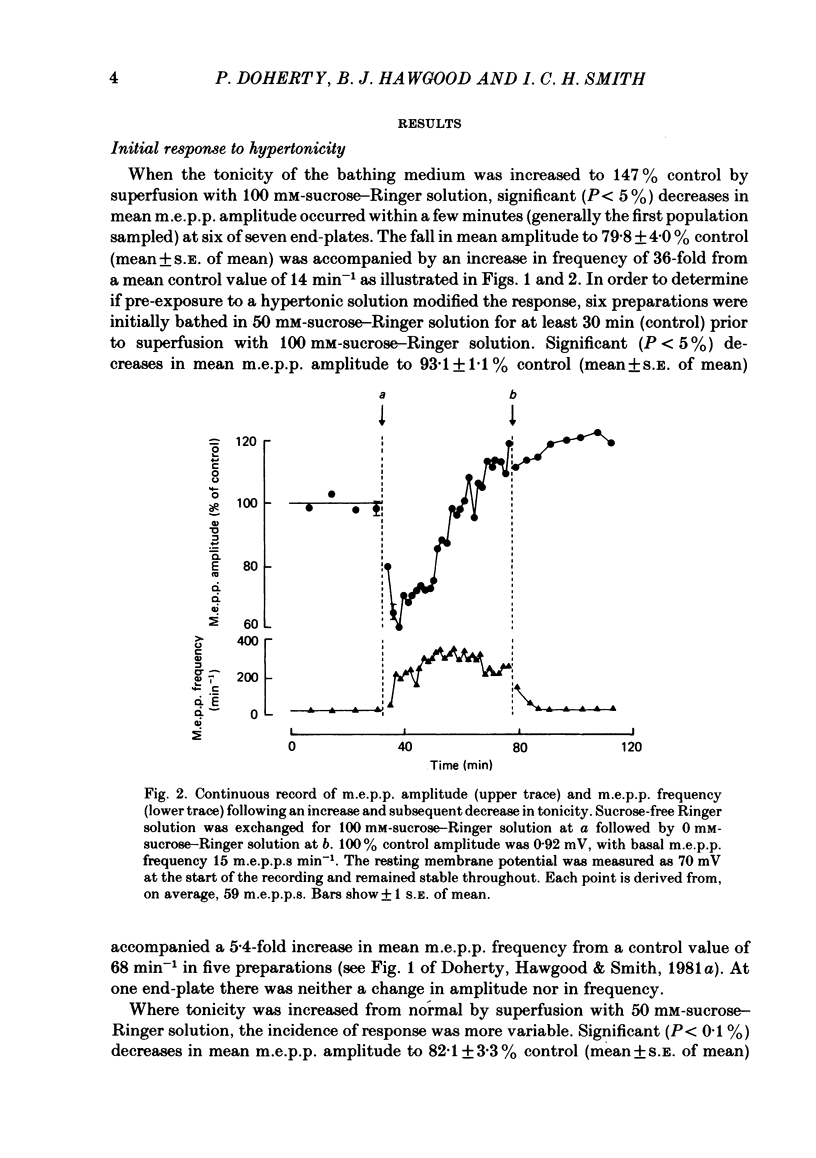
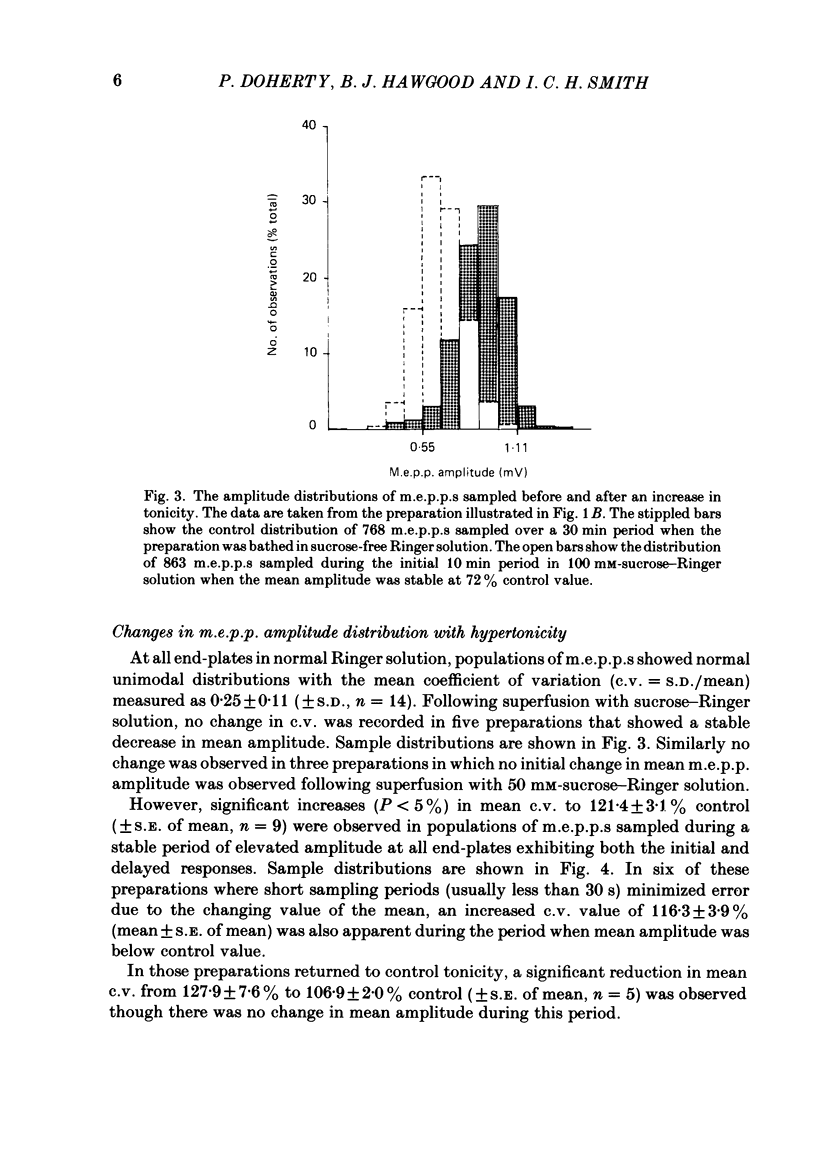
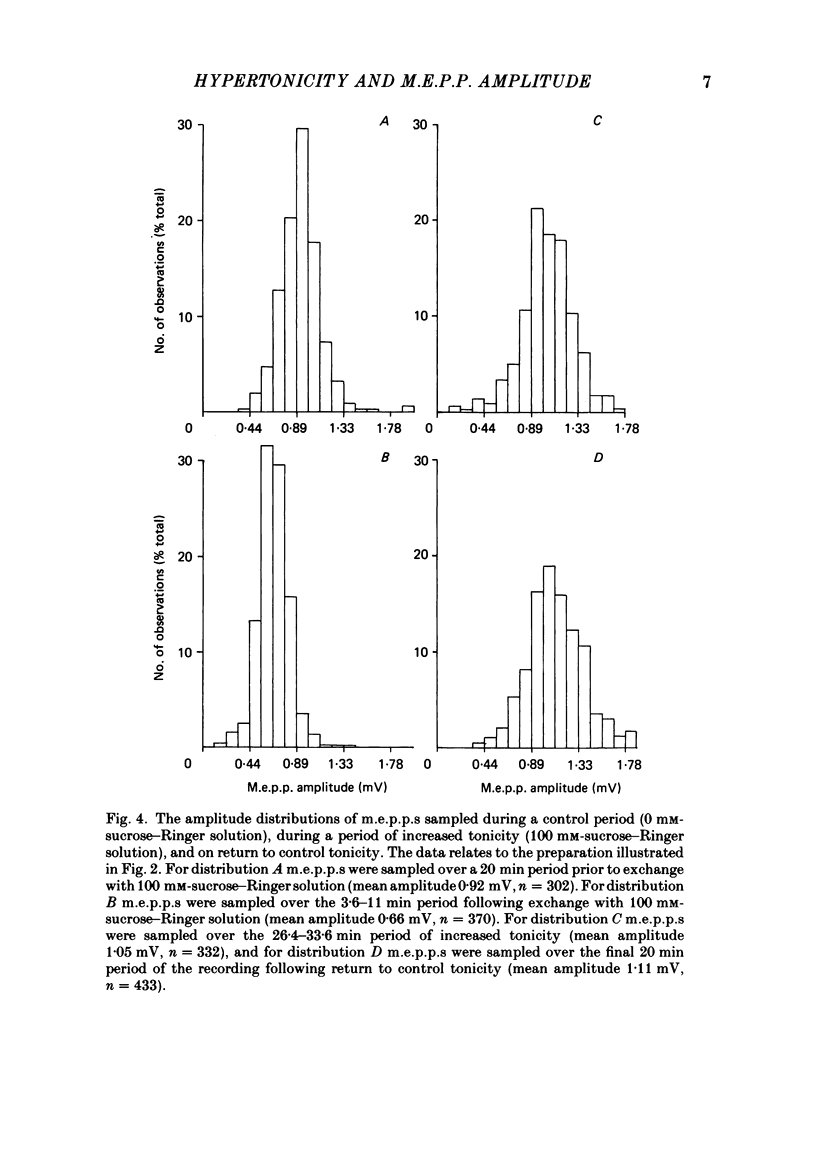
The effect of an increase in tonicity on the amplitude and frequency of miniature end-plate potentials (m.e.p.p.s) following superfusion of 50 mM- or 100 mM-sucrose-Ringer solution was determined from intracellular recording at end-plates of the frog cutaneous pectoris muscle, in the absence of drugs and using on-line statistical analysis. An immediate decrease in mean amplitude of the order of 20% control was associated with a marked increase in mean frequency. A delayed increase in mean amplitude, independent of frequency, followed the initial response in the majority of end-plates exposed to 100 mM-sucrose-Ringer solution. The net gain was of the order of 34% and was attained over 20-40 min. This was not reversible over at least 20 min. There was an increase in the variability of m.e.p.p. amplitudes associated with the increase of mean amplitude but this was reversible. A moderate increase in tonicity is considered to induce two kinds of presynaptic changes. First, an immediate increase in the probability of release of smaller quantal packets and secondly, an increased loading of transmitter into vesicles within the readily releasable store.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceccarelli B., Grohovaz F., Hurlbut W. P. