Abstract
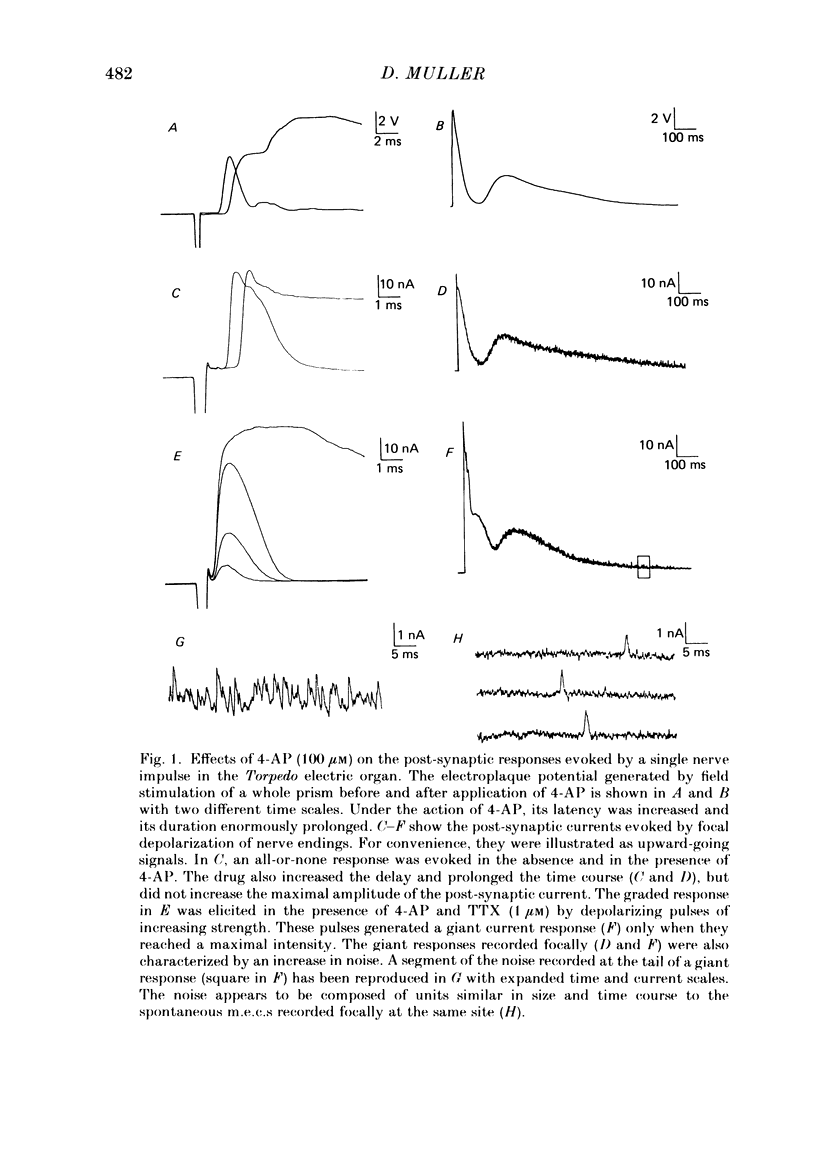
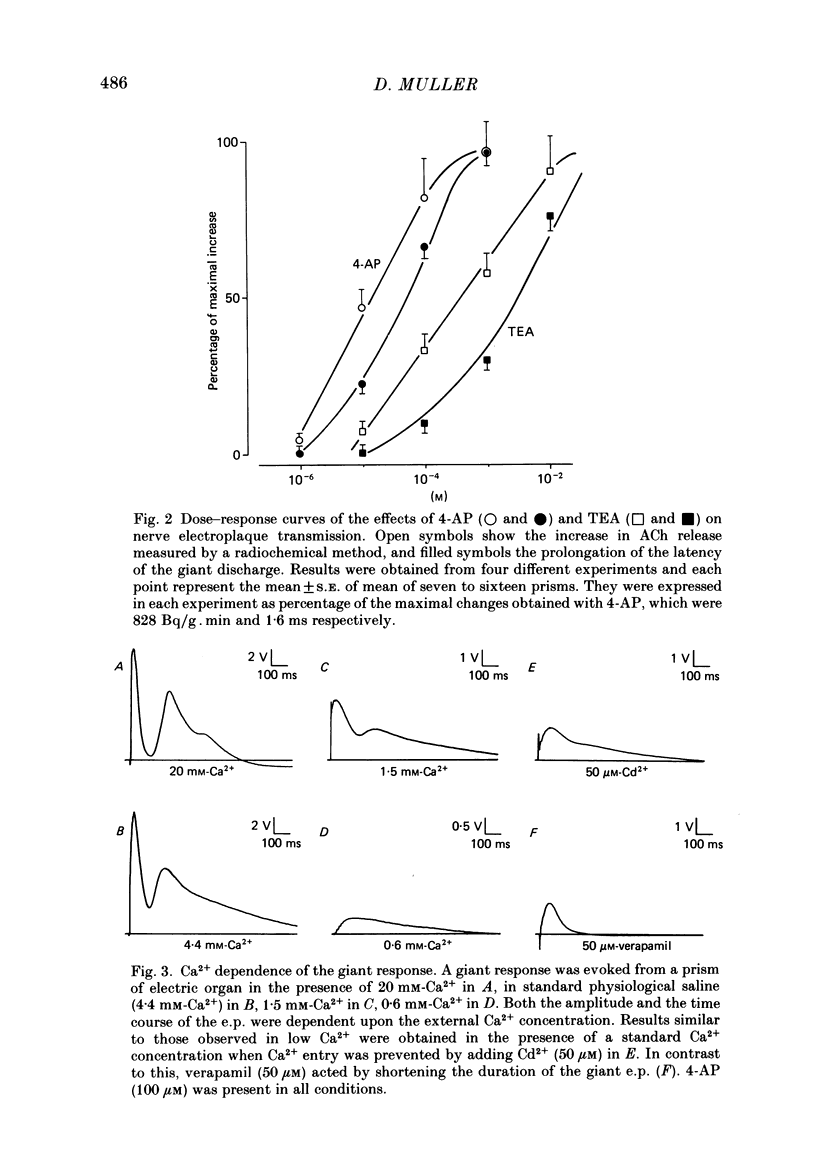
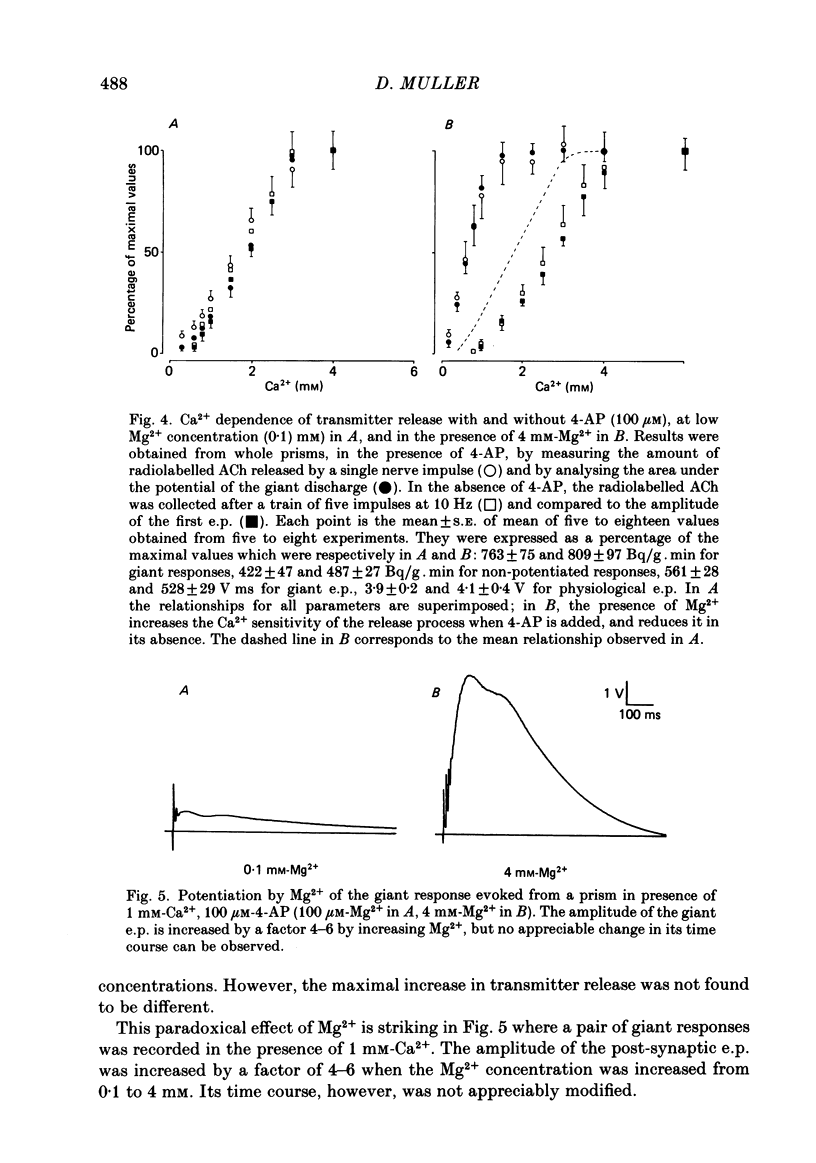
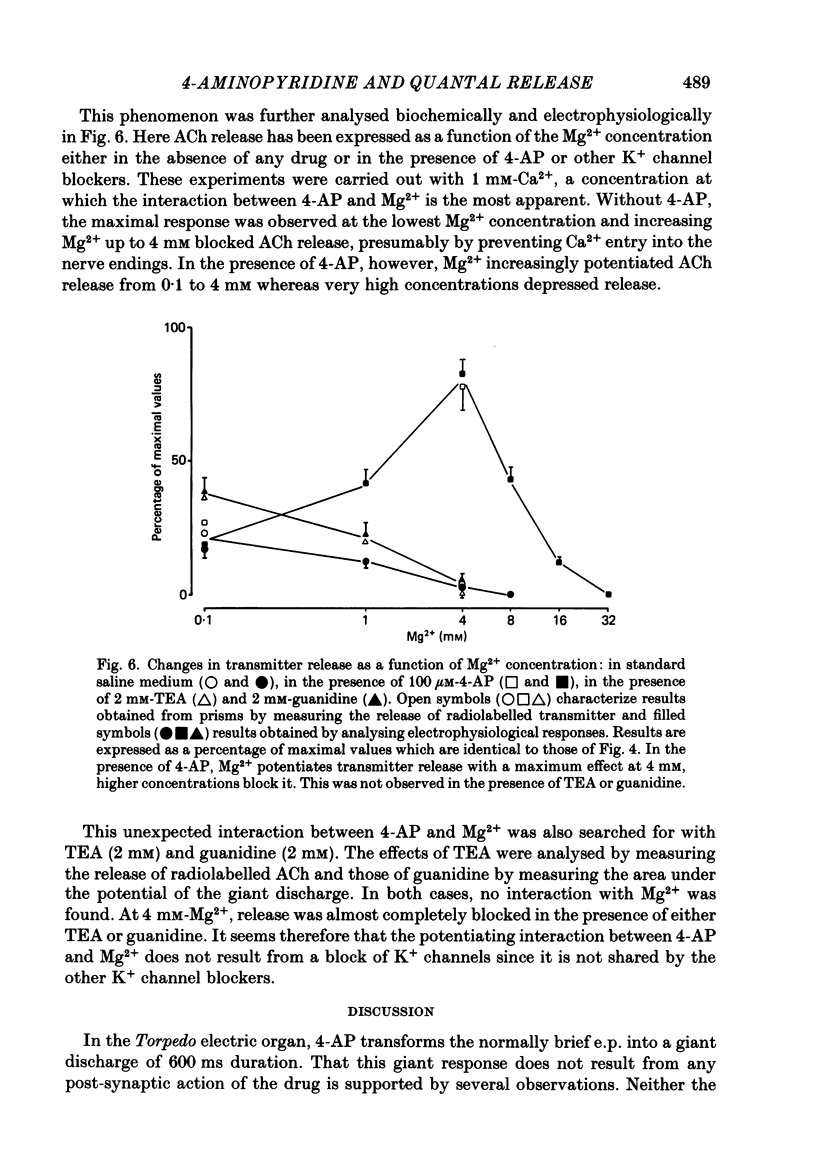
The effects of 4-aminopyridine (4-AP) on electrophysiological post-synaptic responses evoked by field stimulation or evoked focally using a loose patch-clamp technique, and on radiolabelled transmitter release were studied in the Torpedo electric organ. In this preparation, 4-AP had three major effects: it greatly potentiated the amount of acetylcholine (ACh) released by a nerve impulse, it prolonged the duration of the post-synaptic electroplaque current (e.c.) by several hundreds of milliseconds, and it increased the delay of responses triggered by a presynaptic action potential. Noise analysis performed at different times during the focally recorded giant response showed that it was made of a sustained release of ACh quanta. The maximum synchronous release of transmitter, expressed as the maximum number of quanta simultaneously delivered/micron2 of presynaptic membrane, was apparently not modified by 4-AP. A slightly different dose dependence was found for the effects of 4-AP on the potentiation of transmitter release and on the prolongation of the synaptic delay. The effects of tetraethylammonium (TEA) and other K+ channel blockers on these parameters