Abstract
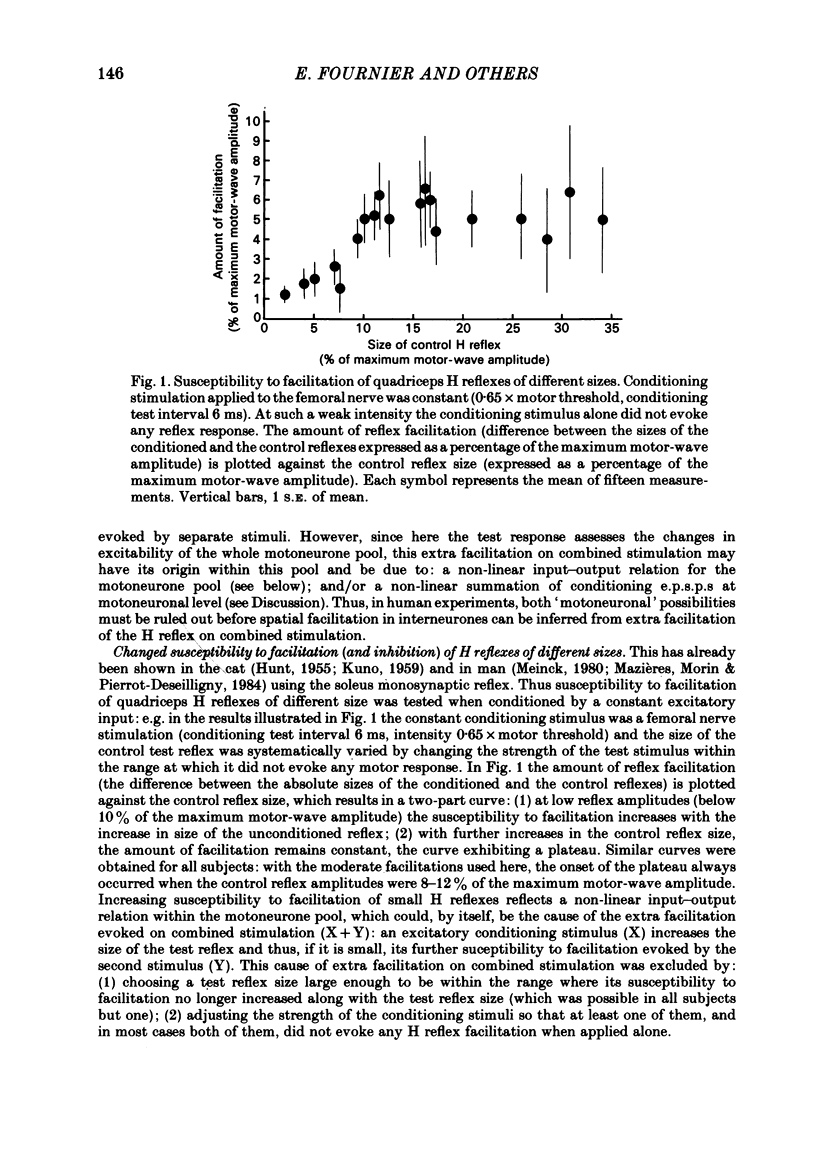
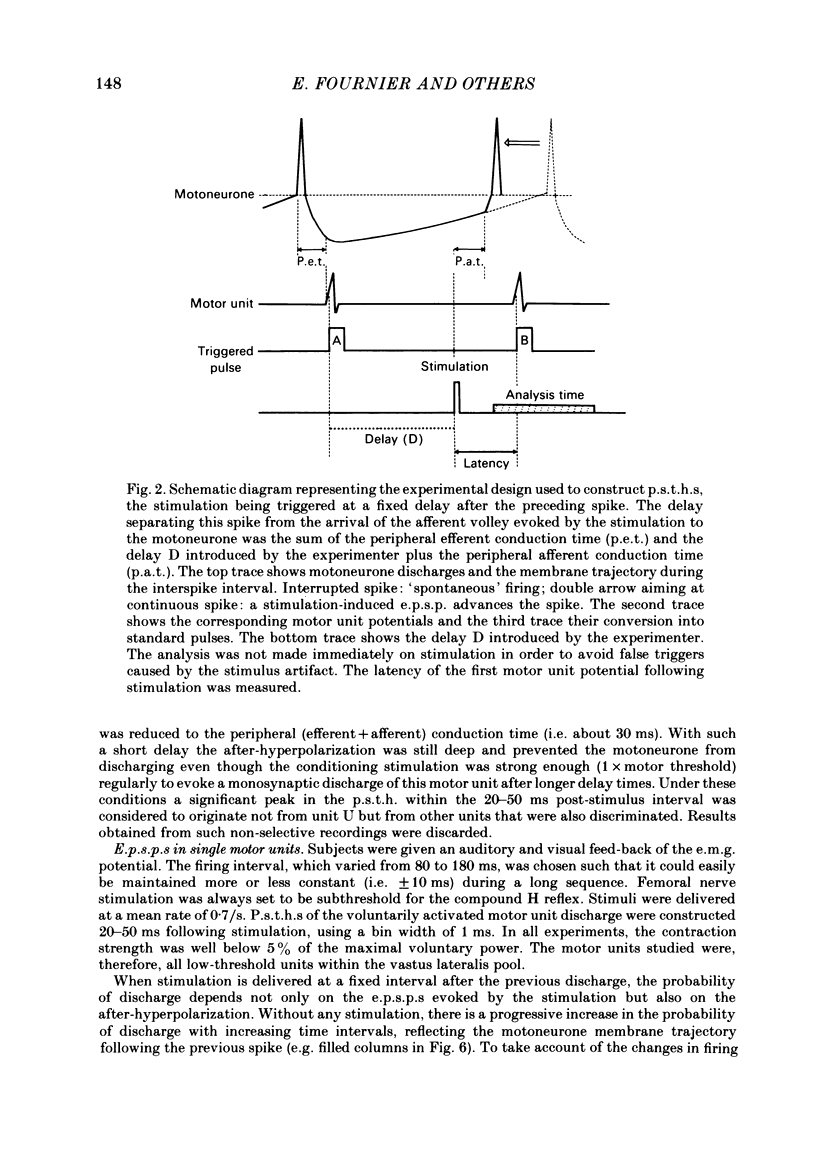
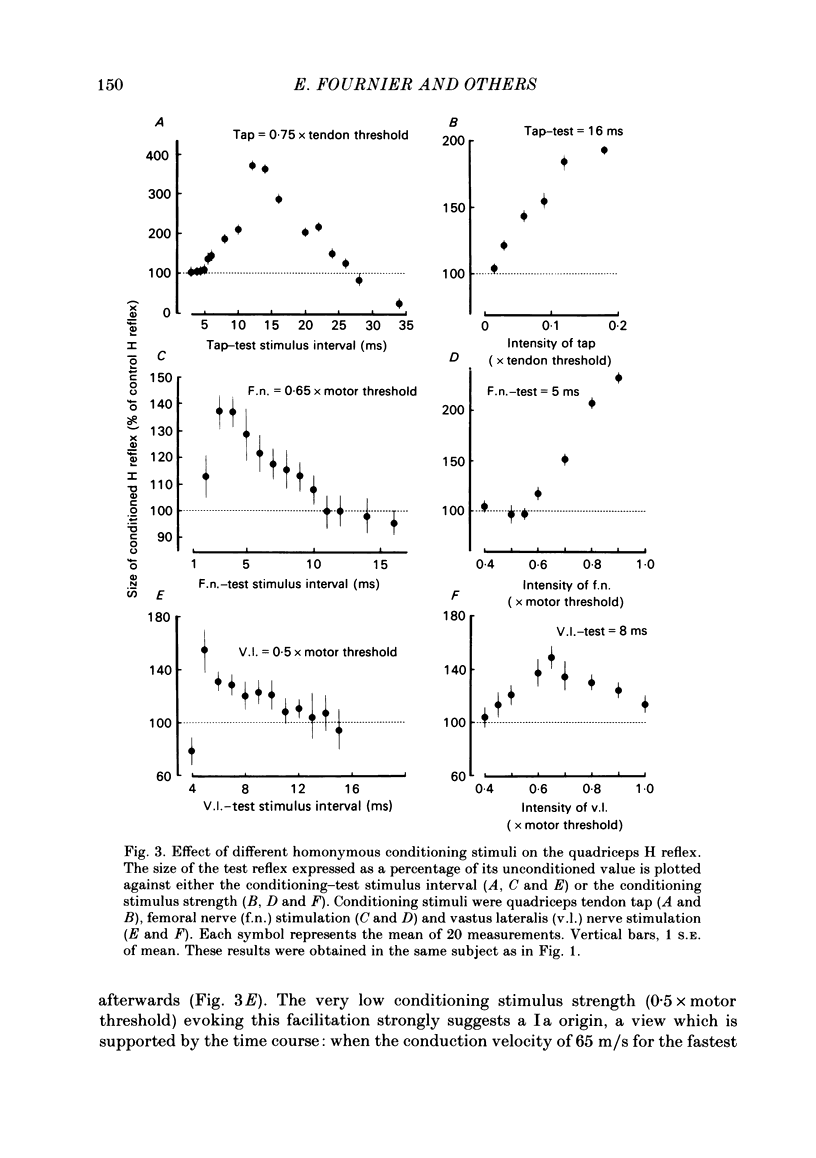
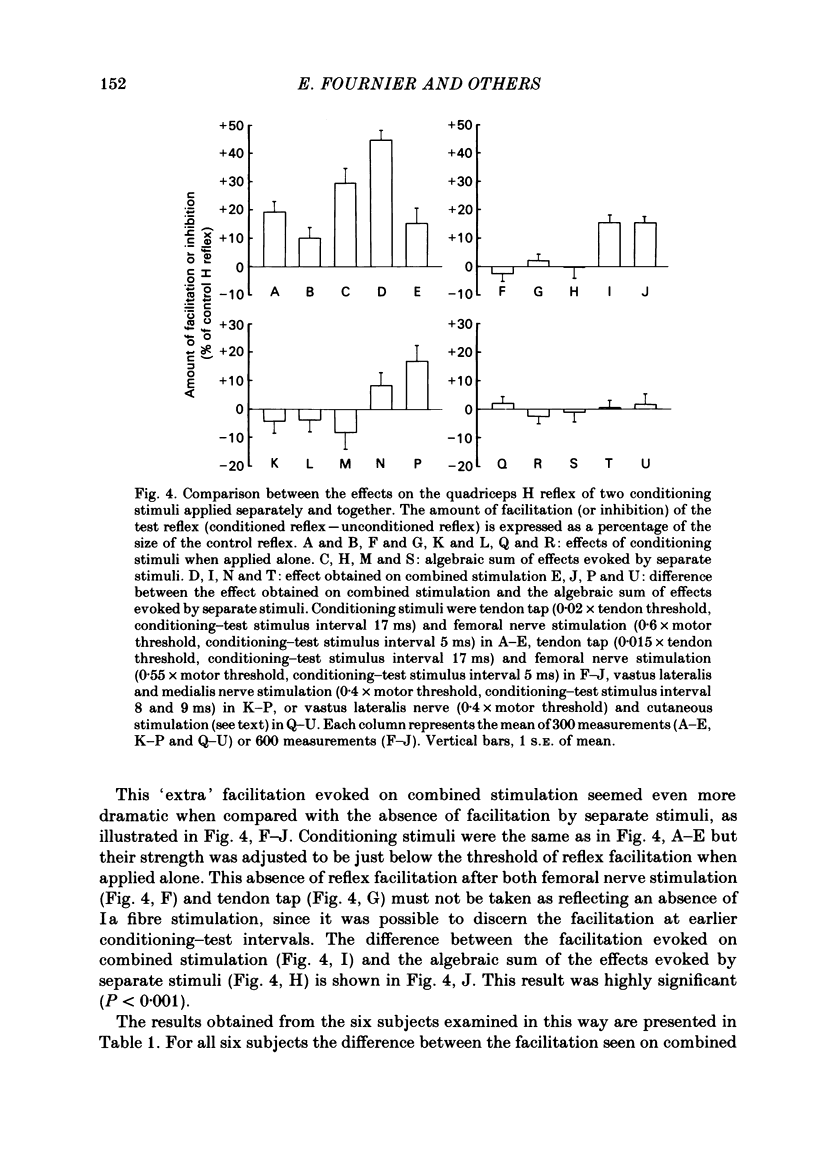
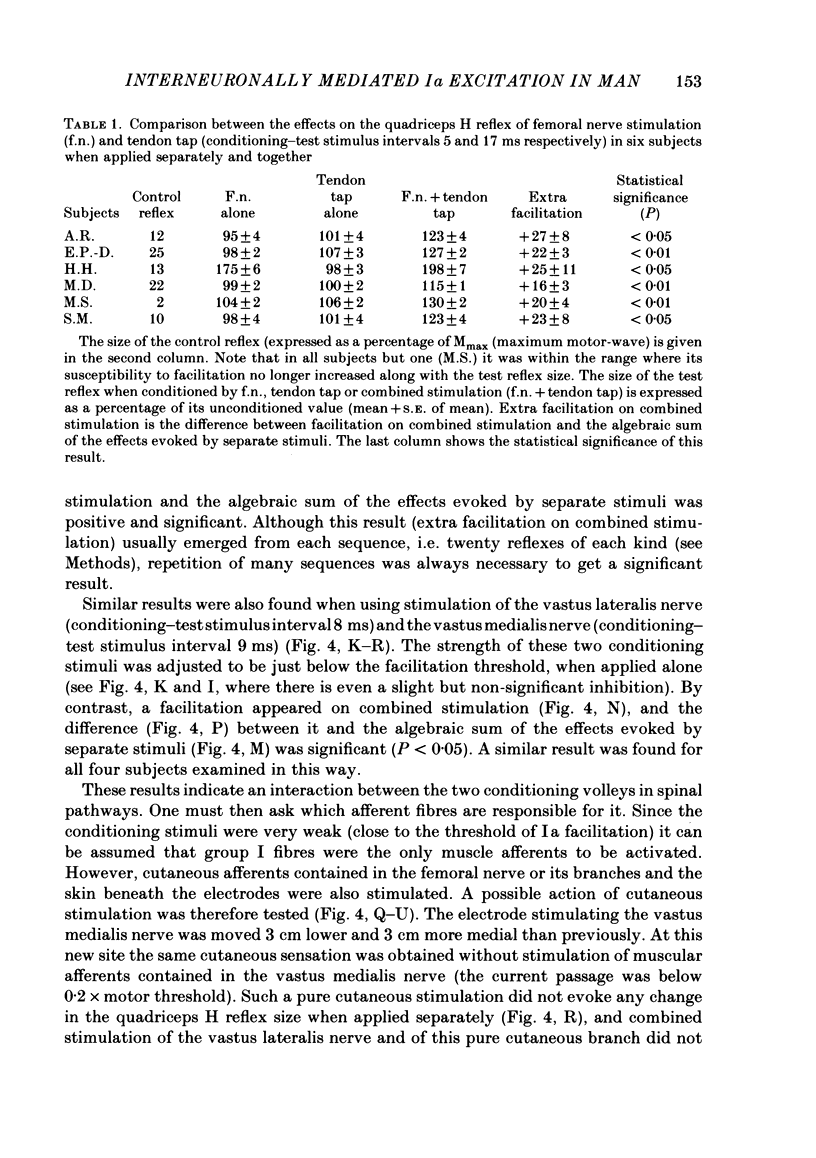
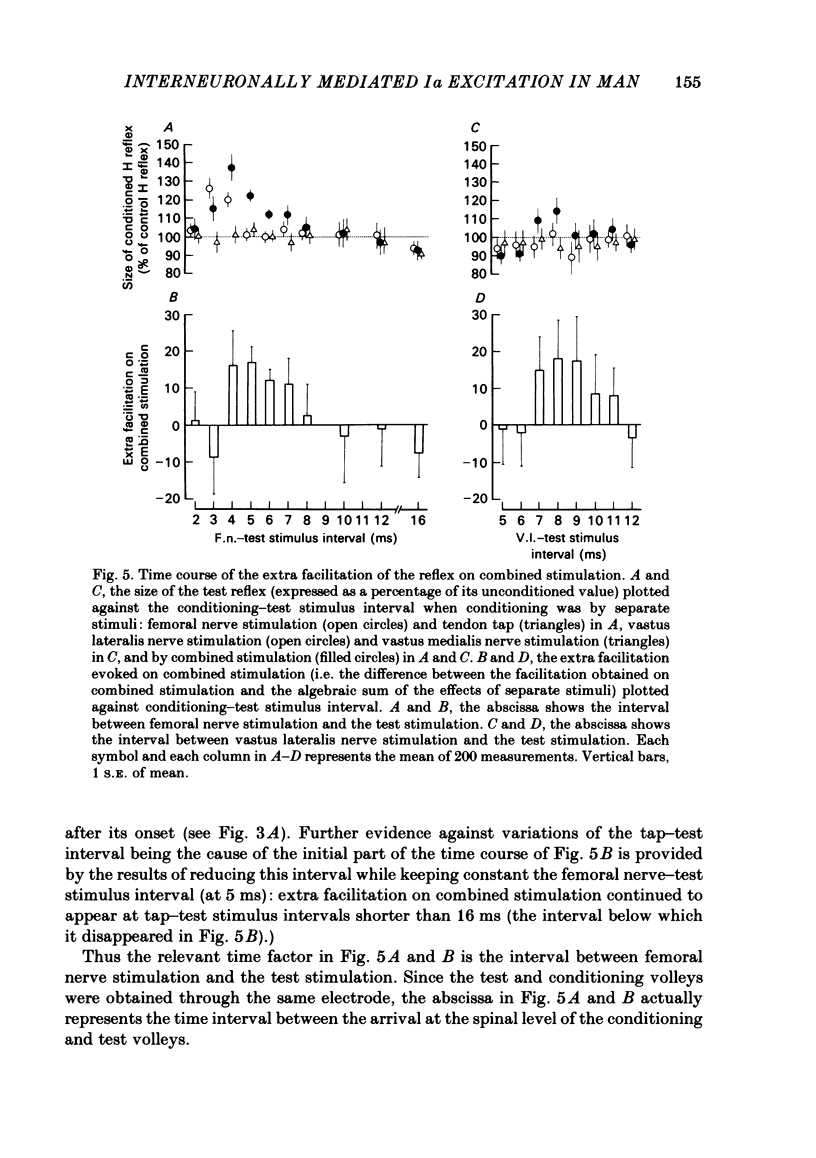
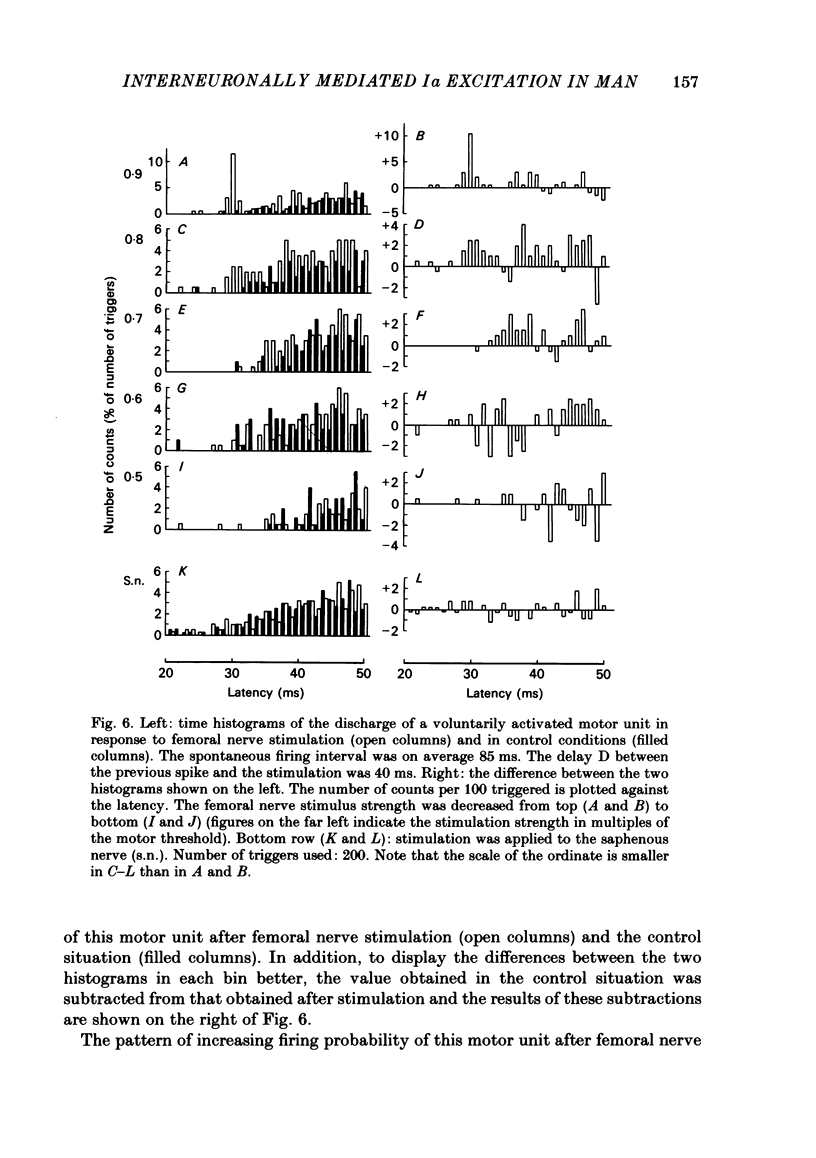
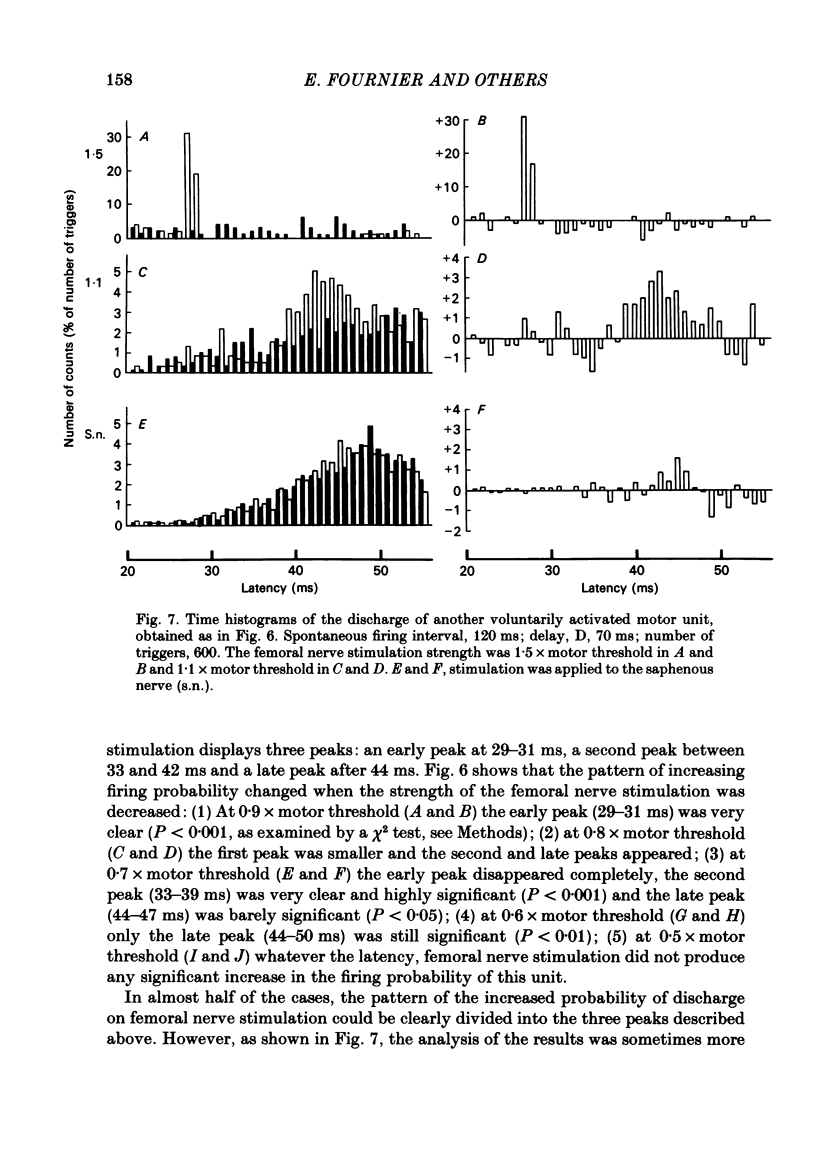
The possibility was investigated that interneuronal pathways contribute to Ia excitation of quadriceps motoneurones in normal man. Two techniques were used: the indirect spatial facilitation technique for investigating summation of Ia excitatory effects in interneurones which may be interposed in pathways to quadriceps motoneurones; the post-stimulus time histogram method for time course measurement of the firing probability of voluntarily activated motor units following femoral nerve stimulation. The spatial facilitation technique was applied while using the quadriceps H reflex to assess the excitability of the whole motoneurone pool: the comparison was made between