Abstract
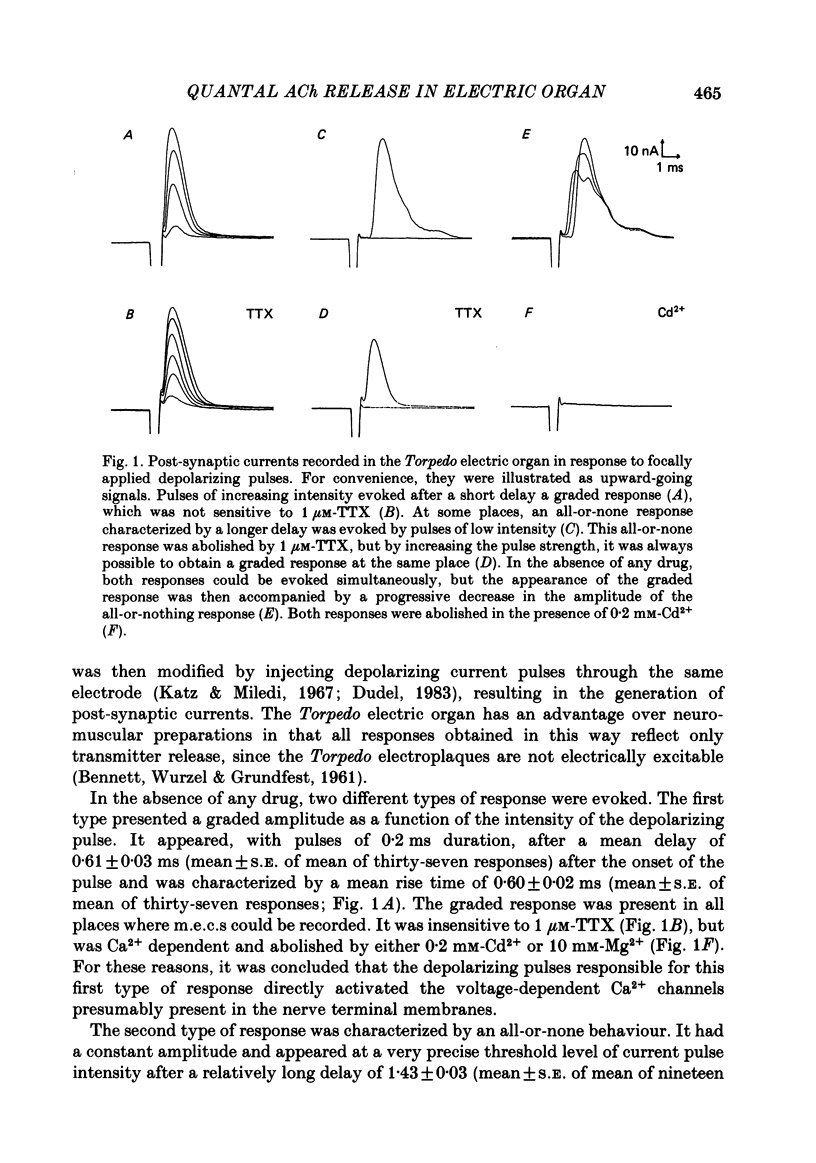
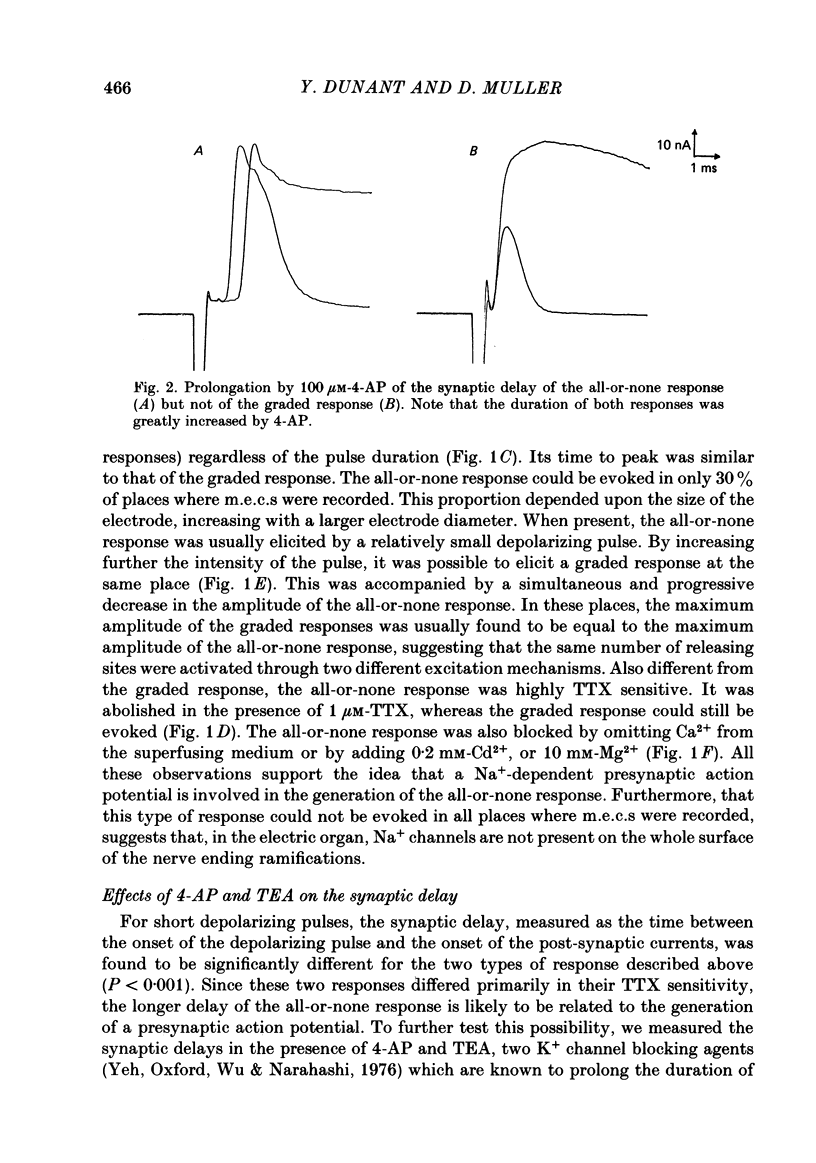
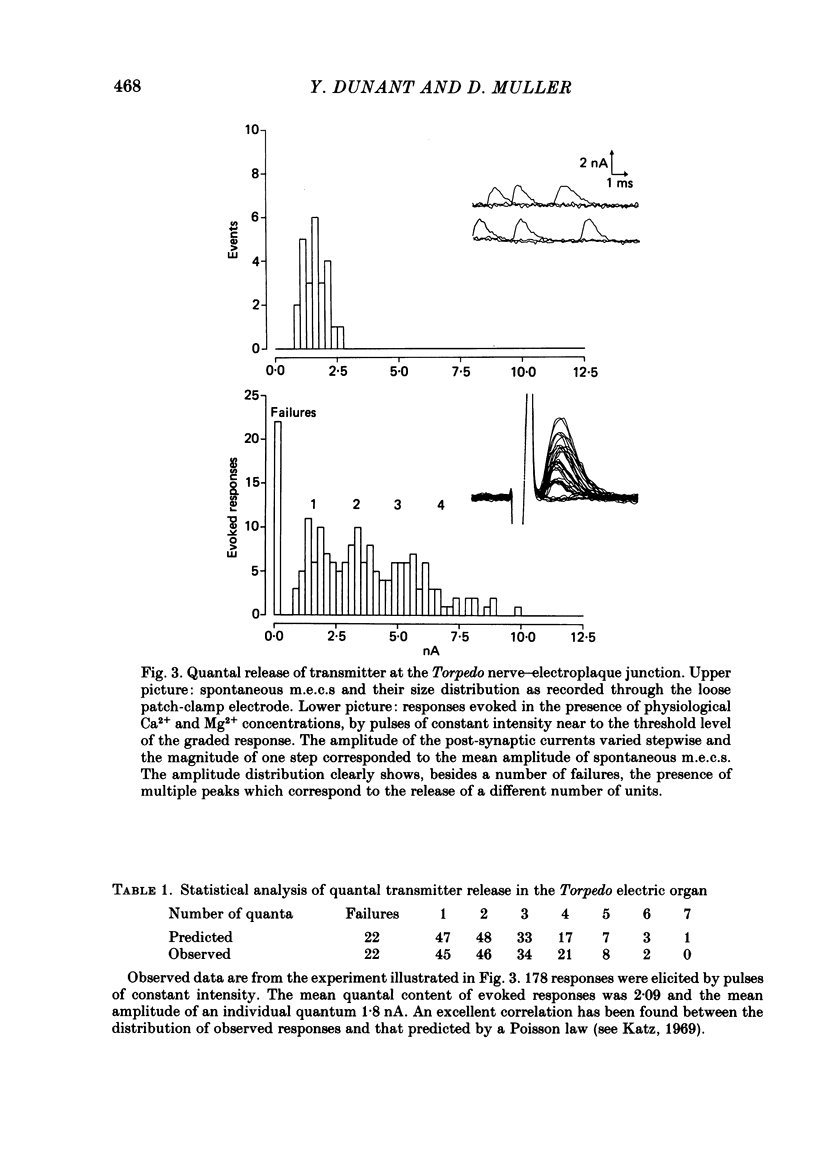
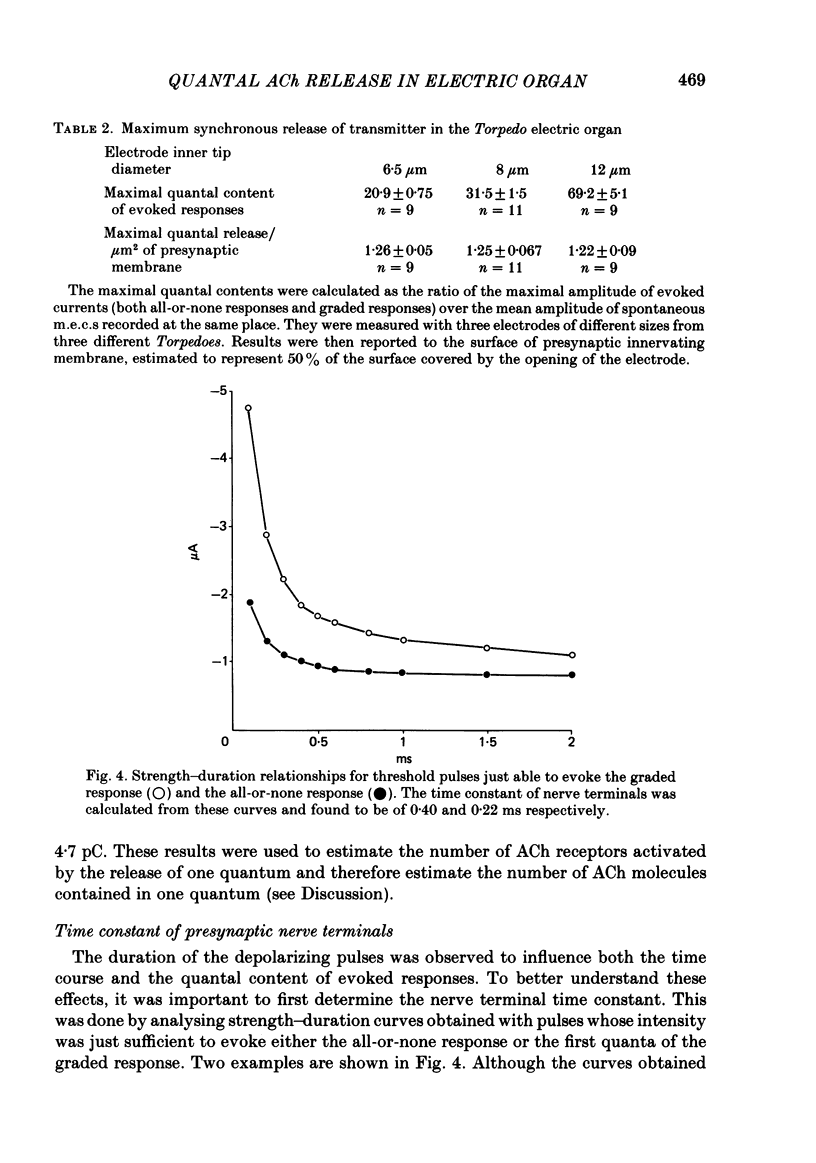
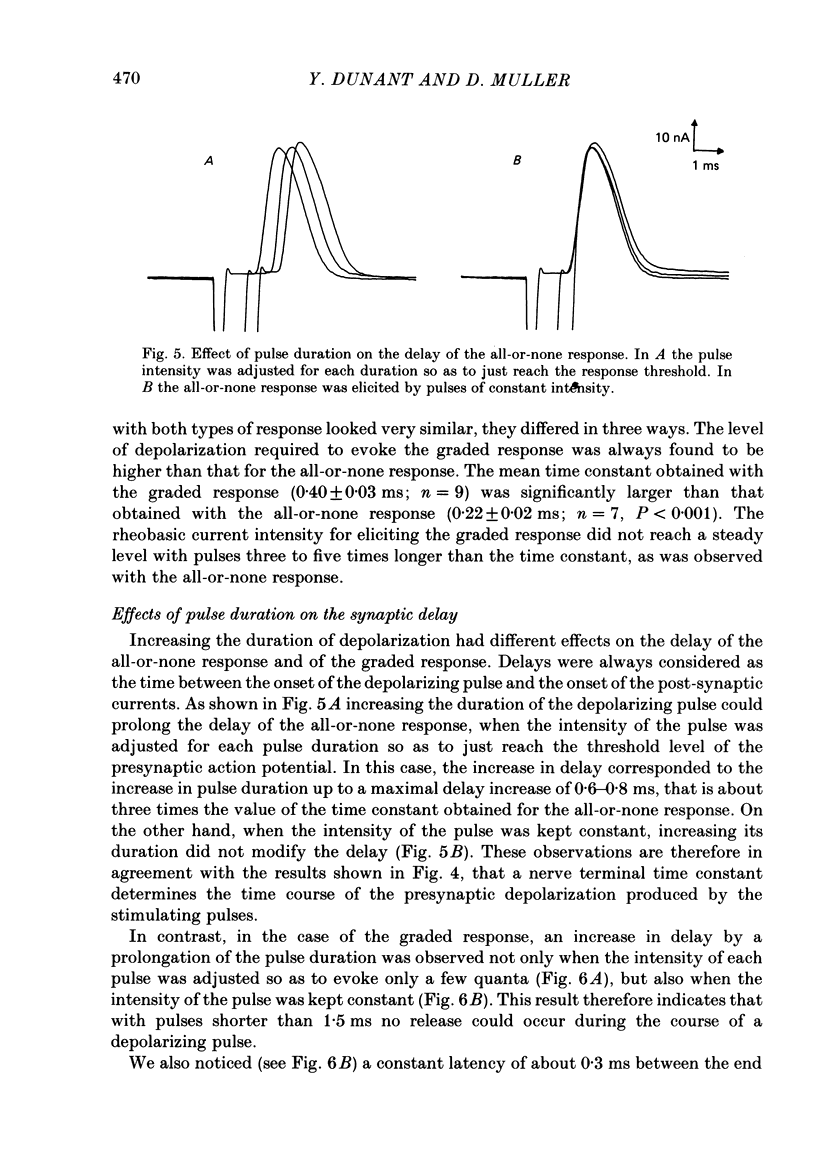
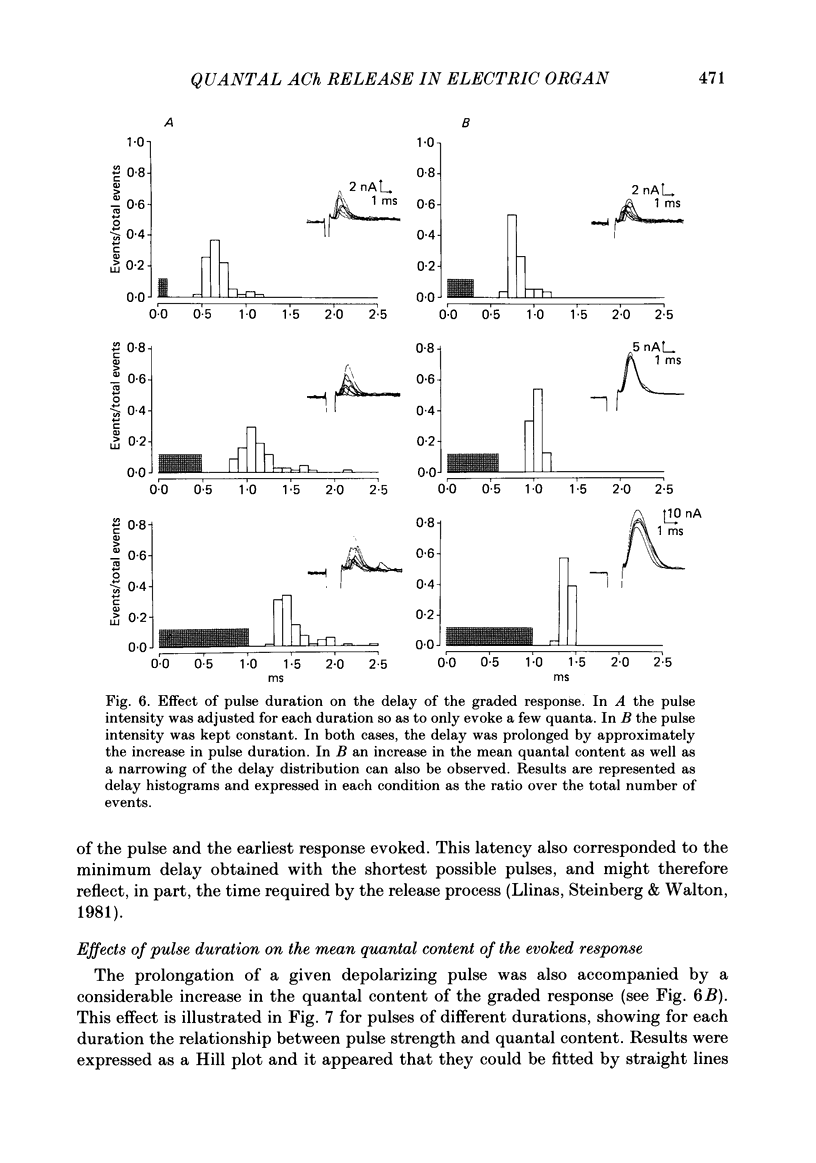
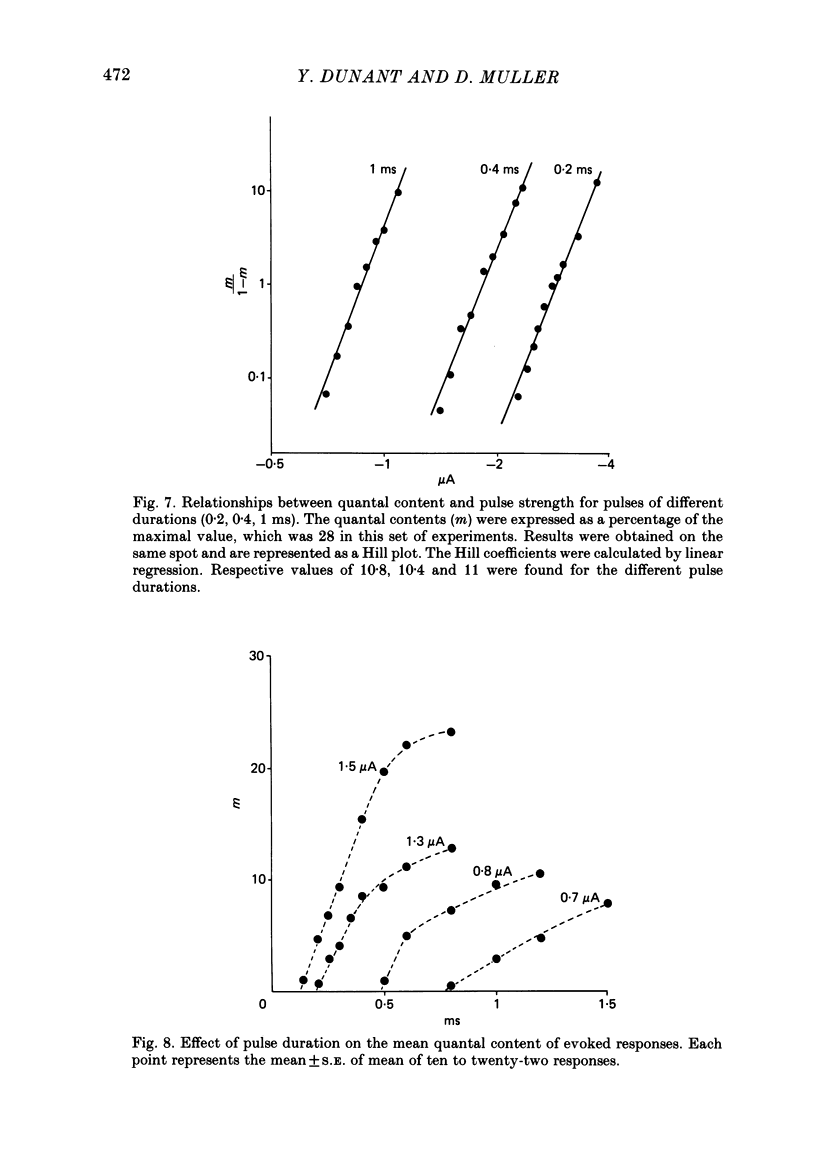
To analyse evoked acetylcholine (ACh) release in the electric organ of Torpedo marmorata, a loose patch-clamp technique was used that allowed with a single extracellular electrode both focal depolarization of nerve endings and recording of the post-synaptic currents produced by the released transmitter. Two different types of post-synaptic response could be evoked by depolarizing pulses of increasing intensity: a graded response appearing with a delay of 0.6 ms (pulses of 0.2 ms duration), and an all-or-none response characterized by a mean delay of 1.4 ms. Both responses had a similar maximal amplitude and a similar rise time of 0.6 ms. The graded response was