were similar to those of 4-AP. Strong depolarizing pulses applied focally to a nerve ending were able to evoke a giant response even in the presence of 1 microM-tetrodotoxin (TTX). The prolongation of the discharge by 4-AP was therefore not caused by repetitive re-excitation of the nerve branches. Both the amplitude and the time course of the giant response were Ca2+ dependent. At a low Mg2+ concentration, the Ca2+ dependence of transmitter release was identical in the presence or absence of 4-AP. Paradoxically, in the presence of 4-AP, addition of 4 mM-Mg2+ considerably increased the Ca2+ dependence of release, whereas in the absence of 4-AP, Mg2+ blocked transmitter release by decreasing its sensitivity to Ca2+. This potentiating interaction between Mg2+ and 4-AP was not seen with TEA or guanidine. In conclusion, 4-AP potentiates ACh release in two different ways in the Torpedo electric organ: it promotes a sustained quantal release of transmitter during several hundreds of milliseconds without any significant change in the maximal synchronous release, it interacts with Mg2+ in such a manner that the sensitivity to Ca2+ of the nerve terminals is increased.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Takeda K., Umbach J. A. Inhibitors of calcium buffering depress evoked transmitter release at the squid giant synapse. J Physiol. 1985 Dec;369:145–159. doi: 10.1113/jphysiol.1985.sp015893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P. R., Mambrini J. Modification of transmitter release by ions which prolong the presynaptic action potential. J Physiol. 1970 Oct;210(3):681–695. doi: 10.1113/jphysiol.1970.sp009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthay J., Dunant Y., Loctin F. Acetylcholine changes underlying transmission of a single nerve impulse in the presence of 4-aminopyridine in Torpedo. J Physiol. 1982 Apr;325:461–479. doi: 10.1113/jphysiol.1982.sp014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Eder L., Servetiadis-Hirt L. Acetylcholine release evoked by single or a few nerve impulses in the electric organ of Torpedo. J Physiol. 1980 Jan;298:185–203. doi: 10.1113/jphysiol.1980.sp013075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Muller D. Quantal release of acetylcholine evoked by focal depolarization at the Torpedo nerve-electroplaque junction. J Physiol. 1986 Oct;379:461–478. doi: 10.1113/jphysiol.1986.sp016264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P., Thesleff S. 4-Aminopyridine and evoked transmitter release from motor nerve endings. Br J Pharmacol. 