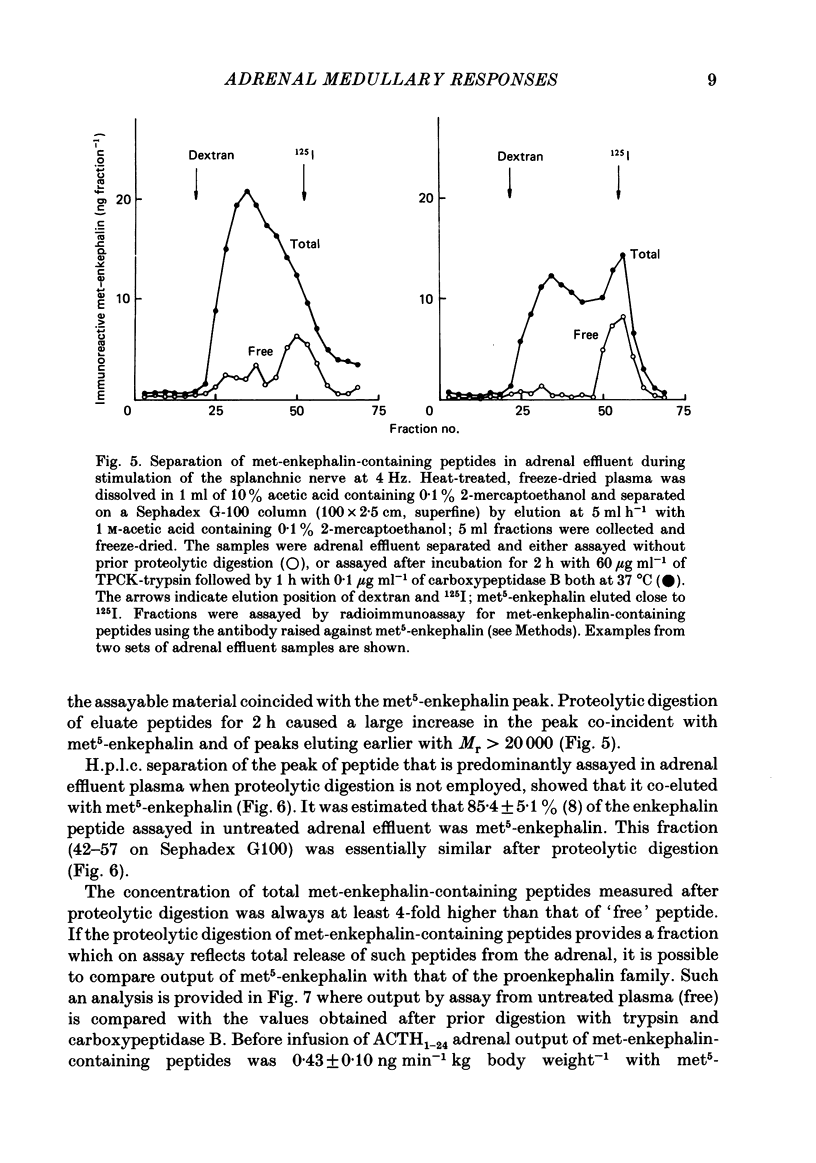
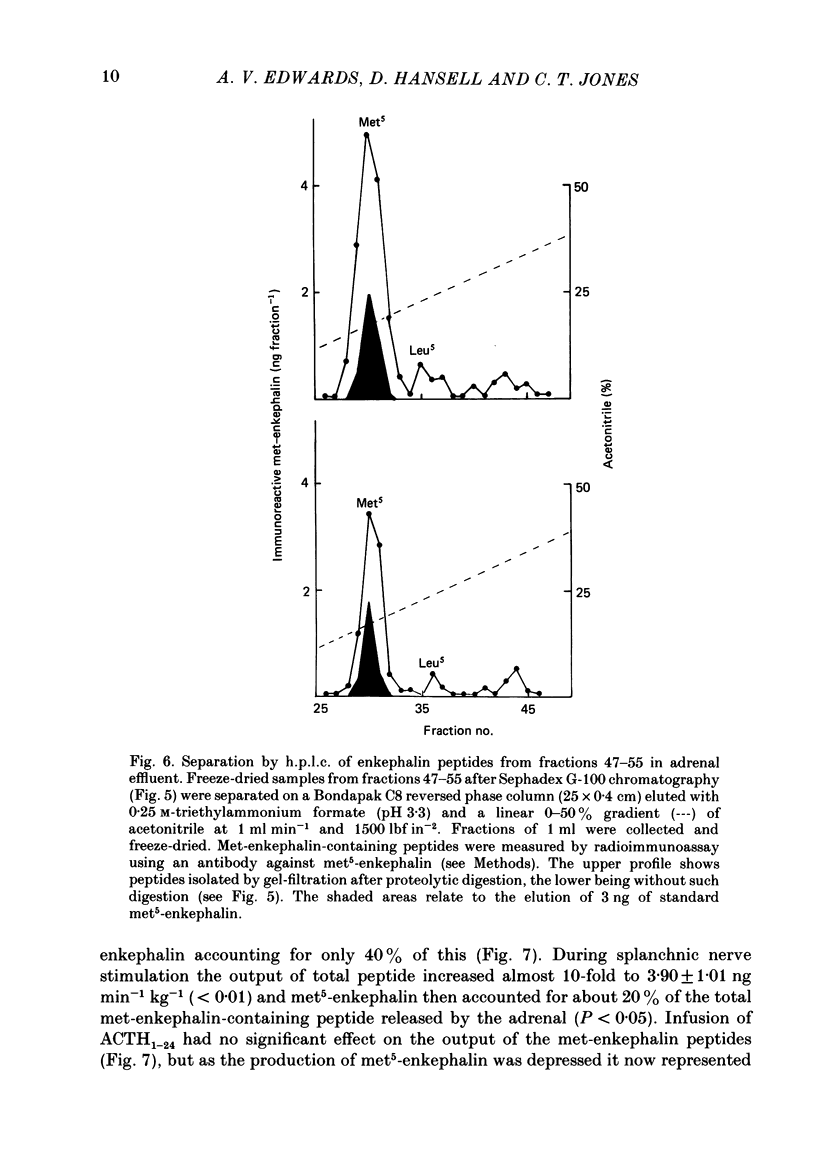
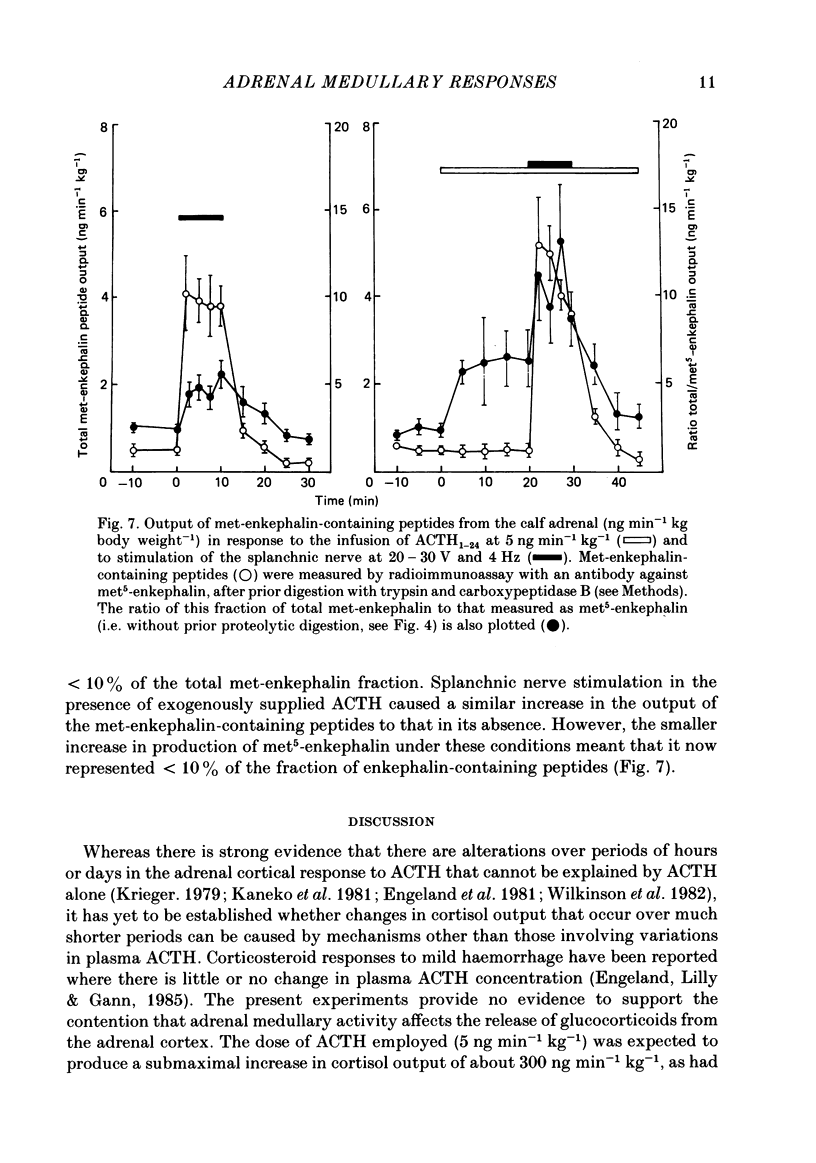
Abstract
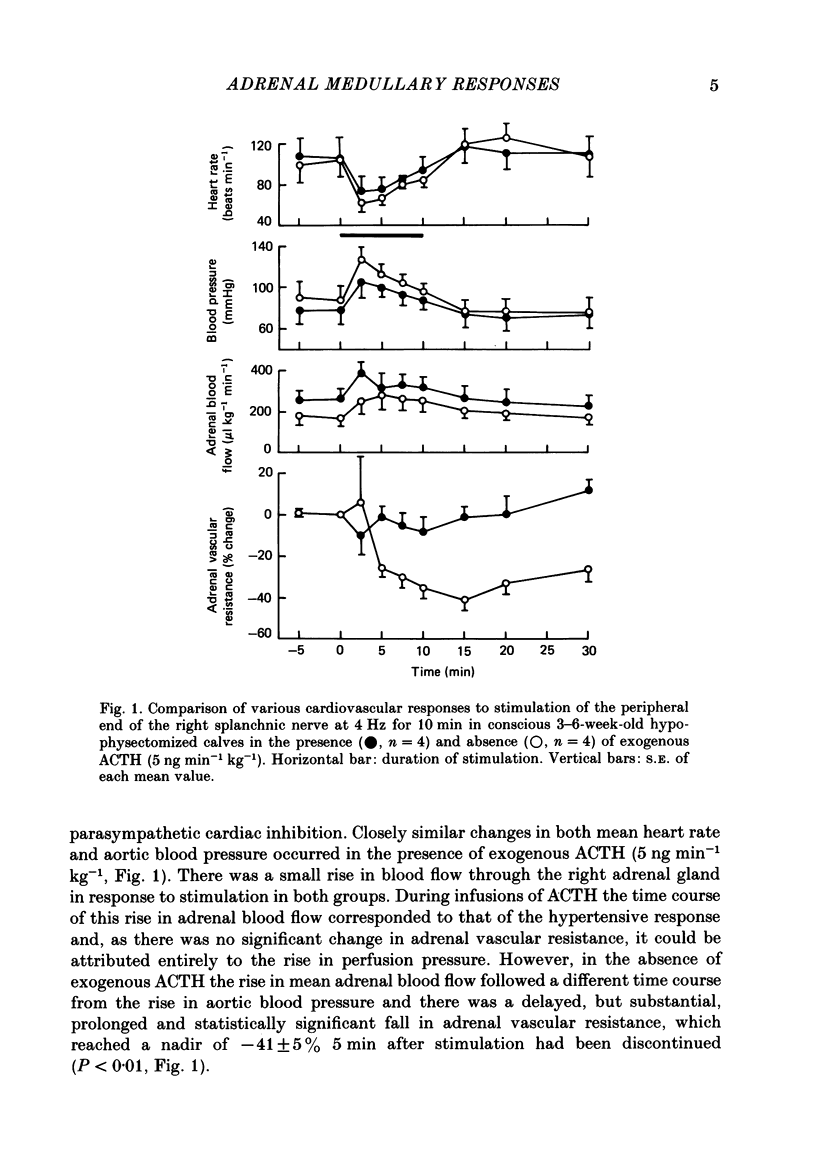
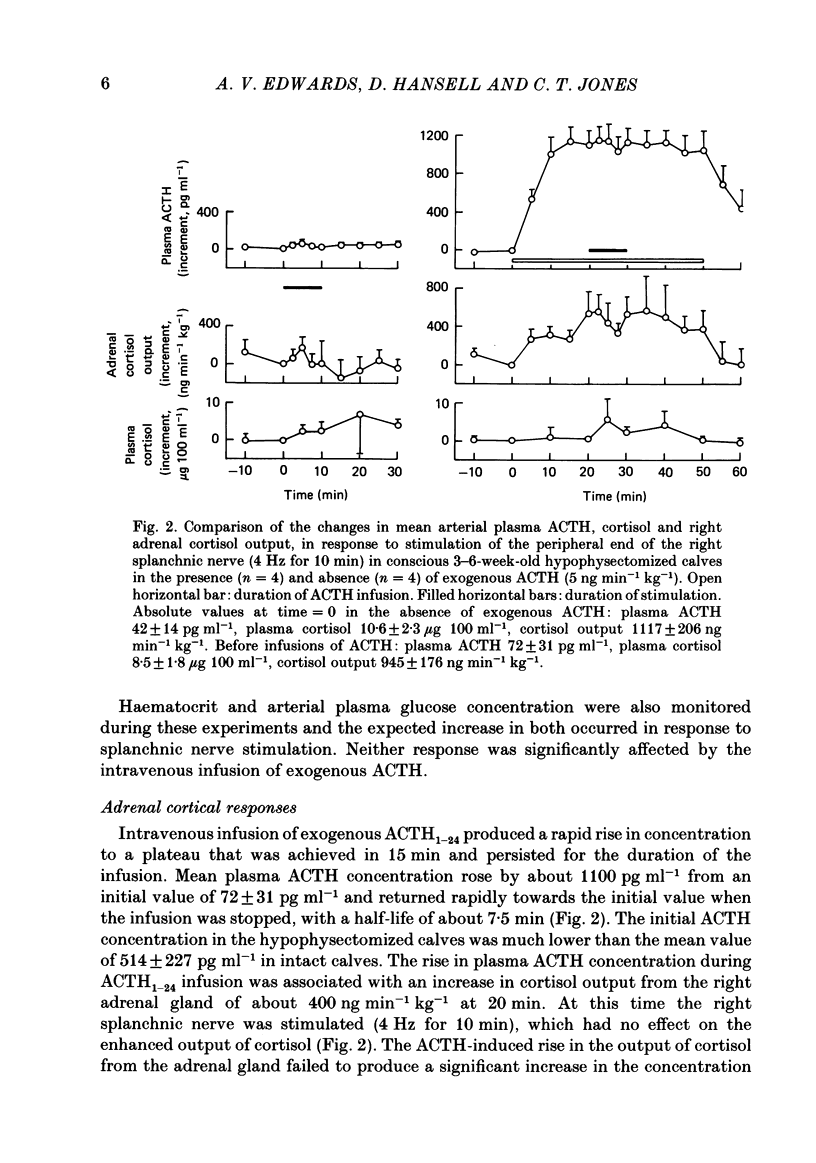
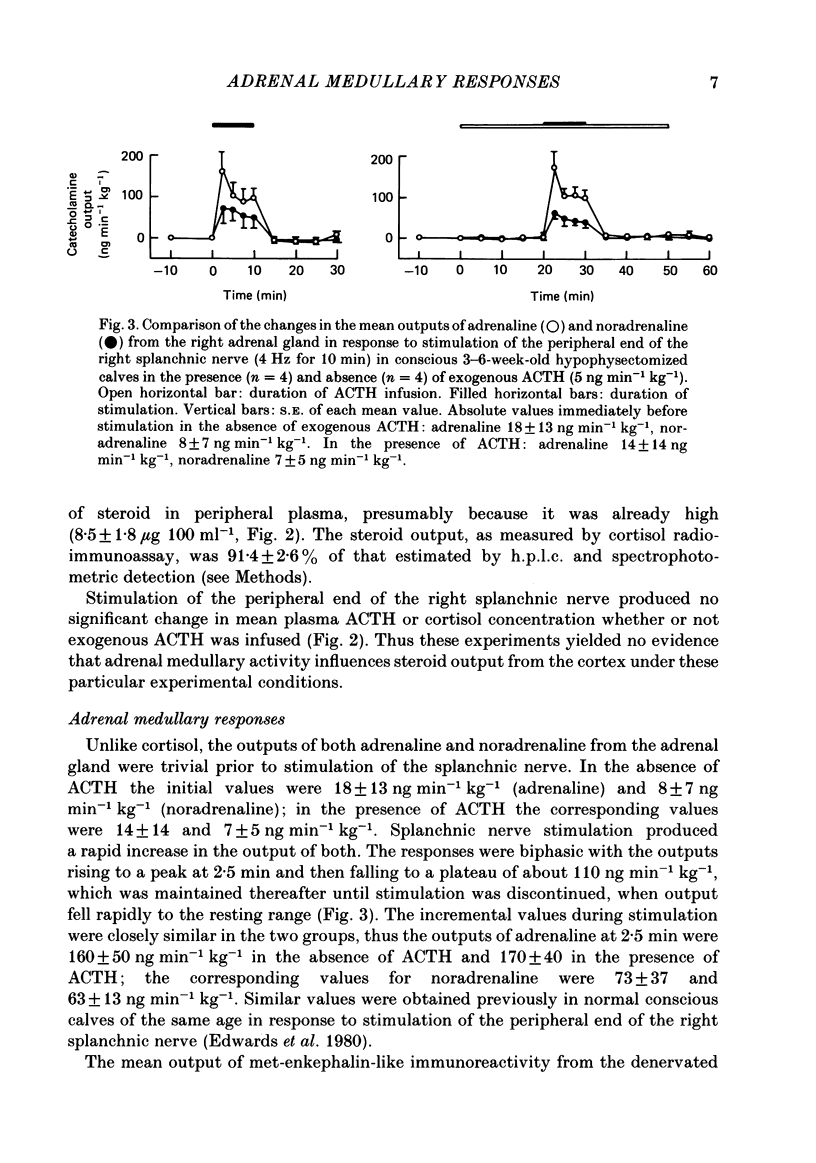
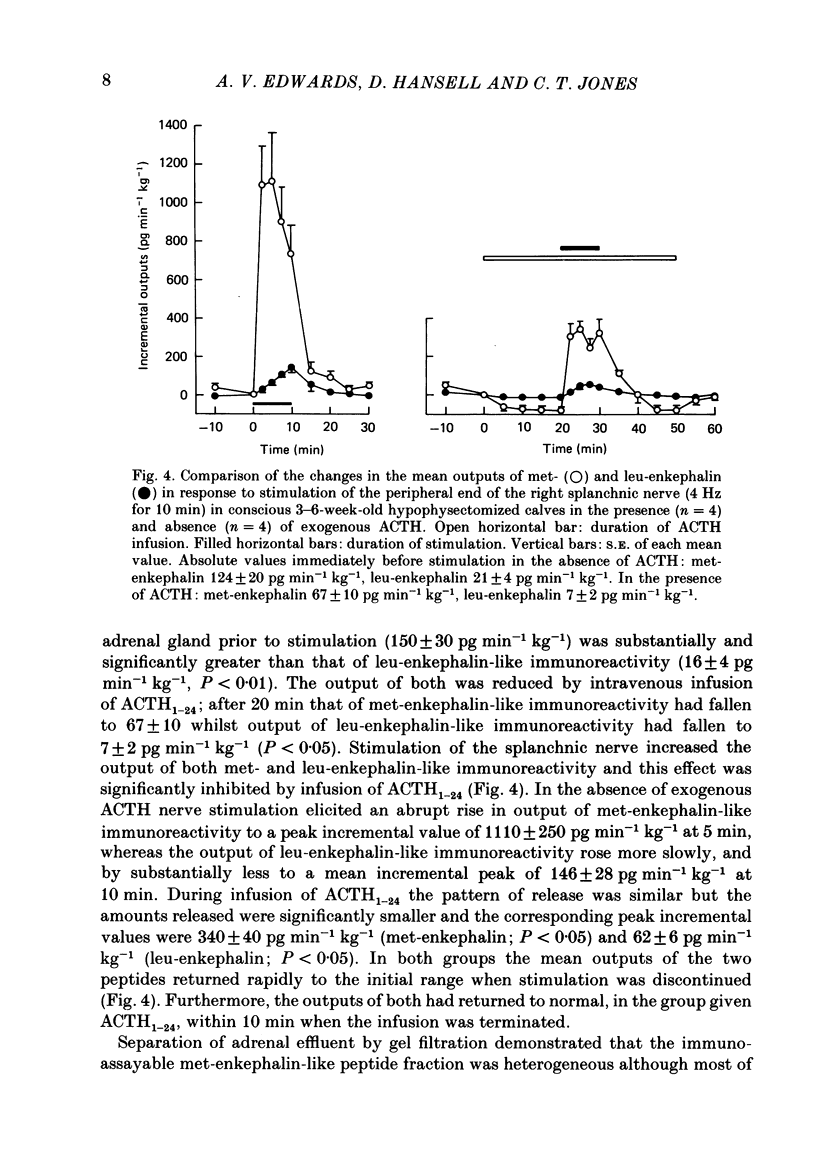
Right medullary and various cardiovascular responses to stimulation of the peripheral end of the splanchnic nerve have been investigated in the presence and absence of exogenous adrenocorticotrophin, ACTH1-24, (5 ng min-1 kg-1). The adrenal-clamp technique was employed in conscious calves, after the pituitary stalk had been cauterized and they had recovered from anaesthesia. The intravenous infusion of ACTH1-24 increased the plasma ACTH concentration by about 1100 pg ml-1 and right adrenal venous output of cortisol by about 400 ng min-1 kg body weight-1. Stimulation of the splanchnic nerve at 4 Hz for 10 min had no effect on either arterial plasma ACTH concentration or the adrenal output of cortisol. Closely similar amounts of both adrenaline and noradrenaline were released in response to nerve stimulation in the presence and absence of exogenous ACTH. In contrast, the fall in adrenal vascular resistance of about 40%, which normally occurred in response to splanchnic nerve stimulation, was completely abolished by ACTH. The adrenal produced relatively large quantities of