Abstract
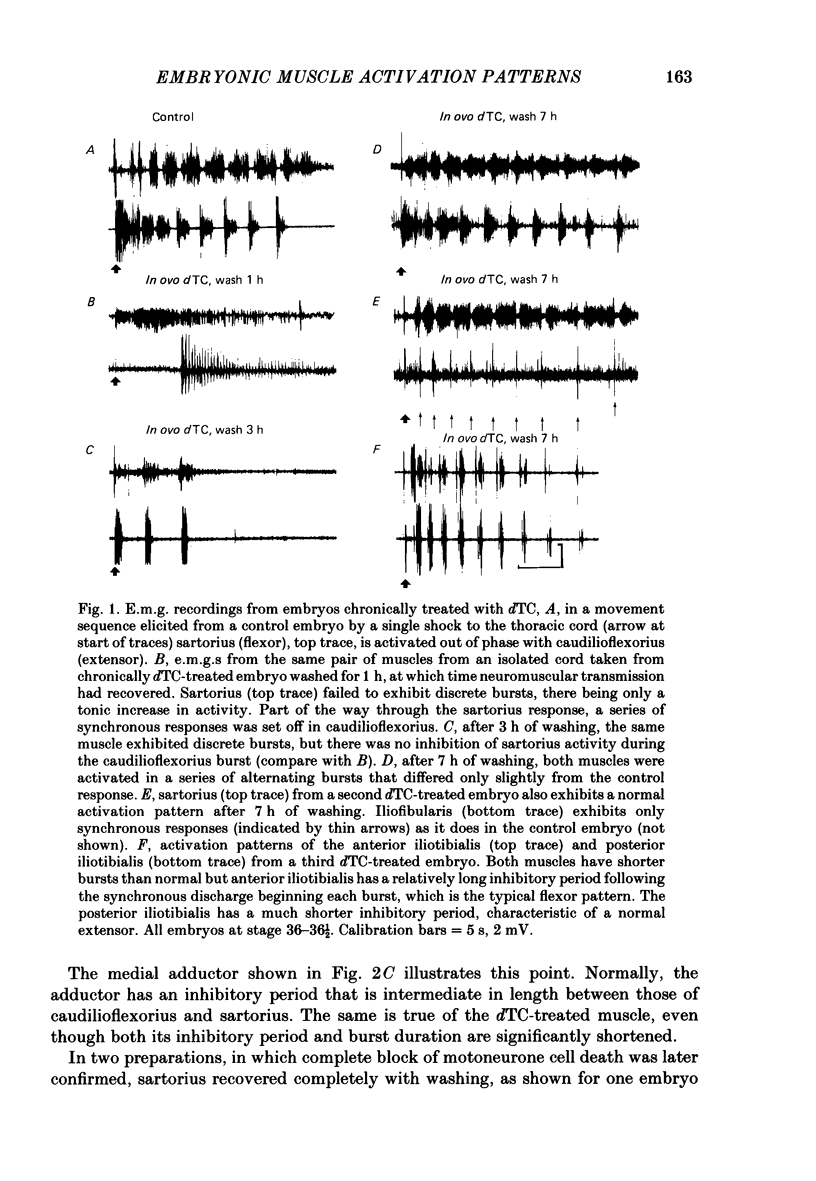
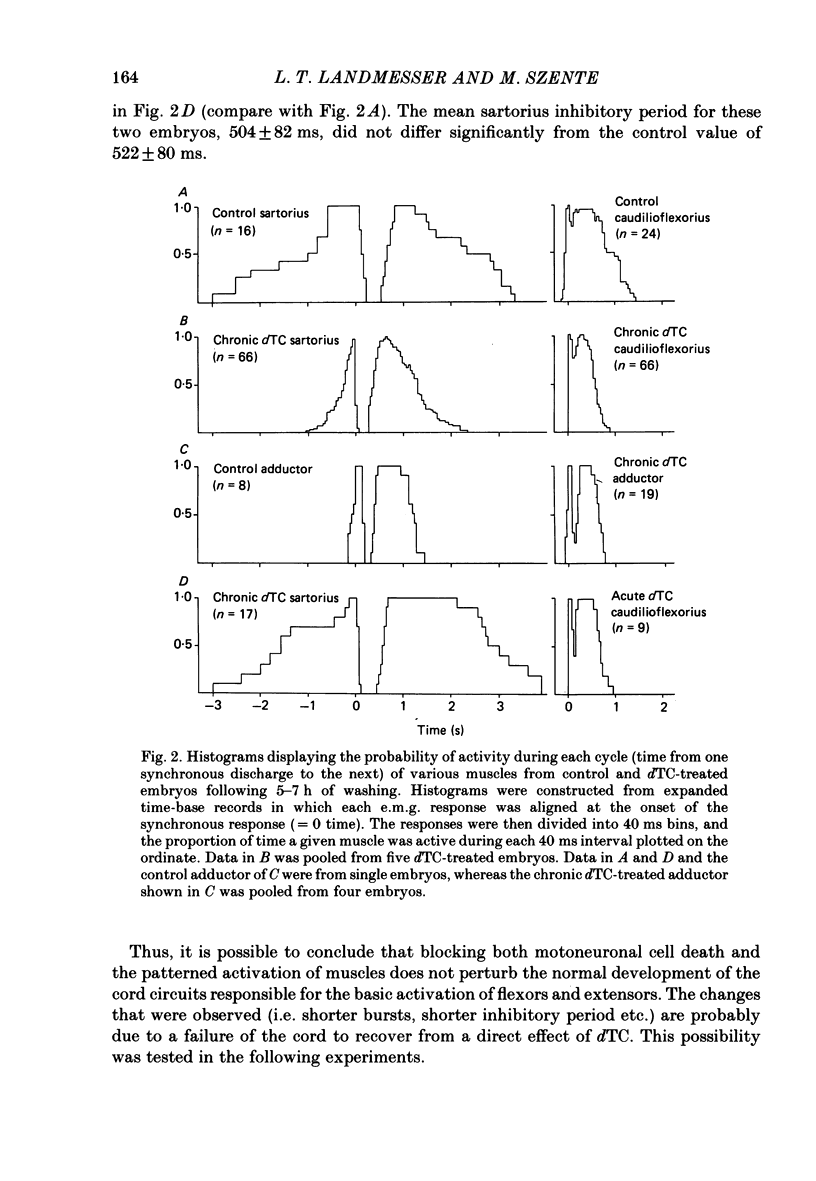
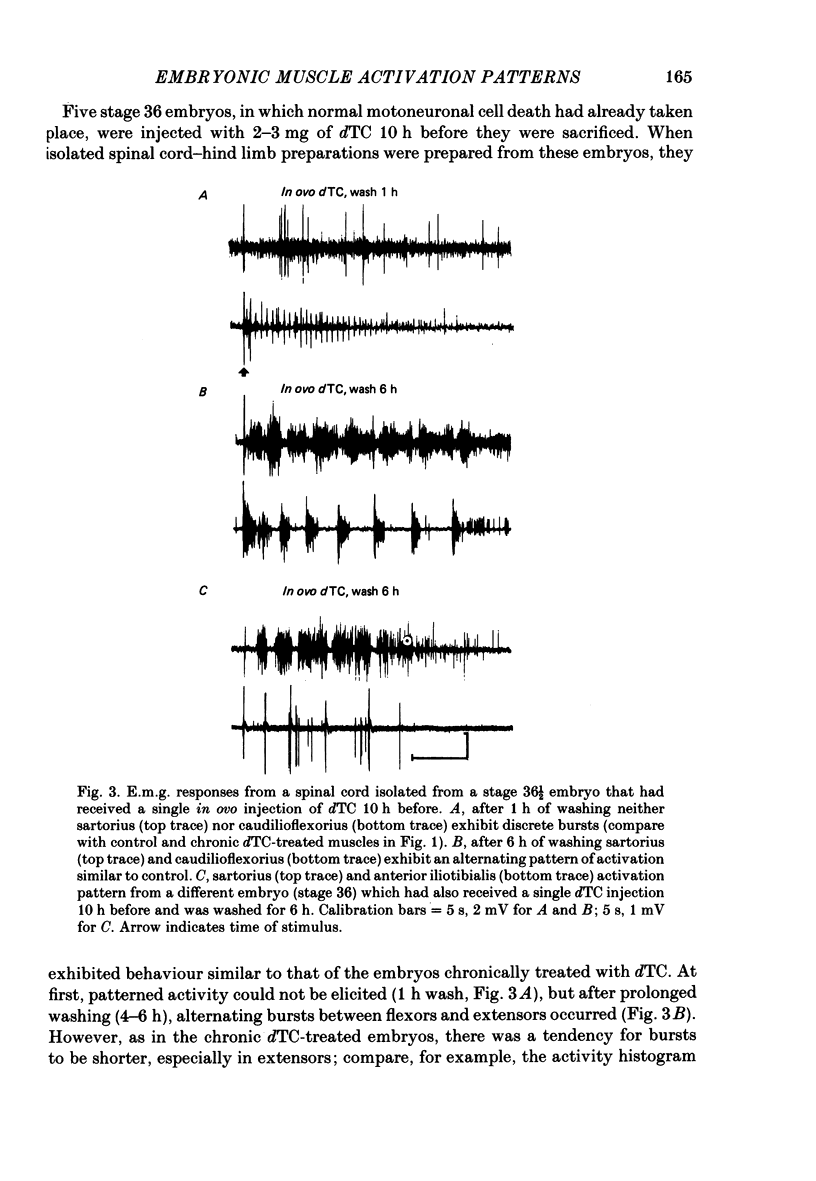
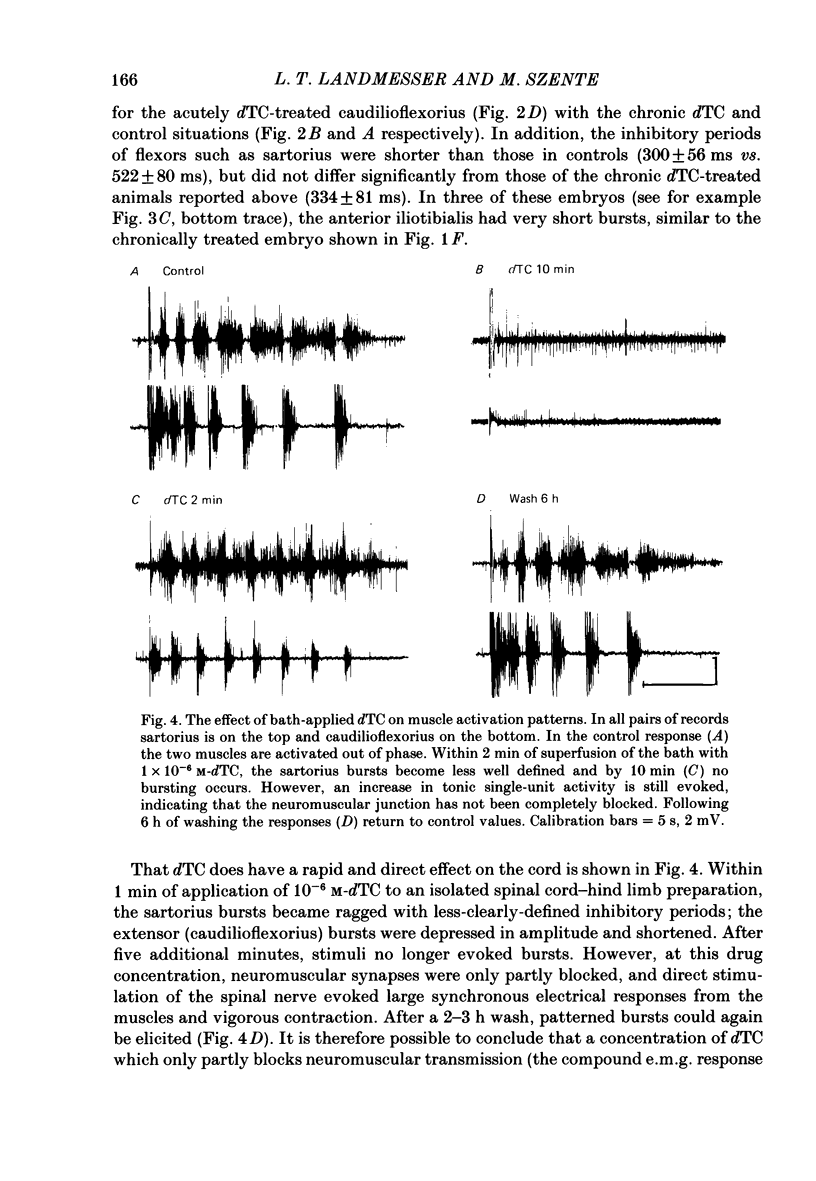
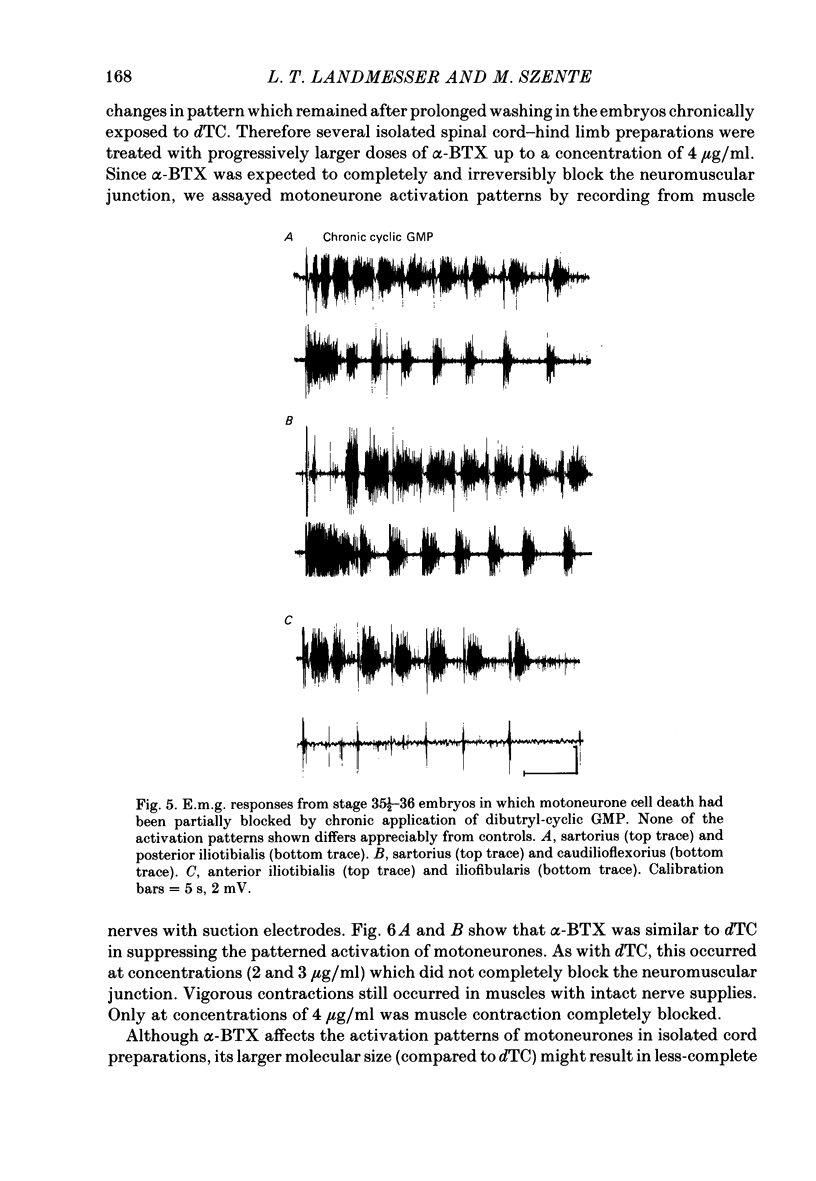
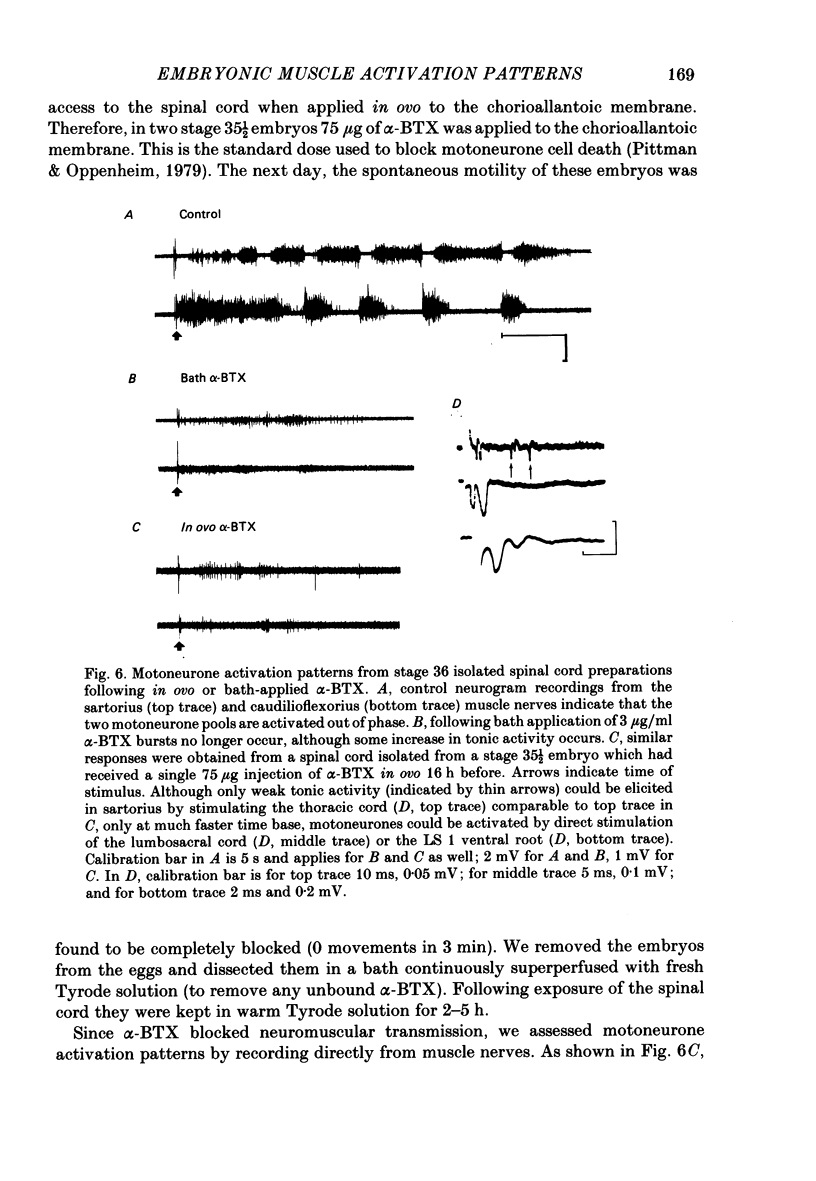
Motoneurone cell death and spontaneous embryonic motility were blocked in chick embryos by daily in ovo injections of d-tubocurarine from stage 28-36 (E5-10). Isolated spinal cord-hind-limb preparations were prepared from these embryos and movement sequences in response to electrical stimulation of the thoracic cord were assessed, after drug wash-out, by electromyogram (e.m.g.) or muscle-nerve recordings. In embryos in which complete blockade of lumbar motoneurone cell death was later confirmed histologically, flexor and extensor motoneurone pools were found to be activated in alternating bursts as occurs in control embryos. Thus the development of the basic cord circuits responsible for these patterns of motoneurone activation does not require motoneurone cell death. Partial blockade of motoneurone cell death by guanosine 3',5'-phosphate (cyclic GMP) was also without effect on muscle activation patterns. In ovo injection of d-tubocurarine or alpha-bungarotoxin in doses sufficient to block embryonic motility was found to have a direct effect on the spinal cord, preventing the patterned activation of motoneurone pools in alternating