Abstract
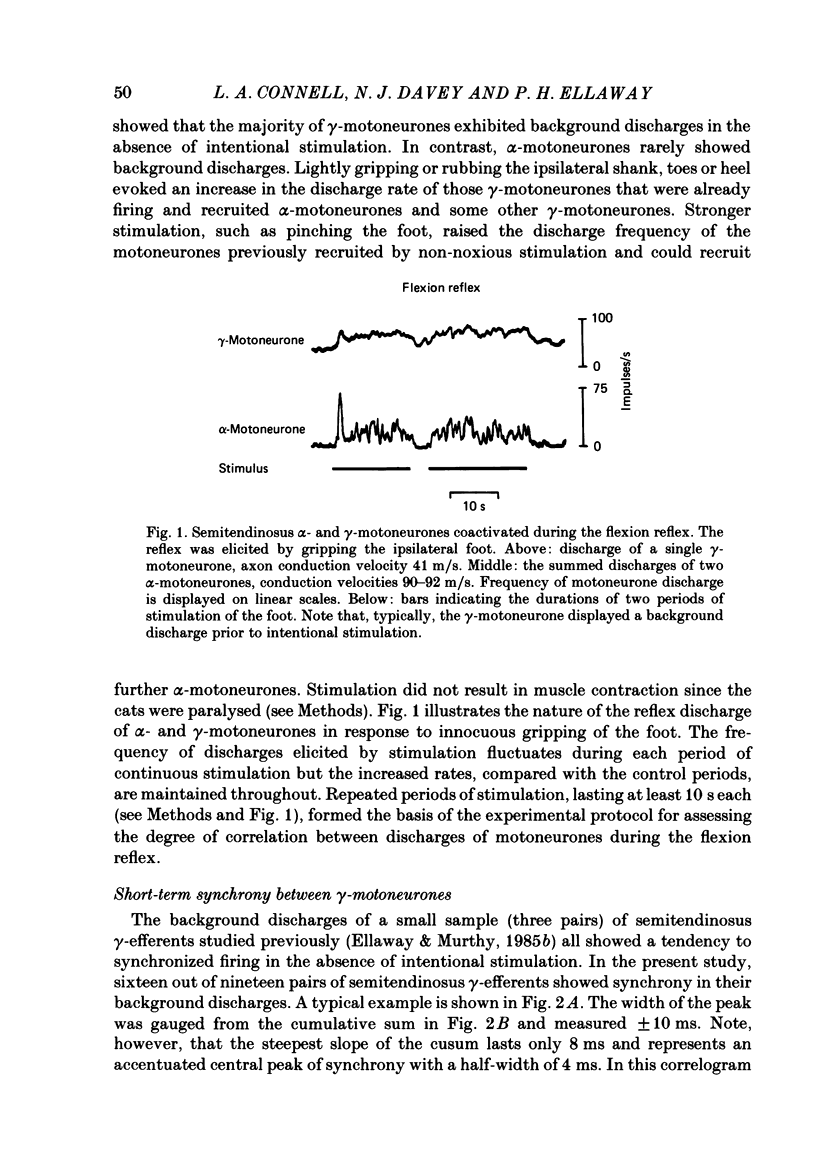
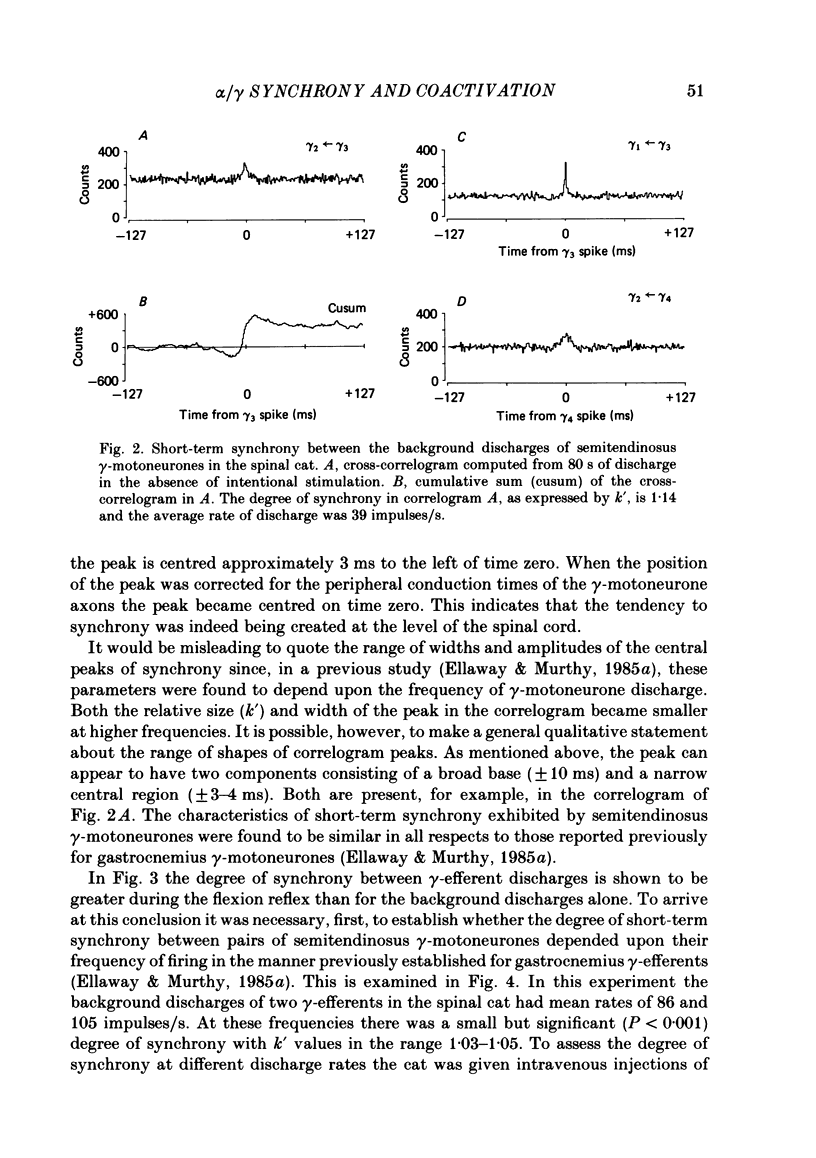
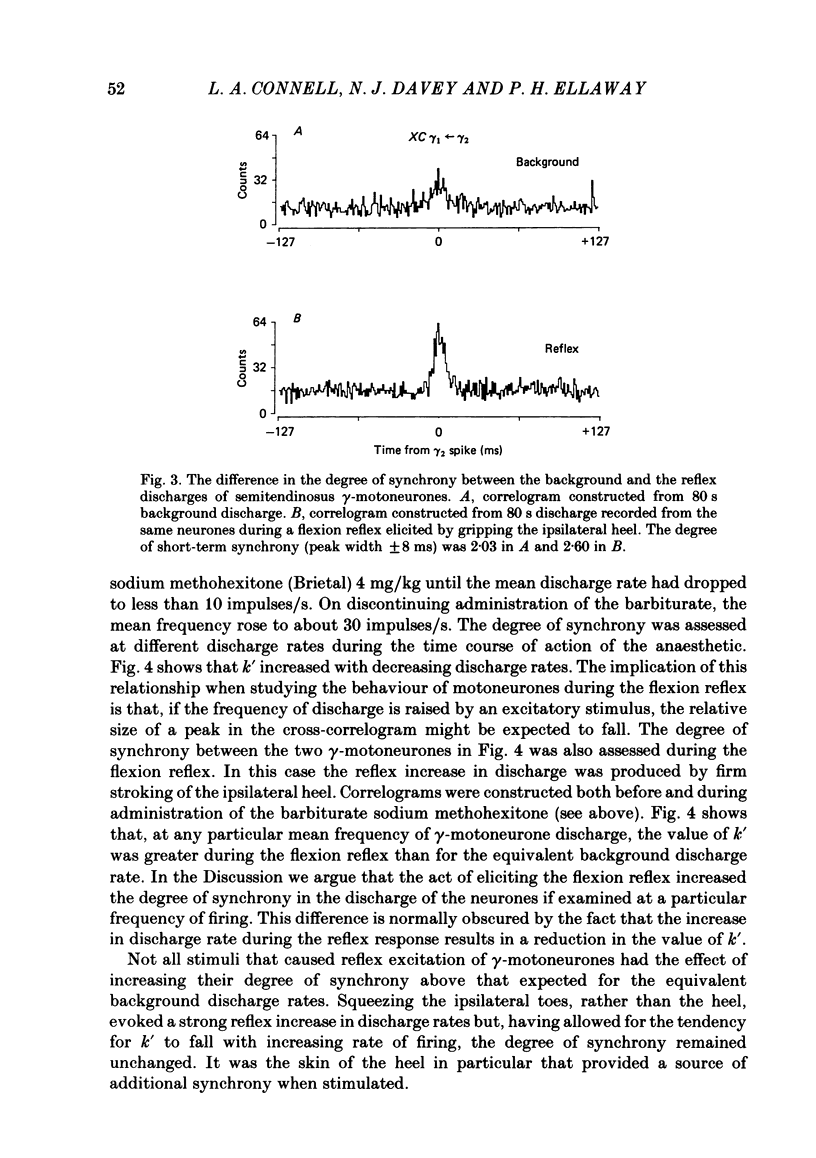
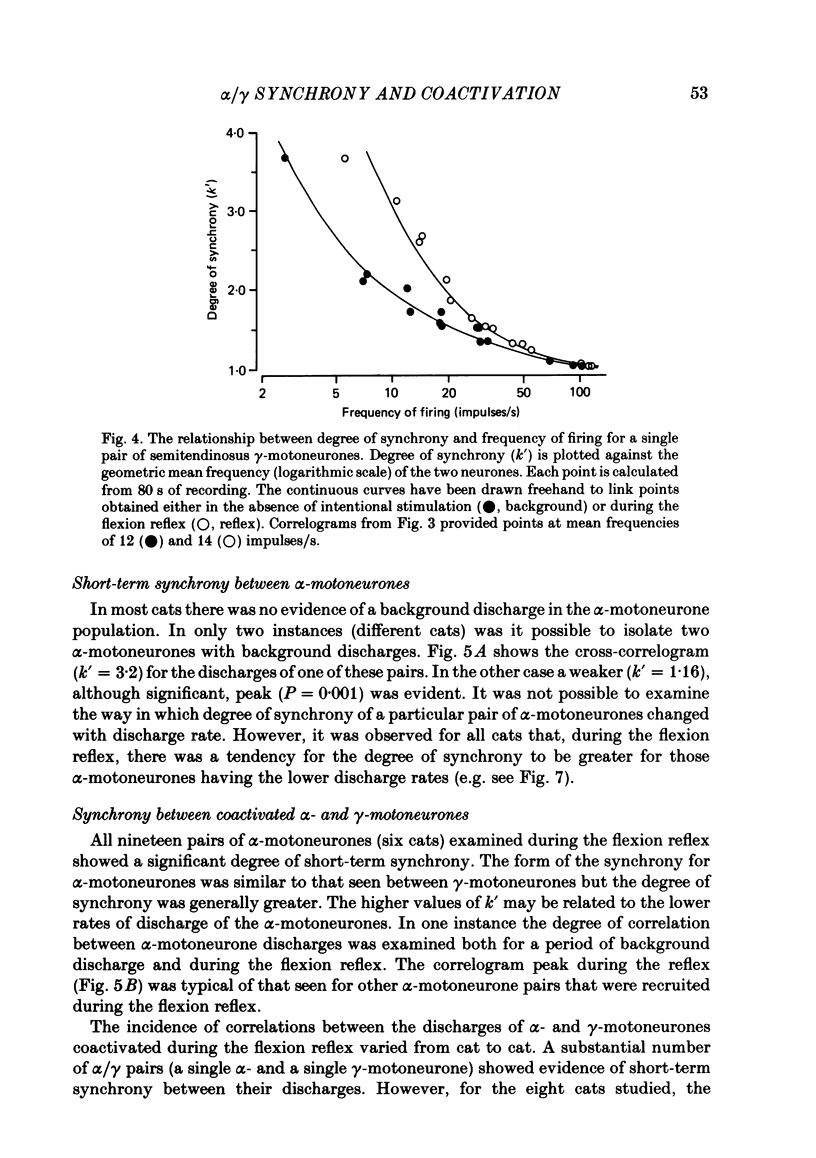
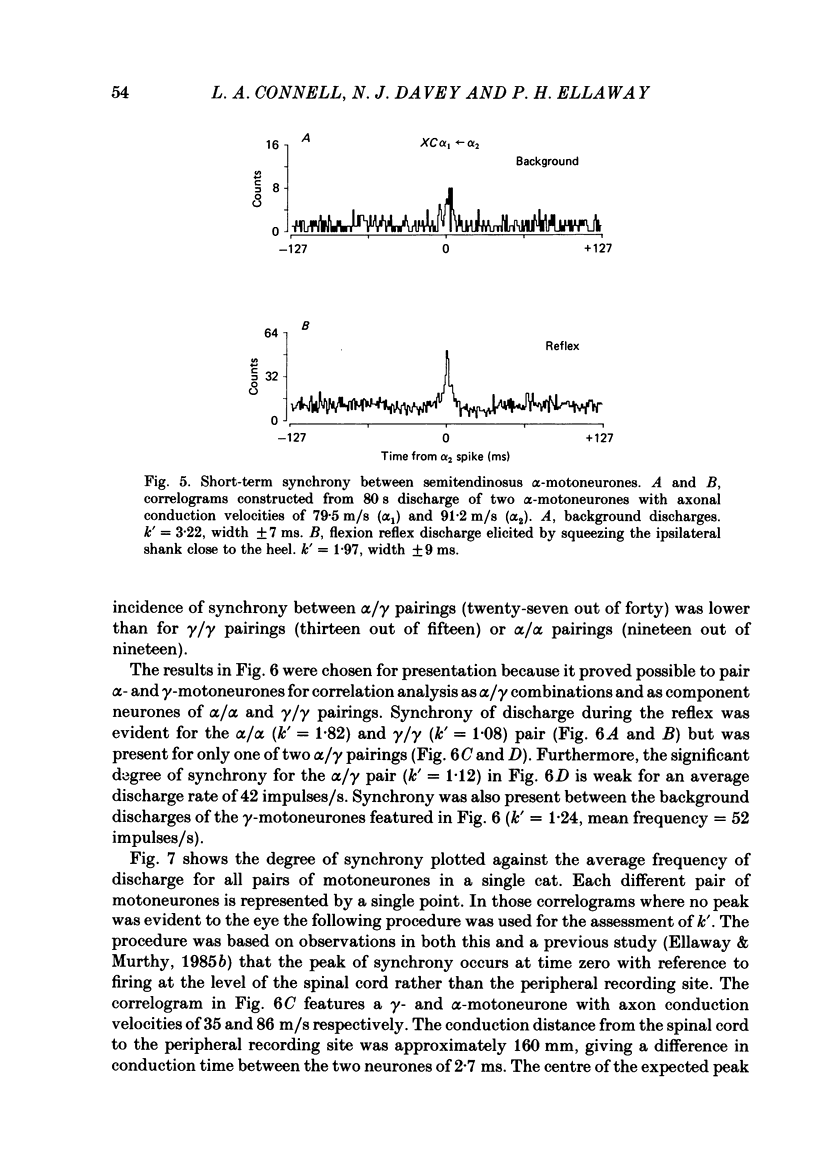
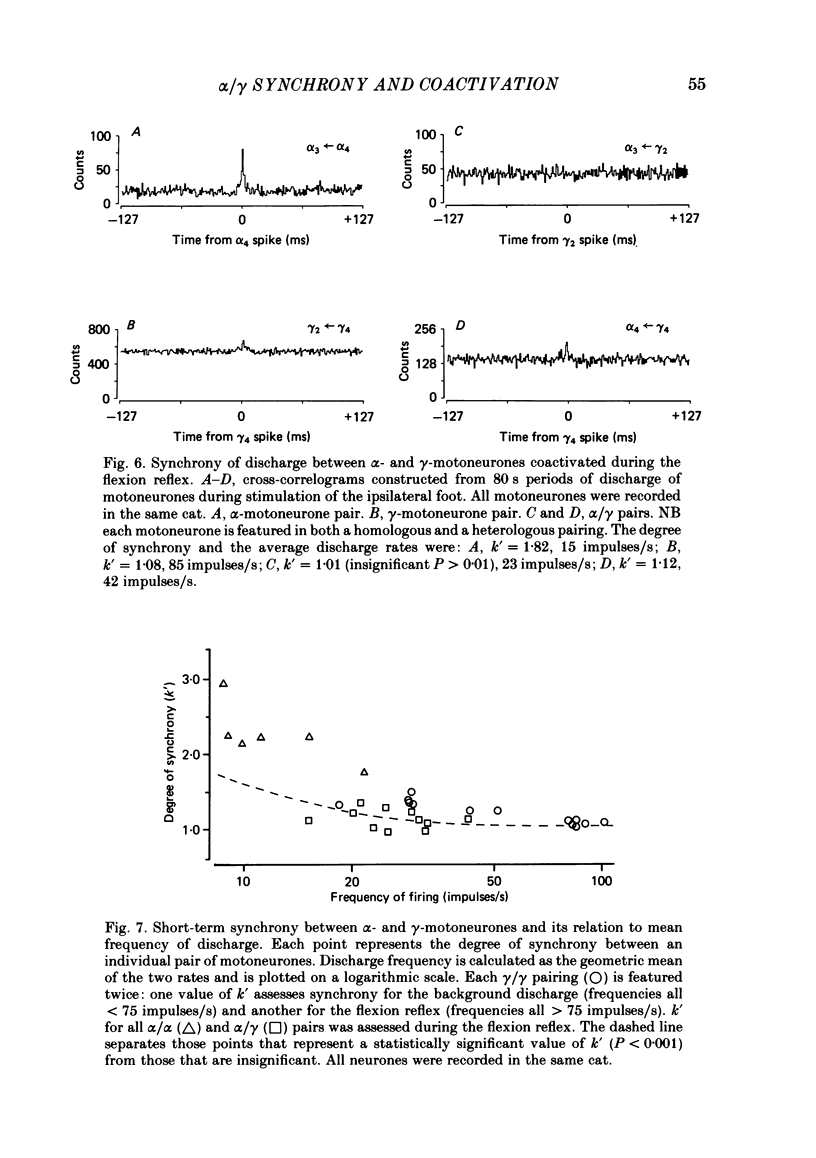
Cross-correlation analysis of unitary neuronal discharges has been used to study the linkage between alpha- and gamma-motoneurones coactivated during the flexion reflex of the semitendinosus muscle in the decerebrated spinal cat. A flexion reflex was elicited by firm grip or squeeze of the ipsilateral heel, shank or foot. The stimulus excited the discharges of both alpha- and gamma-motoneurones and increased the frequency of discharge of those gamma-motoneurones that had shown a background discharge prior to intentional stimulation. Short-term synchrony was present between a high proportion of semitendinosus gamma-motoneurones both for background discharges (sixteen out of nineteen pairs) and during the flexion reflex (thirteen out of fifteen pairs). All nineteen pairs of alpha-motoneurones examined during the flexion reflex showed short-term synchrony of discharge. Few alpha-motoneurones displayed background discharges but synchrony was observed in the two instances studied. The degree of synchrony was measured as the ratio (kappa) of the total counts contributing to the peak of the correlogram over the number expected by chance alone. The ratio was higher when the