Abstract
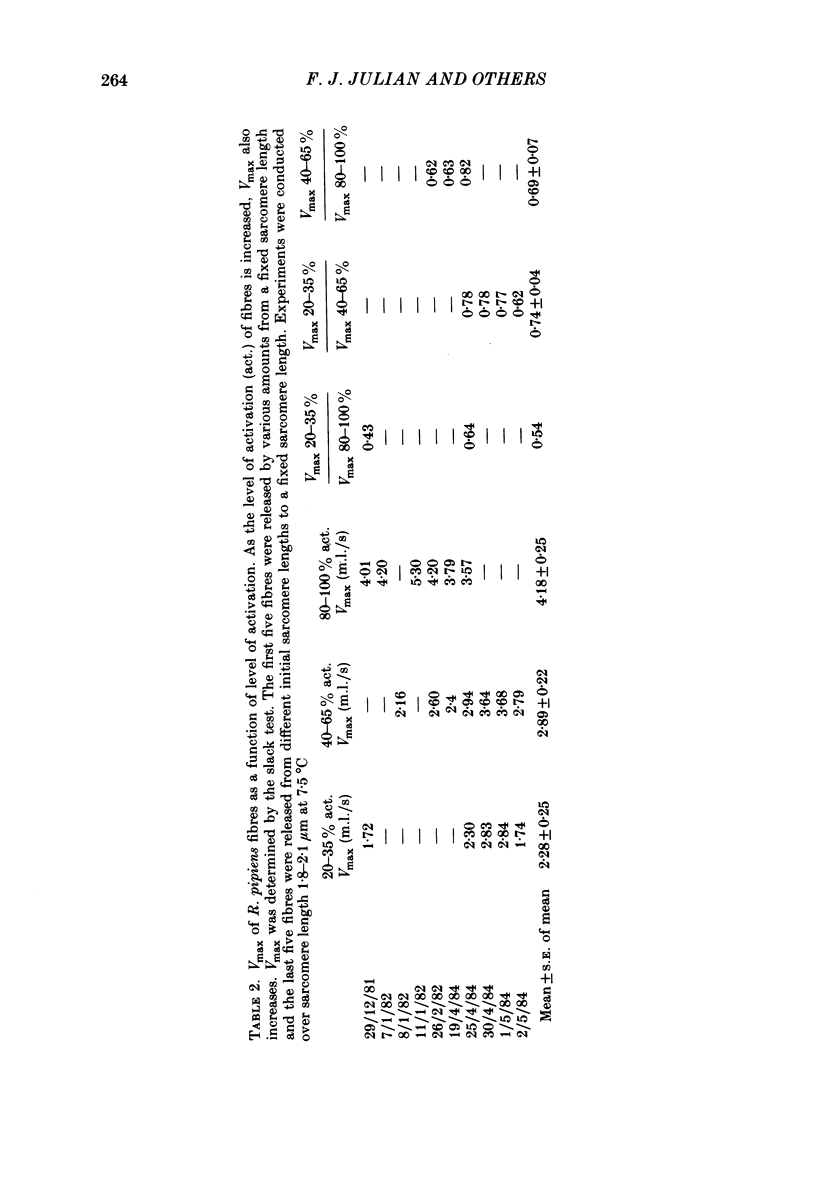
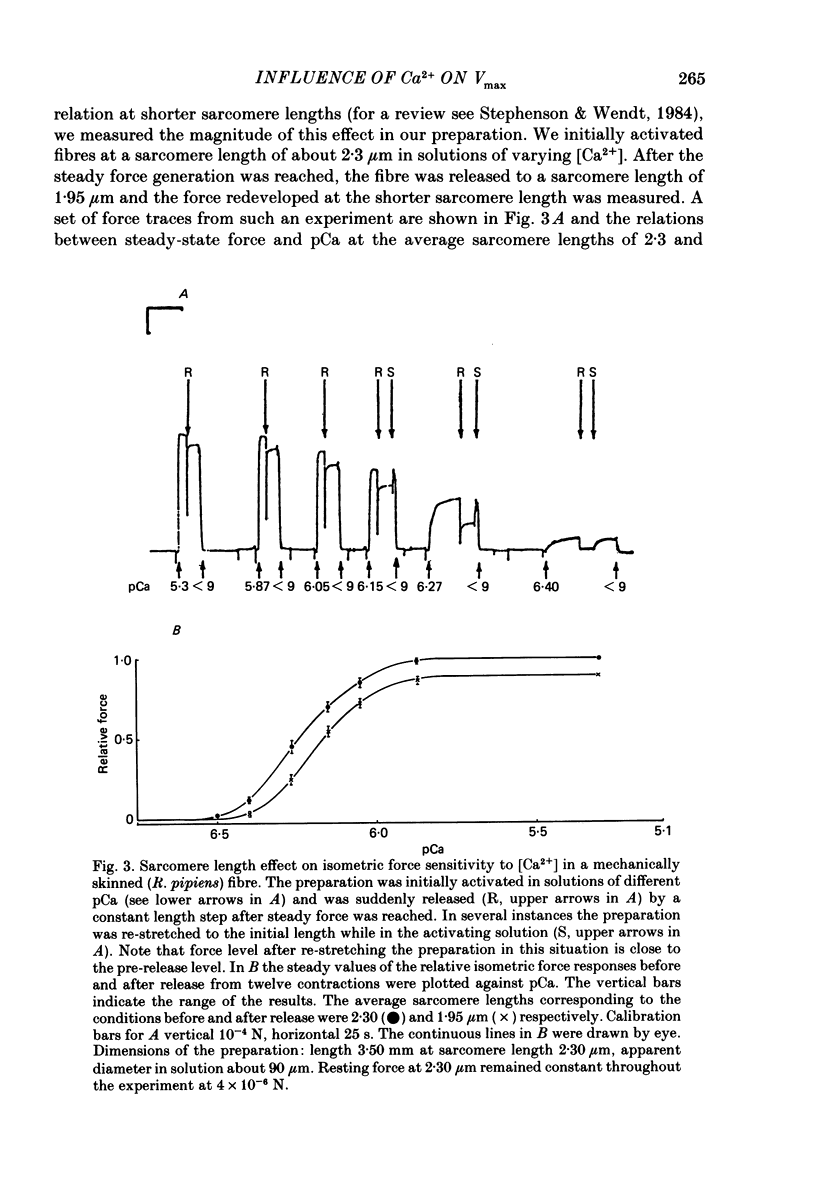
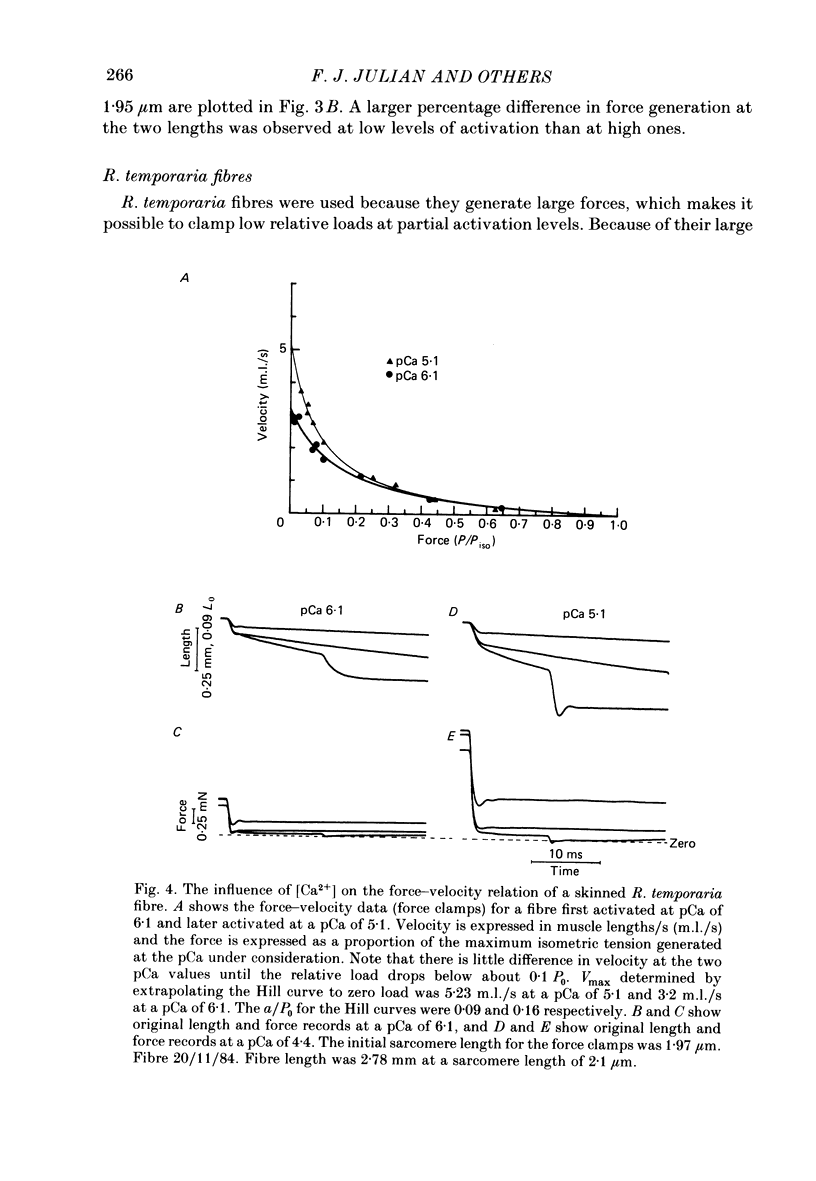
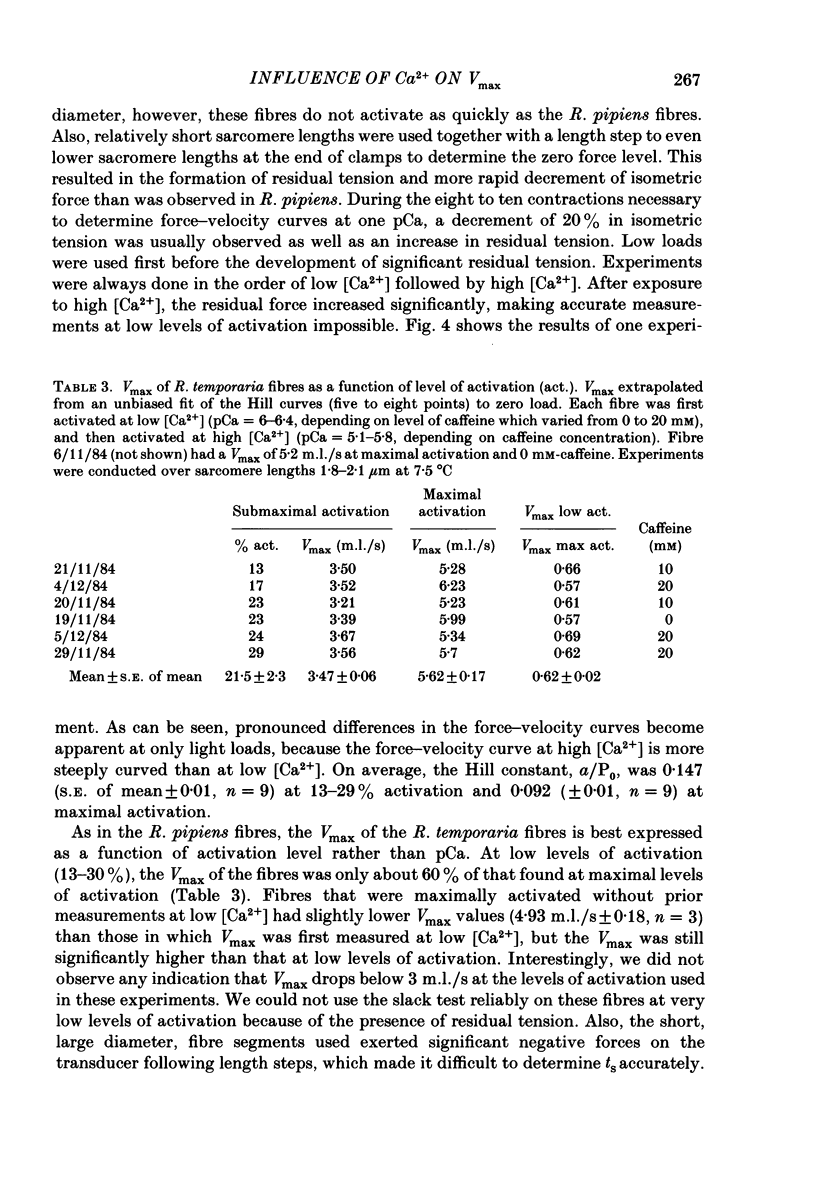
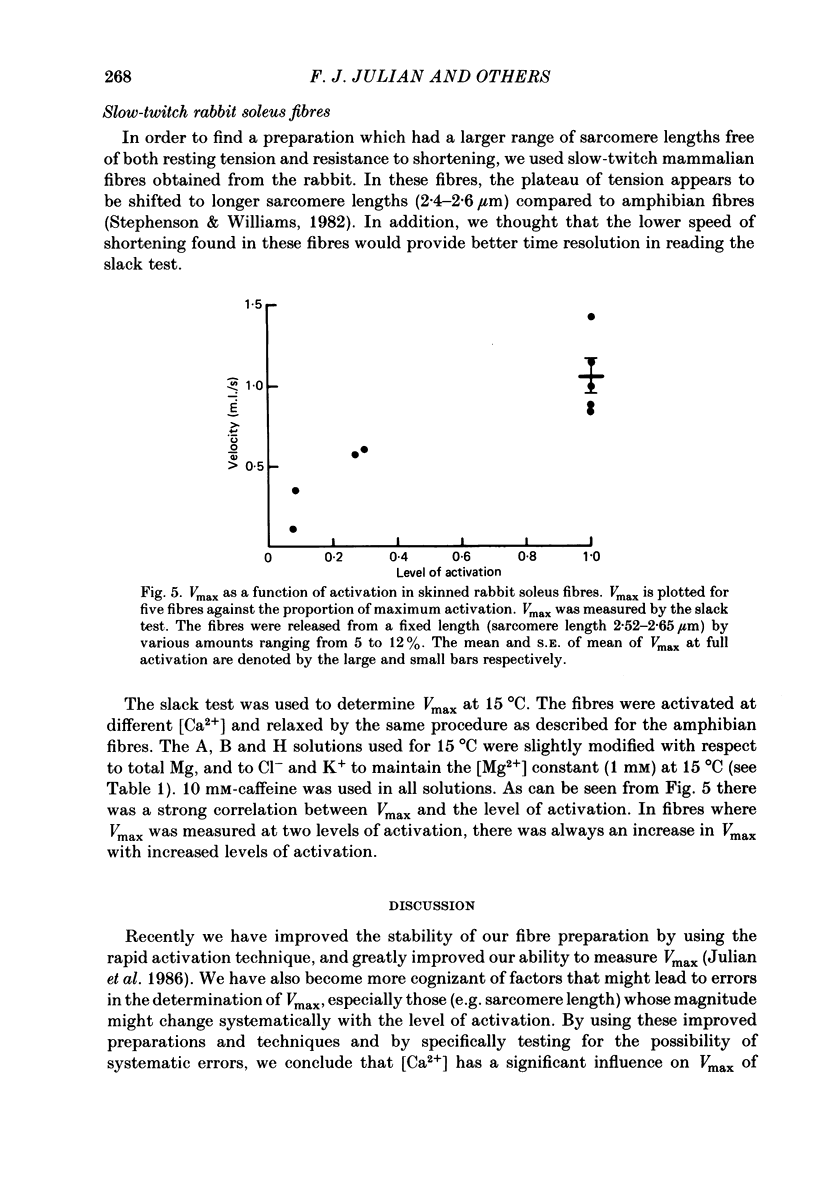
The influence of [Ca2+] on the maximum velocity of shortening (Vmax) was examined in mechanically skinned Rana pipiens and Rana temporaria fibres using improved force clamps and the slack test techniques. All measurements were made at 7.5 degrees C. At low relative loads (P/P0 less than 0.1), maximally activated R. pipiens fibres shortened more rapidly than did submaximally activated fibres. At higher relative loads, however, little difference in the speed of shortening was observed. Vmax (determined by the slack test) of R. pipiens fibres increased as the level of activation increased. Over sarcomere lengths 1.8-2.1 microns it was 2.28 muscle lengths/s (m.l./s) (S.E. of