the excitatory effects of two conditioning stimuli applied either separately or together. Summation of effects at a premotoneuronal level is suggested if facilitation of the reflex evoked on combined conditioning stimulation is larger than the algebraic sum of facilitations evoked by separate stimuli. Quadriceps tendon tap and electrical stimulations applied to either the femoral nerve or to two of its branches, the nerves to the vastus lateralis and vastus medialis muscles, were used as conditioning stimuli. Since these stimuli were very weak (their strength being about at the threshold for facilitation of the test reflex), it can be assumed that they activated predominantly Ia fibres. The facilitation of the quadriceps H reflex evoked on combined stimulation was significantly larger than the algebraic sum of facilitations evoked by separate stimuli. In many experiments, although conditioning stimuli did not evoke any reflex facilitation when applied alone, a significant facilitation appeared on combined stimulation. This 'extra' facilitation of the reflex on combined stimulation appeared with a central latency of 4-5 ms. It is argued that the only mechanism compatible with such a latency is summation at a premotoneuronal level. Post-stimulus time histograms (p.s.t.h.s) of voluntarily activated quadriceps motor units were made following femoral nerve stimulation. Stimulation was triggered at a fixed delay time after the activation of the motor unit. A special attempt was made to set this delay so that the motoneuronal after-hyperpolarization following the spike would partially prevent the discharge evoked by a monosynaptic excitatory post-synaptic potential (e.p.s.p.). At stimulus strengths near motor threshold, femoral nerve stimulation regularly evoked an early increase in firing probability of motor units with the same latency as the H reflex.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVORD E. C., Jr, FUORTES M. G. Reflex activity of extensor motor units following muscular afferent excitation. J Physiol. 