average frequency of motoneurone discharge was low. Kappa was generally higher for alpha-motoneurone pairs than for gamma-motoneurone pairs. The higher degree of synchrony for alpha-motoneurones reflected their lower discharge rates. During the flexion reflex the degree of synchrony between gamma-motoneurones was greater than expected for that same discharge rate in the absence of intentional stimulation. Only twenty-seven out of forty pairings of an alpha- with a gamma-motoneurone showed a significant degree of synchrony of discharge. On average, the degree of synchrony for alpha/gamma pairs was lower than that for either alpha/alpha or gamma/gamma pairings at the equivalent discharge rate. The results support the conclusion that coactivation of alpha- and gamma-motoneurones during the flexion reflex occurs largely through independent sets of interneurones. The possibility is discussed that those alpha-motoneurones which showed short-term synchrony with gamma-motoneurones were skeleto-fusimotor (beta-motoneurones) in nature.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALNAES E., JANSEN J. K., RUDJORD T. FUSIMOTOR ACTIVITY IN THE SPINAL CAT. Acta Physiol Scand. 1965 Mar;63:197–212. doi: 10.1111/j.1748-1716.1965.tb04060.x. [DOI] [PubMed] [Google Scholar]
- APPELBERG B., EMONET-DENAND F. CENTRAL CONTROL OF STATIC AND DYNAMIC SENSITIVITIES OF MUSCLE SPINDLE PRIMARY ENDINGS. Acta Physiol Scand. 1965 Apr;63:487–494. doi: 10.1111/j.1748-1716.1965.tb04093.x. [DOI] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group I muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:237–253. doi: 10.1113/jphysiol.1983.sp014531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenteng K., Morimoto T., Taylor A. Fusimotor activity in masseter nerve of the cat during reflex jaw movements. J Physiol. 1980 Aug;305:415–431. doi: 10.1113/jphysiol.1980.sp013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans J., Grillner S. Changes in dynamic sensitivity of primary endings of muscle spindle afferents induced by DOPA. Acta Physiol Scand. 1968 Dec;74(4):629–636. doi: 10.1111/j.1748-1716.1968.tb04273.x. [DOI] [PubMed] [Google Scholar]
- Bessou P., Emonet-Dénand F., Laporte Y. Motor fibres innervating extrafusal and intrafusal muscle fibres in the cat. J Physiol. 1965 Oct;180(3):649–672. doi: 10.1113/jphysiol.1965.sp007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D. M., Pascoe J. E. The reflex effects of sural nerve stimulation upon gastrocnemius fusimotor neurones of the rabbit [proceedings]. J Physiol. 1978 Mar;276:32P–32P. [PubMed] [Google Scholar]
- Ellaway P. H. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol. 1978 Aug;45(2):302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Emonet-Denand F., Joffroy M., Laporte Y. Lack of exclusively fusimotor -axons in flexor and extensor leg muscles of the cat. J Neurophysiol. 