Abstract
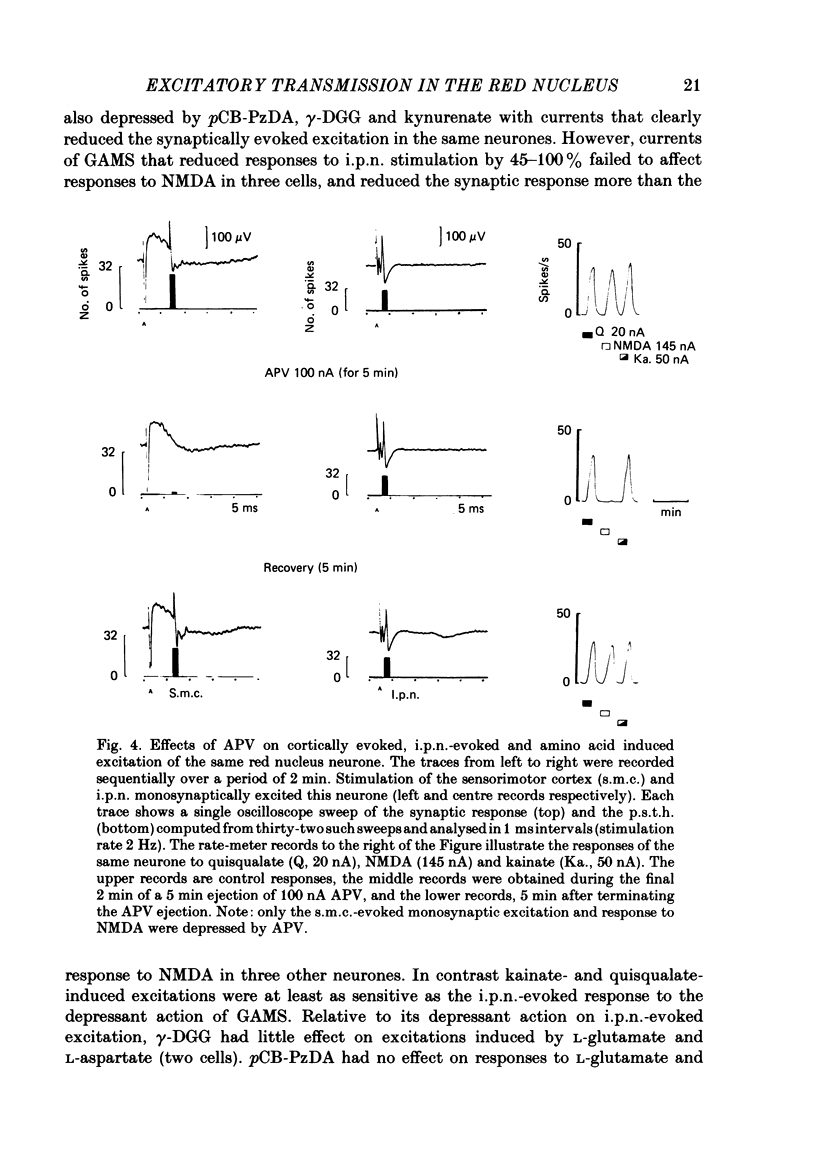
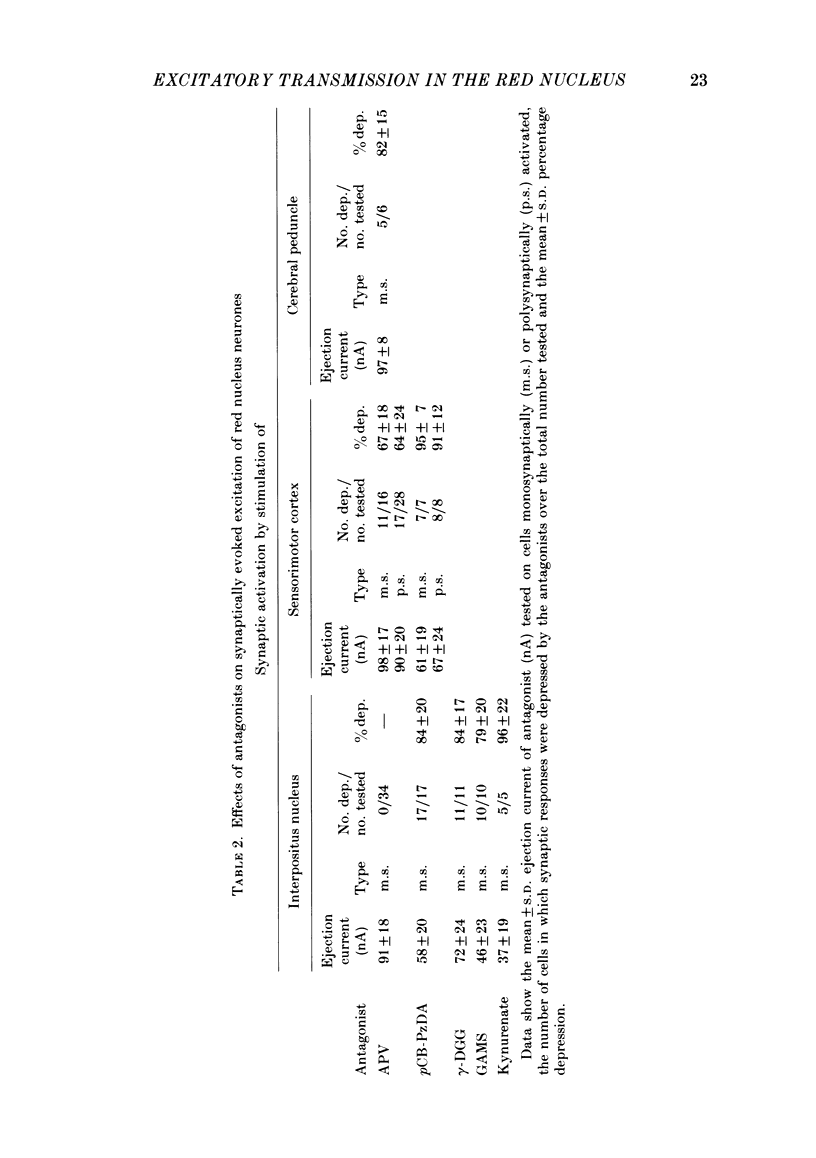
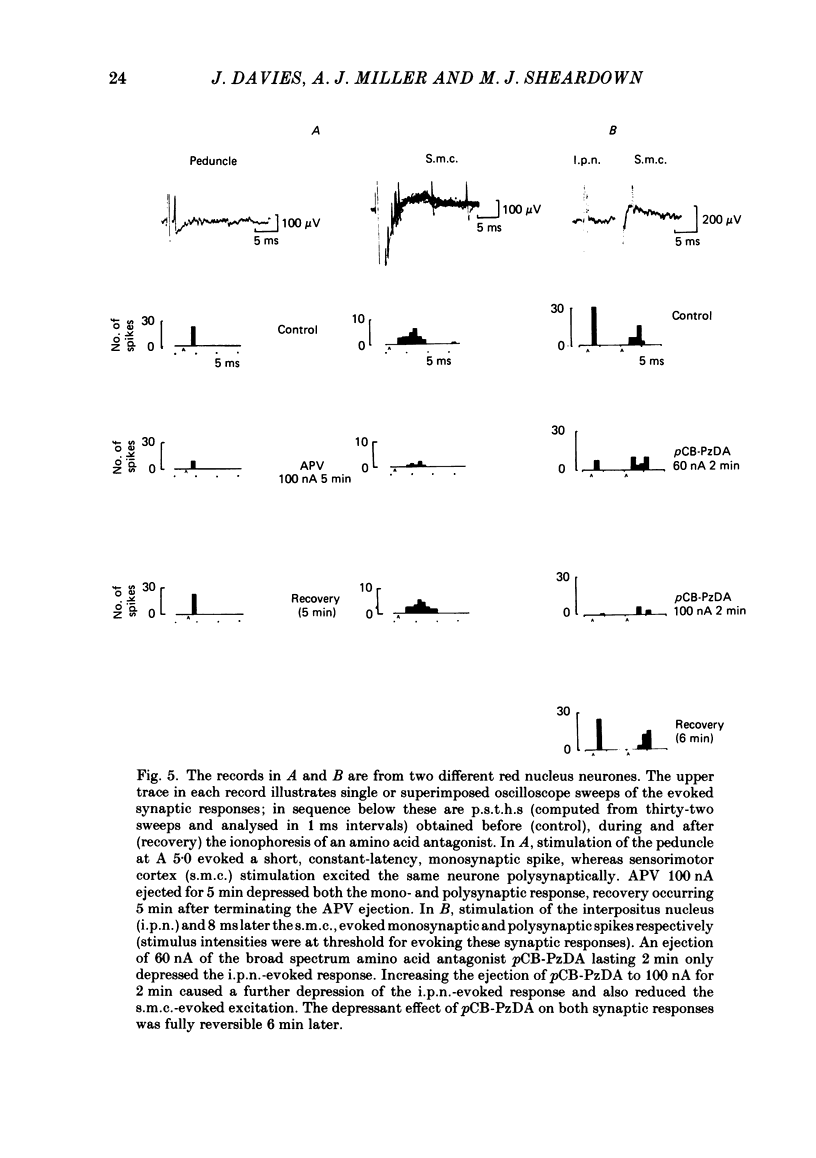
A study has been made of the effects of the selective N-methyl-D-aspartate receptor antagonist, 2-amino-5-phosphonovalerate (APV), and the broad spectrum excitatory amino acid antagonists, gamma-D-glutamylglycine (gamma-DGG), gamma-D-glutamylaminomethylsulphonate (GAMS), 4(p-chlorobenzoyl)-cis-piperazine-2, 3-dicarboxylate (pCB-PzDA) and kynurenate, have been examined on excitation evoked on neurones in the magnocellular red nucleus (m.r.n.) of the anaesthetized cat by stimulation of the interpositus nucleus (i.p.n.) and sensorimotor cortex, and by ionophoresed excitant amino acid agonists. The profile of activity of the excitatory amino acid antagonists on m.r.n. neurones was similar to that described on neurones in other areas of the central nervous system. APV selectively depressed responses to N-methyl-D-aspartate (NMDA), whereas the broader spectrum antagonists reduced responses to kainate and quisqualate as well as to NMDA. Neuronal responses to L-glutamate and L-aspartate were depressed by all the antagonists tested. I.p.n.-evoked monosynaptic responses of m.r.n. neurones were reversibly reduced by the broad spectrum antagonists, but were unaffected by APV. Cortically evoked mono- and polysynaptic excitatory responses were reversibly depressed by APV and the broad spectrum antagonist, pCB-PzDA. The action of APV corresponded with its ability to antagonize responses to NMDA. However, the cortically evoked responses appeared to be more sensitive to the actions of pCB-PzDA than to those of APV, although the former is a less effective antagonist of NMDA-induced excitation compared with APV. APV depressed excitation induced by cortical stimuli and L-glutamate and L-aspartate. However, there was no obvious correlation between the actions of the broad spectrum amino acid antagonists on synaptically evoked responses and those induced by L-glutamate or L-aspartate on the few neurones tested. These results are consistent with an amino acid being the transmitter in the interposito-rubral and cortico-rubral excitatory pathways which interacts with non-NMDA and both NMDA and non-NMDA receptors respectively. However, the identity of the transmitter acting at these receptors remains to be determined.