bursts. Cords removed from treated embryos behaved similarly to cords in which these drugs were applied acutely in the bath. Minor changes in muscle activation patterns that occurred with chronic drug treatment were also observed in acutely treated cords and appear to be a direct and persistent effect of the drugs on cord circuits. It is possible to conclude that cholinergic circuits within the chick lumbar cord play a role in the normal patterned activation of flexor and extensor motoneurone pools. Systemically applied drugs can have access to these circuits, indicating a need for caution when interpreting the results of drugs applied in this manner to developing embryos. We also conclude that neither the activation of motoneurones in patterned bursts, nor the afferent feed-back from the movements that result, are required to form the basic spinal cord circuits responsible for the activation of extensor and flexor motoneurone pools in alternating bursts.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekoff A. Ontogeny of leg motor output in the chick embryo: a neural analysis. Brain Res. 1976 Apr 23;106(2):271–291. doi: 10.1016/0006-8993(76)91025-8. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. Development of the topographical projection of motor neurons to a rat muscle accompanies loss of polyneuronal innervation. J Neurosci. 1984 Sep;4(9):2204–2212. doi: 10.1523/JNEUROSCI.04-09-02204.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. Development of the topographical projection of motor neurons to amphibian muscle accompanies motor neuron death. Brain Res. 1981 Oct;254(3):448–452. doi: 10.1016/0165-3806(81)90053-5. [DOI] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of tenotomy on the evolution of multiinnervation in developing rat soleus muscle. Brain Res. 1975 Dec 5;99(2):354–358. doi: 10.1016/0006-8993(75)90036-0. [DOI] [PubMed] [Google Scholar]
- Boss V. C., Schmidt J. T. Activity and the formation of ocular dominance patches in dually innervated tectum of goldfish. J Neurosci. 1984 Dec;4(12):2891–2905. doi: 10.1523/JNEUROSCI.04-12-02891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Booth C. M. Postnatal development of the adult pattern of motor axon distribution in rat muscle. Nature. 1983 Aug 25;304(5928):741–742. doi: 10.1038/304741a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursztajn S., Gershon M. D. Discrimination between nicotinic receptors in vertebrate ganglia and skeletal muscle by alpha-bungarotoxin and cobra venoms. J Physiol. 1977 Jul;269(1):17–31. doi: 10.1113/jphysiol.1977.sp011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappinelli V. A., Cohen J. B., Zigmond R. E. The effects of alpha- and beta-neurotoxins from the venoms of various snakes on transmission in autonomic ganglia. Brain Res. 1981 Apr 27;211(1):107–126. doi: 10.1016/0006-8993(81)90070-6. [DOI] [PubMed] [Google Scholar]