met-enkephalin-containing peptides. During splanchnic nerve stimulation the output of these increased 2-100-fold, at which time free met5-enkephalin accounted for only 10-20% of total. During ACTH infusion the output of free met5-enkephalin was reduced at rest and during nerve stimulation, but that of total met-enkephalin-containing peptides was unaffected. These results indicate that ACTH or an adrenal steroid may alter the processing of proenkephalin in the adrenal medulla acutely but not total opiate secretion. Alternatively, the presence of ACTH could act by influencing the population of chromaffin cells activated by splanchnic nerve stimulation.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom S. R., Edwards A. V., Hardy R. N., Malinowska K. W., Silver M. Endocrine responses to insulin hypoglycaemia in the young calf. J Physiol. 1975 Jan;244(3):783–803. doi: 10.1113/jphysiol.1975.sp010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom S. R., Edwards A. V. Pancreatic endocrine responses to stimulation of the peripheral ends of the splanchnic nerves in the conscious adrenalectomized calf. J Physiol. 1980 Nov;308:39–48. doi: 10.1113/jphysiol.1980.sp013460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloux P. M., Grossman A., Lytras N., Besser G. M. Evidence for the participation of endogenous opioids in the sympathoadrenal response to hypoglycaemia in man. Clin Endocrinol (Oxf) 1985 Jan;22(1):49–56. doi: 10.1111/j.1365-2265.1985.