1953 Nov 28;122(2):302–321. doi: 10.1113/jphysiol.1953.sp005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARAKI T., EOCLES J. C., ITO M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. J Physiol. 1960 Dec;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P., Labelle K. Effects of extensor and flexor group I afferent volleys on the excitability of individual soleus motoneurones in man. J Neurol Neurosurg Psychiatry. 1977 Sep;40(9):910–919. doi: 10.1136/jnnp.40.9.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P., Zilm D. Characteristics of postsynaptic potentials produced in single human motoneurons by homonymous group 1 volleys. Exp Brain Res. 1982;47(1):41–48. doi: 10.1007/BF00235884. [DOI] [PubMed] [Google Scholar]
- Burke D., Gandevia S. C., McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol. 1984 Sep;52(3):435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Burke D., Gandevia S. C., McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol. 1983 Jun;339:535–552. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Spatial facilitation in the direct inhibitory pathway. Nature. 1957 Jun 22;179(4573):1305–1306. doi: 10.1038/1791305a0. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Gustafsson B. Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol. 1983 Aug;341:387–410. doi: 10.1113/jphysiol.1983.sp014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier E., Katz R., Pierrot-Deseilligny E. A re-evaluation of the pattern of group I fibre projections in the human lower limb on using randomly alternated stimulations. Exp Brain Res. 1984;56(1):193–195. doi: 10.1007/BF00237457. [DOI] [PubMed] [Google Scholar]
- Fukushima Y., Yamashita N., Shimada Y. Facilitation of H-reflex by homonymous Ia-afferent fibers in man. J Neurophysiol. 1982 Nov;48(5):1079–1088. doi: 10.1152/jn.1982.48.5.1079. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984 Feb;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. Monosynaptic reflex response of spinal motoneurons to graded afferent stimulation. J Gen Physiol. 