- Chu-Wang I. W., Oppenheim R. W. Cell death of motoneurons in the chick embryo spinal cord. II. A quantitative and qualitative analysis of degeneration in the ventral root, including evidence for axon outgrowth and limb innervation prior to cell death. J Comp Neurol. 1978 Jan 1;177(1):59–85. doi: 10.1002/cne.901770106. [DOI] [PubMed] [Google Scholar]
- Ding R., Jansen J. K., Laing N. G., Tønnesen H. The innervation of skeletal muscles in chickens curarized during early development. J Neurocytol. 1983 Dec;12(6):887–919. doi: 10.1007/BF01153341. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Balaban M., Oppenheim R., Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool. 1965 Jun;159(1):1–13. doi: 10.1002/jez.1401590102. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975 Apr 15;160(4):535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- Hollyday M. Development of motor innervation of chick limbs. Prog Clin Biol Res. 1983;110(Pt A):183–193. [PubMed] [Google Scholar]
- Lamb A. H. The projection patterns of the ventral horn to the hind limb during development. Dev Biol. 1976 Nov;54(1):82–99. doi: 10.1016/0012-1606(76)90288-8. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C., Landmesser L. Pathway selection by embryonic chick motoneurons in an experimentally altered environment. Proc R Soc Lond B Biol Sci. 1981 Dec 9;214(1194):19–52. doi: 10.1098/rspb.1981.0080. [DOI] [PubMed] [Google Scholar]