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Chaminade M., Foutz A. S., Rossier J. Co-release of enkephalins and precursors with catecholamines from the perfused cat adrenal gland in situ. J Physiol. 1984 Aug;353:157–169. doi: 10.1113/jphysiol.1984.sp015329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Guidotti A., Hanbauer I., Saiani L. Modulation of nicotinic receptor function by opiate recognition sites highly selective for Met5-enkephalin[Arg6Phe7]. Fed Proc. 1983 Sep;42(12):2946–2952. [PubMed] [Google Scholar]
- Dallman M. F., Engeland W. C., McBride M. H. The neural regulation of compensatory adrenal growth. Ann N Y Acad Sci. 1977 Oct 28;297:373–392. doi: 10.1111/j.1749-6632.1977.tb41869.x. [DOI] [PubMed] [Google Scholar]
- De Souza E. B., Van Loon G. R. D-Ala2-Met-enkephalinamide, a potent opioid peptide, alters pituitary-adrenocortical secretion in rats. Endocrinology. 1982 Nov;111(5):1483–1490. doi: 10.1210/endo-111-5-1483. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Furness P. N., Helle K. B. Adrenal medullary responses to stimulation of the splanchnic nerve in the conscious calf. J Physiol. 1980 Nov;308:15–27. doi: 10.1113/jphysiol.1980.sp013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The effects of infusions of synthetic adrenocorticotrophin in the conscious calf. J Physiol. 