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann H., ten Bruggencate G., Pickelmann P., Steinberg R. Effects of glutamate, aspartate, and two-presumed antagonists on feline rubrospinal neurones. Pflugers Arch. 1976 Aug 24;364(3):249–255. doi: 10.1007/BF00581763. [DOI] [PubMed] [Google Scholar]
- Anderson M. E. Cerebellar and cerebral inputs to physiologically identified efferent cell groups in the red nucleus of the cat. Brain Res. 1971 Jul 9;30(1):49–66. doi: 10.1016/0006-8993(71)90005-9. [DOI] [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The detection of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:33–62. doi: 10.1113/jphysiol.1985.sp015845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Jones A. W., Sheardown M. J., Smith D. A., Watkins J. C. Phosphono dipeptides and piperazine derivatives as antagonists of amino acid-induced and synaptic excitation in mammalian and amphibian spinal cord. Neurosci Lett. 1984 Nov 23;52(1-2):79–84. doi: 10.1016/0304-3940(84)90354-9. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982 Mar 11;235(2):378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Depressant actions of gamma-D-glutamylaminomethyl sulfonate (GAMS) on amino acid-induced and synaptic excitation in the cat spinal cord. Brain Res. 1985 Feb 18;327(1-2):113–120. doi: 10.1016/0006-8993(85)91505-7. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Differentiation of kainate and quisqualate receptors in the cat spinal cord by selective antagonism with gamma-D(and L)-glutamylglycine. Brain Res. 1981 Feb 9;206(1):172–177. doi: 10.1016/0006-8993(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Role of excitatory amino acid receptors in mono- and polysynaptic excitation in the cat spinal cord. Exp Brain Res. 1983;49(2):280–290. doi: 10.1007/BF00238587. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Scheid P., Táboríková H. Responses of red nucleus neurons to antidromic and synaptic activation. J Neurophysiol. 1975 Jul;38(4):947–964. doi: 10.1152/jn.1975.38.4.947. [DOI] [PubMed] [Google Scholar]