evoked in all places where spontaneous miniature electroplaque currents (m.e.e.s) could be recorded. It was not modified by 1 microM-tetrodotoxin (TTX), but was Ca2+ dependent and was abolished by Cd2+ (0.2 mM) or Mg2+ (10 mM). The all-or-none response could be evoked in only 30% of places where m.e.c.s. were recorded, it was highly TTX sensitive, Ca2+ dependent, and abolished by Cd2+ (0.2 mM) or Mg2+ (10 mM). K+ channel blocking agents, such as 4-aminopyridine (4-AP) or tetraethylammonium (TEA), which are known to prolong the duration of action potentials, prolonged the delay of the all-or-none response, but not that of the graded response. At low strength stimulation, the graded response was clearly evoked in a quantal way, with the quantum corresponding to the amplitude of spontaneous m.e.c.s. The amplitude distribution of the evoked responses closely followed a Poisson distribution. The maximum synchronous release of transmitter was found to be approximately 1.3 quanta/micron2 of presynaptic membrane and a mean quantal size of about 7000 ACh molecules was estimated from the charge transfer of m.e.c.s. The nerve terminal time constant was calculated from strength-duration curves obtained with depolarizing pulses just able to evoke either the all-or-none response or the first few quanta of the graded response. Respective mean values of 0.22 and 0.40 ms were found. Increasing the duration of the depolarizing pulse had two consequences: it differently affected the delay of the all-or-none response and that of the graded response; it increased the mean quantal content of the graded response. Both effects could not simply be accounted for by the influence of the nerve terminal time constant.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Wurzel M., Grundfest H. The Electrophysiology of Electric Organs of Marine Electric Fishes : I. Properties of electroplaques of Torpedo nobiliana. J Gen Physiol. 