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. II. Effects of electrical stimulation and high potassium. J Cell Biol. 1979 Apr;81(1):178–192. doi: 10.1083/jcb.81.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W. Changes in the structure of neuromuscular junctions caused by variations in osmotic pressure. J Cell Biol. 1976 Jun;69(3):521–538. doi: 10.1083/jcb.69.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P., Hawgood B. J., Smith I. C. Changes in miniature end-plate potentials after brief nervous stimulation at the frog neuromuscular junction. J Physiol. 1984 Nov;356:349–358. doi: 10.1113/jphysiol.1984.sp015469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Structural changes after transmitter release at the frog neuromuscular junction. J Cell Biol. 1981 Mar;88(3):564–580. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. J Physiol. 1968 Aug;197(3):639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. Some effects of nerve stimulation andhemicholinium on quantal transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Mar;207(1):51–61. doi: 10.1113/jphysiol.1970.sp009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S., Robbins N. Bimodal miniature and evoked end-plate potentials in adult mouse neuromuscular junctions. J Physiol. 1984 Jan;346:353–363. doi: 10.1113/jphysiol.1984.sp015027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., van der Kloot W. Time course and magnitude of effects of changes in tonicity on acetylcholine release at frog neuromuscular junction. J Neurophysiol. 1977 Mar;40(2):212–224. doi: 10.1152/jn.1977.40.2.212. [DOI] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A., Rang H. P. Factors affecting the rate of incorporation of a false transmitter into mammalian motor nerve terminals. J Physiol. 1978 Dec;285:1–24. doi: 10.1113/jphysiol.1978.sp012553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. A study of desensitization of acetylcholine receptors using nerve-released transmitter in the frog. J Physiol. 1981 Jul;316:225–250. doi: 10.1113/jphysiol.1981.sp013784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y., Alnaes E., Rahamimoff R. Is hyperosmotic neurosecretion from motor nerve endings a calcium-dependent process? Nature. 1977 May 12;267(5607):170–172. doi: 10.1038/267170a0. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. Quantal size is not altered by abrupt changes in nerve terminal volume. Nature. 1978 Feb 9;271(5645):561–562. doi: 10.1038/271561a0. [DOI] [PubMed] [Google Scholar]
- Wernig A. Localization of active sites in the neuromuscular junction of the frog. Brain Res. 1976 Dec 10;118(1):63–72. doi: 10.1016/0006-8993(76)90841-6. [DOI] [PubMed] [Google Scholar]