1972 Jan;35(1):149–153. doi: 10.1152/jn.1972.35.1.149. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Murthy K. S. The origins and characteristics of cross-correlated activity between gamma-motoneurones in the cat. Q J Exp Physiol. 1985 Apr;70(2):219–232. doi: 10.1113/expphysiol.1985.sp002905. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Murthy K. S. The source and distribution of short-term synchrony between gamma-motoneurones in the cat. Q J Exp Physiol. 1985 Apr;70(2):233–247. doi: 10.1113/expphysiol.1985.sp002906. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y., Matthews P. B., Petit J. On the subdivision of static and dynamic fusimotor actions on the primary ending of the cat muscle spindle. J Physiol. 1977 Jul;268(3):827–861. doi: 10.1113/jphysiol.1977.sp011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y. Proportion of muscles spindles supplied by skeletofusimotor axons (beta-axons) in peroneus brevis muscle of the cat. J Neurophysiol. 1975 Nov;38(6):1390–1394. doi: 10.1152/jn.1975.38.6.1390. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. The influence of DOPA on the static and the dynamic fusimotor activity to the triceps surae of the spinal cat. Acta Physiol Scand. 1969 Dec;77(4):490–509. doi: 10.1111/j.1748-1716.1969.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984 Feb;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Further study of efferent small-nerve fibers to mammalian muscle spindles; multiple spindle innervation and activity during contraction. J Physiol. 