1961 Mar 1;44(4):757–804. doi: 10.1085/jgp.44.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloedel J., Gage P. W., Llinás R., Quastel D. M. Transmitter release at the squid giant synapse in the presence of tetrodotoxin. Nature. 1966 Oct 1;212(5057):49–50. doi: 10.1038/212049a0. [DOI] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthay J., Dunant Y., Loctin F. Acetylcholine changes underlying transmission of a single nerve impulse in the presence of 4-aminopyridine in Torpedo. J Physiol. 1982 Apr;325:461–479. doi: 10.1113/jphysiol.1982.sp014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteaux R., Pécot-Dechavassine M. Vésicules synaptiques et poches au niveau des "zones actives" de la jonction neuromusculaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2346–2349. [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Secretion of acetylcholine in response to graded depolarization of motor nerve terminals. J Physiol (Paris) 1982;78(4):412–416. [PubMed] [Google Scholar]
- Den Hertog A., Pielkenrood J., Biessels P., Agoston S. The effect of some new aminopyridines on mammalian non-myelinated nerve fibres. Eur J Pharmacol. 1983 Oct 28;94(3-4):353–355. doi: 10.1016/0014-2999(83)90428-4. [DOI] [PubMed] [Google Scholar]
- Dudel J. Control of quantal transmitter release at frog's motor nerve terminals. I. Dependence on amplitude and duration of depolarization. Pflugers Arch. 1984 Nov;402(3):225–234. doi: 10.1007/BF00585504. [DOI] [PubMed] [Google Scholar]
- Dudel J. Control of quantal transmitter release at frog's motor nerve terminals. II. Modulation by de- or hyperpolarizing pulses. Pflugers Arch. 1984 Nov;402(3):235–243. doi: 10.1007/BF00585505. [DOI] [PubMed] [Google Scholar]
- Dudel J. Graded or all-or-nothing release of transmitter quanta by local depolarizations of nerve terminals on crayfish muscle? Pflugers Arch. 1983 Jul;398(2):155–164. doi: 10.1007/BF00581065. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Gautron J., Israël M., Lesbats B., Manaranche R. Les compartiments d'acetylcholine de l'organe electrique de la torpille et leurs modifications par la stimulation. J Neurochem. 1972 Aug;19(8):1987–2002. doi: 10.1111/j.1471-4159.1972.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Jones G. J., Loctin F. Acetylcholine measured at short time intervals during transmission of nerve impulses in the electric organ of Torpedo. J Physiol. 1982 Apr;325:441–460. doi: 10.1113/jphysiol.1982.sp014161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël M., Lesbats B. Continuous determination by a chemiluminescent method of acetylcholine release and compartmentation in Torpedo electric organ synaptosomes. J Neurochem. 1981 Dec;37(6):1475–1483. doi: 10.1111/j.1471-4159.1981.tb06317.x. [DOI] [PubMed] [Google Scholar]
- Israël M., Manaranche R., Morel N., Dedieu J. C., Gulik-Krzywicki T., Lesbats B. Redistribution of intramembrane particles related to acetylcholine release by cholinergic synaptosomes. J Ultrastruct Res. 1981 May;75(2):162–178. doi: 10.1016/s0022-5320(81)80132-3. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi T., Sears T. A. Electrical activity of mouse motor nerve terminals. Proc R Soc Lond B Biol Sci. 1984 Jul 23;222(1226):115–120. doi: 10.1098/rspb.1984.0052. [DOI] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier F. M. Relationship between presynaptic membrane potential and acetylcholine release in synaptosomes from Torpedo electric organ. J Physiol. 1984 Sep;354:121–137. doi: 10.1113/jphysiol.1984.sp015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D. Potentiation by 4-aminopyridine of quantal acetylcholine release at the Torpedo nerve-electroplaque junction. J Physiol. 1986 Oct;379:479–493. doi: 10.1113/jphysiol.1986.sp016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J. 1976 Jan;16(1):77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]