mean +/- 0.25, n = 5) at 20-35% activation, 2.89 m.l./s (+/- 0.22, n = 7) at 40-60% activation, and 4.18 m.l./s (+/- 0.25, n = 6) at 100% activation. At longer sarcomere lengths (2.2-2.6 microns), higher Vmax values were observed at all levels of activation, but the influence of Ca2+ on Vmax persisted. Vmax was 3.54 m.l./s (+/- 0.41, n = 4) at 20-30% activation and 5.15 m.l./s (+/- 0.22, n = 5) at 100% activation. In R. temporaria fibres, Vmax (determined by force clamps over sarcomere lengths 1.8-2.1 micron) also increased as the level of activation increased, from 3.47 m.l./s (+/- 0.06, n = 6) at 13-29% activation to 5.62 m.l./s (+/- 0.17, n = 6) at 100% activation. Vmax was also determined (using the slack test) in mechanically and chemically skinned rabbit soleus fibres. Vmax at 15 degrees C (1.05 m.l./s, +/- 0.11, n = 5) at full activation decreased by more than 3-fold as the level of activation was reduced to 10%. We conclude that the level of activation influences the Vmax of skinned skeletal muscle fibres. This has now been demonstrated in three different preparations and by a variety of techniques. This effect is most pronounced at low relative loads, and might not be observed if there are experimental limitations which prevent making velocity measurements at low relative loads.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Podolsky R. J. Contraction transients of skinned muscle fibers: effects of calcium and ionic strength. J Gen Physiol. 1978 Nov;72(5):701–715. doi: 10.1085/jgp.72.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. Effects of calcium and ionic strength on shortening velocity and tension development in frog skinned muscle fibres. J Physiol. 