1974 Jun;239(3):477–498. doi: 10.1113/jphysiol.1974.sp010579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A. V., Hardy R. N., Malinowska K. W. The sensitivity of adrenal responses to synthetic adrenocorticotrophin in the conscious unrestrained calf. J Physiol. 1975 Mar;245(3):639–653. doi: 10.1113/jphysiol.1975.sp010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland W. C., Byrnes G. J., Presnell K., Gann D. S. Adrenocortical sensitivity to adrenocorticotropin (ACTH) in awake dogs changes as a function of the time of observation and after hemorrhage independently of changes in ACTH. Endocrinology. 1981 Jun;108(6):2149–2153. doi: 10.1210/endo-108-6-2149. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., LEWIS G. P. THE ACTION OF PEPTIDES ON THE ADRENAL MEDULLA. RELEASE OF ADRENALINE BY BRADYKININ AND ANGIOTENSIN. J Physiol. 1964 May;171:98–108. doi: 10.1113/jphysiol.1964.sp007364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLKOW B., VON EULER U. S. Selective activation of noradrenaline and adrenaline producing cells in the cat's adrenal gland by hypothalamic stimulation. Circ Res. 1954 May;2(3):191–195. doi: 10.1161/01.res.2.3.191. [DOI] [PubMed] [Google Scholar]