- Landmesser L. T., O'Donovan M. J. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol. 1984 Feb;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. T., O'Donovan M. J. The activation patterns of embryonic chick motoneurones projecting to inappropriate muscles. J Physiol. 1984 Feb;347:205–224. doi: 10.1113/jphysiol.1984.sp015062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L., Morris D. G. The development of functional innervation in the hind limb of the chick embryo. J Physiol. 1975 Jul;249(2):301–326. doi: 10.1113/jphysiol.1975.sp011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. The distribution of motoneurones supplying chick hind limb muscles. J Physiol. 1978 Nov;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. L. Tetrodotoxin blocks the formation of ocular dominance columns in goldfish. Science. 1982 Nov 5;218(4572):589–591. doi: 10.1126/science.7123262. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbová G. Observations on the elimination of polyneuronal innervation in developing mammalian skeletal muscle. J Physiol. 1978 Sep;282:571–582. doi: 10.1113/jphysiol.1978.sp012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W. Cell death of motoneurons in the chick embryo spinal cord. V. Evidence on the role of cell death and neuromuscular function in the formation of specific peripheral connections. J Neurosci. 1981 Feb;1(2):141–151. doi: 10.1523/JNEUROSCI.01-02-00141.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W. The role of supraspinal input in embryonic motility: a re-examination in the chick. J Comp Neurol. 1975 Mar 1;160(1):37–50. doi: 10.1002/cne.901600104. [DOI] [PubMed] [Google Scholar]
- Pittman R. H., Oppenheim R. W. Neuromuscular blockade increases motoneurone survival during normal cell death in the chick embryo. Nature. 1978 Jan 26;271(5643):364–366. doi: 10.1038/271364a0. [DOI] [PubMed] [Google Scholar]
- Pittman R., Oppenheim R. W. Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol. 1979 Sep 15;187(2):425–446. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- Ravdin P. M., Berg D. K. Inhibition of neuronal acetylcholine sensitivity by alpha-toxins from Bungarus multicinctus venom. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2072–2076. doi: 10.1073/pnas.76.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh T. A., Constantine-Paton M. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J Neurosci. 1985 May;5(5):1132–1143. doi: 10.1523/JNEUROSCI.05-05-01132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester R. R., Taxt T. Repression of inactive motor nerve terminals in partially denervated rat muscle after regeneration of active motor axons. J Physiol. 