1955 Jul 20;38(6):813–852. doi: 10.1085/jgp.38.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma S., Mizote M., Watanabe S. Participation of mono- and polysynaptic transmission during tonic activation of the stretch reflex arcs. Jpn J Physiol. 1975;25(2):135–146. doi: 10.2170/jjphysiol.25.135. [DOI] [PubMed] [Google Scholar]
- Jankowska E., McCrea D., Mackel R. Oligosynaptic excitation of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol. 1981 Jul;316:411–425. doi: 10.1113/jphysiol.1981.sp013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda K. Contribution of polysynaptic pathways to the tonic vibration reflex. Jpn J Physiol. 1972 Aug;22(4):367–377. doi: 10.2170/jjphysiol.22.367. [DOI] [PubMed] [Google Scholar]
- Katz R., Morin C., Pierrot-Deseilligny E., Hibino R. Conditioning of H reflex by a preceding subthreshold tendon reflex stimulus. J Neurol Neurosurg Psychiatry. 1977 Jun;40(6):575–580. doi: 10.1136/jnnp.40.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982 Jan;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The effects of single afferent impulses on the probability of firing of external intercostal motoneurones in the cat. J Physiol. 1982 Jan;322:315–336. doi: 10.1113/jphysiol.1982.sp014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhanov V. M., Shapovalov A. I. Sinapticheskie éffekty, vyzyvaemye v motoneironakh razdrazheniem odinochnykh propriospinal'nykh neironov. Neirofiziologiia. 1977;9(3):300–306. [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- MAGLADERY J. W., McDOUGAL D. B., Jr Electrophysiological studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull Johns Hopkins Hosp. 1950 May;86(5):265–290. [PubMed] [Google Scholar]
- MAGLADERY J. W., PORTER W. E., PARK A. M., TEASDALL R. D. Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull Johns Hopkins Hosp. 1951 Jun;88(6):499–519. [PubMed] [Google Scholar]
- Mao C. C., Ashby P., Wang M., McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res. 1984;56(2):341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Meinck H. M. Facilitation and inhibition of the human H reflex as a function of the amplitude of the control reflex. Electroencephalogr Clin Neurophysiol. 1980 Feb;48(2):203–211. doi: 10.1016/0013-4694(80)90305-3. [DOI] [PubMed] [Google Scholar]
- Pacheco P., Guzmán-Flores C. Intracellular recording in extensor motoneurons of spastic cats. Exp Neurol. 1969 Dec;25(4):472–481. doi: 10.1016/0014-4886(69)90091-0. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Ohye C. Polysynaptic activation of extensor motorneurones from group Ia fibres in the cat spinal cord. Experientia. 1964 Nov 15;20(11):628–629. doi: 10.1007/BF02144828. [DOI] [PubMed] [Google Scholar]
- Táboríková H., Sax D. S. Conditioning of H-reflexes by a preceding subthreshold H-reflex stimulus. Brain. 1969 Mar;92(1):203–212. doi: 10.1093/brain/92.1.203. [DOI] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]