- Feuerstein G., Gutman Y. Preferential secretion of adrenaline or noradrenaline by the cat adrenal in vivo in response to different stimuli. Br J Pharmacol. 1971 Dec;43(4):764–775. doi: 10.1111/j.1476-5381.1971.tb07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone G. L., Payvar F., Yamamoto K. R. Glucocorticoid regulation of protein processing and compartmentalization. Nature. 1982 Nov 18;300(5889):221–225. doi: 10.1038/300221a0. [DOI] [PubMed] [Google Scholar]
- Fleminger G., Howells R. D., Kilpatrick D. L., Udenfriend S. Intact proenkephalin is the major enkephalin-containing peptide produced in rat adrenal glands after denervation. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7985–7988. doi: 10.1073/pnas.81.24.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni S., Hanbauer I., Hexum T. D., Yang H. Y., Kelly G. D., Costa E. In vivo characterization of the mechanisms that secrete enkephalin-like peptides stored in dog adrenal medulla. Neuropharmacology. 1981 Jul;20(7):639–645. doi: 10.1016/0028-3908(81)90110-6. [DOI] [PubMed] [Google Scholar]
- Gros C., Pradelles P., Rouget C., Bepoldin O., Dray F., Fournie-Zaluski M. C., Roques B. P., Pollard H., Llorens-Cortes C., Schwartz J. C. Radioimmunoassay of methionine- and leucine-enkephalins in regions of rat brain and comparison with endorphins estimated by a radioreceptor assay. J Neurochem. 1978 Jul;31(1):29–39. doi: 10.1111/j.1471-4159.1978.tb12429.x. [DOI] [PubMed] [Google Scholar]