1984 Feb;347:497–511. doi: 10.1113/jphysiol.1984.sp015078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge R. M., Betz W. J. The effect of selective, chronic stimulation on motor unit size in developing rat muscle. J Neurosci. 1984 Oct;4(10):2614–2620. doi: 10.1523/JNEUROSCI.04-10-02614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley K. L., Provine R. R. Neural correlates of embryonic motility in the chick. Brain Res. 1972 Oct 13;45(1):127–134. doi: 10.1016/0006-8993(72)90220-x. [DOI] [PubMed] [Google Scholar]
- Sanes D. H., Constantine-Paton M. The sharpening of frequency tuning curves requires patterned activity during development in the mouse, Mus musculus. J Neurosci. 1985 May;5(5):1152–1166. doi: 10.1523/JNEUROSCI.05-05-01152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W., Kuffler D. P., Jansen J. K. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature. 1983 Apr 14;302(5909):614–616. doi: 10.1038/302614a0. [DOI] [PubMed] [Google Scholar]
- Tosney K. W., Landmesser L. T. Specificity of early motoneuron growth cone outgrowth in the chick embryo. J Neurosci. 1985 Sep;5(9):2336–2344. doi: 10.1523/JNEUROSCI.05-09-02336.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill C. L., Greene D. P. Prevention of natural motoneurone cell death by dibutyryl cyclic GMP. 1984 Mar 29-Apr 4Nature. 308(5958):452–454. doi: 10.1038/308452a0. [DOI] [PubMed] [Google Scholar]