1951 Apr;113(2-3):283–297. doi: 10.1113/jphysiol.1951.sp004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., PAINTAL A. S. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958 Sep 23;143(2):195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm T. M., Reinking R. M., Roscoe D. D., Stuart D. G. Synchronous afferent discharge from a passive muscle of the cat: significance for interpreting spike-triggered averages. J Physiol. 1985 Aug;365:77–102. doi: 10.1113/jphysiol.1985.sp015760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol. 1984;101:1–110. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The central control of the dynamic response of muscle spindle receptors. J Physiol. 1962 May;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami L., Murthy K. S., Petit J. A quantitative study of skeletofusimotor innervation in the cat peroneus tertius muscle. J Physiol. 1982 Apr;325:125–144. doi: 10.1113/jphysiol.1982.sp014140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A. On the use and interpretation of cross-correlations measurements in the mammalian central nervous system. J Neurosci Methods. 1979 Aug;1(2):107–132. doi: 10.1016/0165-0270(79)90009-8. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982 Jan;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D. Proceedings: Synchronized firing of respiratory motoneurones during spontaneous breathing in the anaesthetized cat. J Physiol. 1974 May;239(1):11P–13P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K. Cross-correlation functions for a neuronal model. Biophys J. 1974 Aug;14(8):567–582. doi: 10.1016/S0006-3495(74)85936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox C. K., Poppele R. E. Correlation analysis of stimulus-evoked changes in excitability of spontaneously firing neurons. J Neurophysiol. 1977 May;40(3):616–625. doi: 10.1152/jn.1977.40.3.616. [DOI] [PubMed] [Google Scholar]
- Murthy K. S. Vertebrate fusimotor neurones and their influences on motor behavior. Prog Neurobiol. 1978;11(3-4):249–307. doi: 10.1016/0301-0082(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Noth J. Autogenetic inhibition of extensor gamma-motoneurones revealed by electrical stimulation of group I fibres in the cat. J Physiol. 1983 Sep;342:51–65. doi: 10.1113/jphysiol.1983.sp014839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. Activity of fusimotor fibres innervating muscle spindles in the intercostal muscles of the cat. Nature. 1963 Mar 9;197:1013–1014. doi: 10.1038/1971013a0. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo A. B. Discharge patterns in human muscle spindle afferents during isometric voluntary contractions. Acta Physiol Scand. 1970 Dec;80(4):552–566. doi: 10.1111/j.1748-1716.1970.tb04823.x. [DOI] [PubMed] [Google Scholar]