- Ganong A. H., Lanthorn T. H., Cotman C. W. Kynurenic acid inhibits synaptic and acidic amino acid-induced responses in the rat hippocampus and spinal cord. Brain Res. 1983 Aug 22;273(1):170–174. doi: 10.1016/0006-8993(83)91108-3. [DOI] [PubMed] [Google Scholar]
- Herrling P. L. Pharmacology of the corticocaudate excitatory postsynaptic potential in the cat: evidence for its mediation by quisqualate- or kainate-receptors. Neuroscience. 1985 Feb;14(2):417–426. doi: 10.1016/0306-4522(85)90301-x. [DOI] [PubMed] [Google Scholar]
- Huffman R. D., Davis R. Pharmacology of the brachium conjunctivum: red nucleus synaptic system in the baboon. J Neurosci Res. 1977;3(3):175–192. doi: 10.1002/jnr.490030302. [DOI] [PubMed] [Google Scholar]
- Jeneskog T., Padel Y. Cerebral cortical areas of origin of excitation and inhibition of rubrospinal cells in the cat. Exp Brain Res. 1983;50(2-3):309–320. doi: 10.1007/BF00239195. [DOI] [PubMed] [Google Scholar]
- Kerkerian L., Nieoullon A., Dusticier N. Topographic changes in high-affinity glutamate uptake in the cat red nucleus, substantia nigra, thalamus, and caudate nucleus after lesions of sensorimotor cortical areas. Exp Neurol. 1983 Sep;81(3):598–612. doi: 10.1016/0014-4886(83)90329-1. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Mixed-agonist action of excitatory amino acids on mouse spinal cord neurones under voltage clamp. J Physiol. 1984 Sep;354:29–53. doi: 10.1113/jphysiol.1984.sp015360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Mizuno N. An electron microscopic study of the interposito-rubral connections in the cat and rabbit. Brain Res. 1971 Dec 10;35(1):283–286. doi: 10.1016/0006-8993(71)90619-6. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Kerkerian L., Dusticier N. High affinity glutamate uptake in the red nucleus and ventrolateral thalamus after lesion of the cerebellum in the adult cat: biochemical evidence for functional changes in the deafferented structures. Exp Brain Res. 1984;55(3):409–419. doi: 10.1007/BF00235271. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olverman H. J., Jones A. W., Watkins J. C. L-glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature. 1984 Feb 2;307(5950):460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- Perkins M. N., Stone T. W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982 Sep 9;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Berry K., Hansen S., Diamond S., Mok C. Regional distribution of amino acids in human brain obtained at autopsy. J Neurochem. 1971 Mar;18(3):513–519. doi: 10.1111/j.1471-4159.1971.tb11979.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H., Kubota M., Nakamura M., Tsukahara N. Effects of amino acids on cat red nucleus neurons in vitro. Exp Brain Res. 1984;54(1):150–156. doi: 10.1007/BF00235826. [DOI] [PubMed] [Google Scholar]
- Sawada S., Yamamoto C. Gamma-D-glutamylglycine and cis-2,3-piperidine dicarboxylate as antagonists of excitatory amino acids in the hippocampus. Exp Brain Res. 1984;55(2):351–358. doi: 10.1007/BF00237285. [DOI] [PubMed] [Google Scholar]
- Toyama K., Tsukahara N., Kosaka K., Matsunami K. Synaptic excitation of red nucleus neurones by fibres from interpositus nucleus. Exp Brain Res. 1970;11(2):187–198. doi: 10.1007/BF00234322. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Fuller D. R., Brooks V. B. Collateral pyramidal influences on the corticorubrospinal system. J Neurophysiol. 1968 May;31(3):467–484. doi: 10.1152/jn.1968.31.3.467. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Korn H., Stone J. Pontine relay from cerebral cortex to cerebellar cortex and nucleus interpositus. Brain Res. 1968 Sep;10(3):448–453. doi: 10.1016/0006-8993(68)90213-8. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Kosaka K. The mode of cerebral excitation of red nucleus neurons. Exp Brain Res. 1968;5(2):102–117. doi: 10.1007/BF00238700. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]