1978 Dec;64(4):623–629. doi: 10.1111/j.1476-5381.1978.tb17325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Walton K., Bohr V. Synaptic transmission in squid giant synapse after potassium conductance blockage with external 3- and 4-aminopyridine. Biophys J. 1976 Jan;16(1):83–86. doi: 10.1016/S0006-3495(76)85664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H. Effects of 4-aminopyridine on neuromuscular transmission. Brain Res. 1978 Sep 22;153(2):307–318. doi: 10.1016/0006-8993(78)90409-2. [DOI] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Effects of guanidine on transmitter release and neuronal excitability. J Physiol. 1977 Mar;266(1):69–89. doi: 10.1113/jphysiol.1977.sp011756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgo J., Lemeignan M., Lechat P. Effects of 4-aminopyridine at the frog neuromuscular junction. J Pharmacol Exp Ther. 1977 Dec;203(3):653–663. [PubMed] [Google Scholar]
- Simonneau M., Tauc L., Baux G. Quantal release of acetylcholine examined by current fluctuation analysis at an identified neuro-neuronal synapse of Aplysia. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1661–1665. doi: 10.1073/pnas.77.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff S. Aminopyridines and synaptic transmission. Neuroscience. 1980;5(8):1413–1419. doi: 10.1016/0306-4522(80)90002-0. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J. 1976 Jan;16(1):77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