- Grossman A., Gaillard R. C., McCartney P., Rees L. H., Besser G. M. Opiate modulation of the pituitary-adrenal axis: effects of stress and circadian rhythm. Clin Endocrinol (Oxf) 1982 Sep;17(3):279–286. doi: 10.1111/j.1365-2265.1982.tb01590.x. [DOI] [PubMed] [Google Scholar]
- HILLARP N. A., HOKFELT B. Evidence of adrenaline and noradrenaline in separate adrenal medullary cells. Acta Physiol Scand. 1953 Dec 31;30(1):55–68. doi: 10.1111/j.1748-1716.1954.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Henderson J. R., Daniel P. M. Portal circulations and their relation to counter-current systems. Q J Exp Physiol Cogn Med Sci. 1978 Oct;63(4):355–369. doi: 10.1113/expphysiol.1978.sp002448. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Boddy K., Robinson J. S., Ratcliffe J. G. Developmental changes in the responses of the adrenal glands of foetal sheep to endogenous adrenocorticotrophin, as indicated by hormone responses to hypoxaemia. J Endocrinol. 1977 Mar;72(3):279–292. doi: 10.1677/joe.0.0720279. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Kaneko K., Shinsako J., Dallman M. F. Adrenal sensitivity to adrenocorticotropin varies diurnally. Endocrinology. 1981 Jul;109(1):70–75. doi: 10.1210/endo-109-1-70. [DOI] [PubMed] [Google Scholar]