1981 Feb;311:179–199. doi: 10.1113/jphysiol.1981.sp013580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. Sarcomere length-tension relations of frog skinned muscle fibres at lengths above the optimum. J Physiol. 1980 Jul;304:529–539. doi: 10.1113/jphysiol.1980.sp013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Rome L. C., Stephenson D. G., Striz S. The maximum speed of shortening in living and skinned frog muscle fibres. J Physiol. 1986 Jan;370:181–199. doi: 10.1113/jphysiol.1986.sp015929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G., Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978 Feb;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolin R. A., Ford L. E. The influence of calcium on shortening velocity of skinned frog muscle cells. J Muscle Res Cell Motil. 1983 Jun;4(3):263–282. doi: 10.1007/BF00711996. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Wendt I. R. Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres. J Muscle Res Cell Motil. 1984 Jun;5(3):243–272. doi: 10.1007/BF00713107. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981 Aug;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol. 1982 Dec;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull J. T. Phosphorylation of contractile proteins in relation to muscle function. Adv Cyclic Nucleotide Res. 1980;13:39–93. [PubMed] [Google Scholar]
- Sweeney H. L., Kushmerick M. J. Myosin phosphorylation in permeabilized rabbit psoas fibers. Am J Physiol. 1985 Sep;249(3 Pt 1):C362–C365. doi: 10.1152/ajpcell.1985.249.3.C362. [DOI] [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Wise R. M., Rondinone J. F., Briggs F. N. Effect of calcium on force-velocity characteristics of glycerinated skeletal muscle. Am J Physiol. 1971 Oct;221(4):973–979. doi: 10.1152/ajplegacy.1971.221.4.973. [DOI] [PubMed] [Google Scholar]