- Kumakura K., Karoum F., Guidotti A., Costa E. Modulation of nicotinic receptors by opiate receptor agonists in cultured adrenal chromaffin cells. Nature. 1980 Jan 31;283(5746):489–492. doi: 10.1038/283489a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S., Kimura S., Rossier J., Stein S., Udenfriend S. An about 50,000-dalton protein in adrenal medulla: a common precursor of [Met]- and [Leu]enkephalin. Science. 1980 Jun 27;208(4451):1459–1461. doi: 10.1126/science.7384787. [DOI] [PubMed] [Google Scholar]
- Motta P., Muto M., Fujita T. Three dimensional organization of mammalian adrenal cortex. A scanning electron microscopic study. Cell Tissue Res. 1979 Jan 30;196(1):23–38. doi: 10.1007/BF00236346. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Lundberg J. M., Hökfelt T., Terenius L., Brandt J., Elde R. P., Goldstein M. Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience. 1978;3(12):1169–1186. doi: 10.1016/0306-4522(78)90137-9. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Lewis R. V., Kimura S., Rossier J., Gerber L. D., Brink L., Stein S., Udenfriend S. Isolation of the opioid heptapeptide Met-enkephalin [Arg6,Phe7] from bovine adrenal medullary granules and striatum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6680–6683. doi: 10.1073/pnas.76.12.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs W. A., Delitala G., Jones A., Jeffcoate W. J., Edwards C. R., Ratter S. J., Besser G. M., Bloom S. R., Alberti K. G. Hormonal and metabolic responses to an enkephalin analogue in normal man. Lancet. 1978 Dec 9;2(8102):1225–1227. doi: 10.1016/s0140-6736(78)92100-1. [DOI] [PubMed] [Google Scholar]
- VON EULER U. S., FLODING I. A fluorimetric micromethod for differential estimation of adrenaline and noradrenaline. Acta Physiol Scand Suppl. 1955;33(118):45–56. [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Wilkinson C. W., Shinsako J., Dallman M. F. Rapid decreases in adrenal and plasma corticosterone concentrations after drinking are not mediated by changes in plasma adrenocorticotropin concentration. Endocrinology. 1982 May;110(5):1599–1606. doi: 10.1210/endo-110-5-1599. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Unsworth C. D., Viveros O. H. Regulation of opioid peptide synthesis and processing in adrenal chromaffin cells by catecholamines and cyclic adenosine 3':5'-monophosphate. J Neurosci. 1984 Dec;4(12):2993–3001. doi: 10.1523/JNEUROSCI.04-12-02993.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman R. J., Axelrod J. Control of enzymatic synthesis of adrenaline in the adrenal medulla by adrenal cortical steroids. J Biol Chem. 1966 May 25;241(10):2301–2305. [PubMed] [Google Scholar]
- del Pozo E., Martin-Perez J., Stadelmann A., Girard J., Brownell J. Inhibitory action of a met-enkephalin on ACTH release in man. J Clin Invest. 1980 Jun;65(6):1531–1534. doi: 10.1172/JCI109820. [DOI] [PMC free article] [PubMed] [Google Scholar]


