Abstract
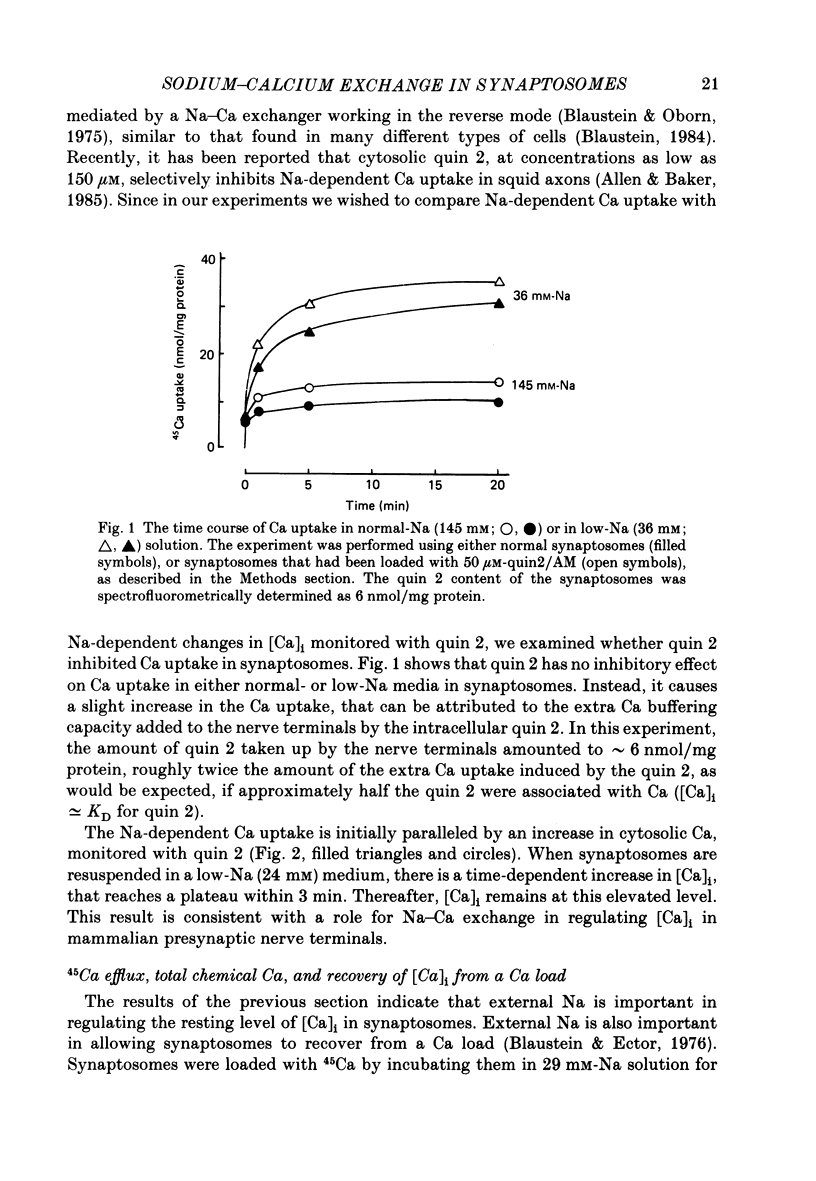
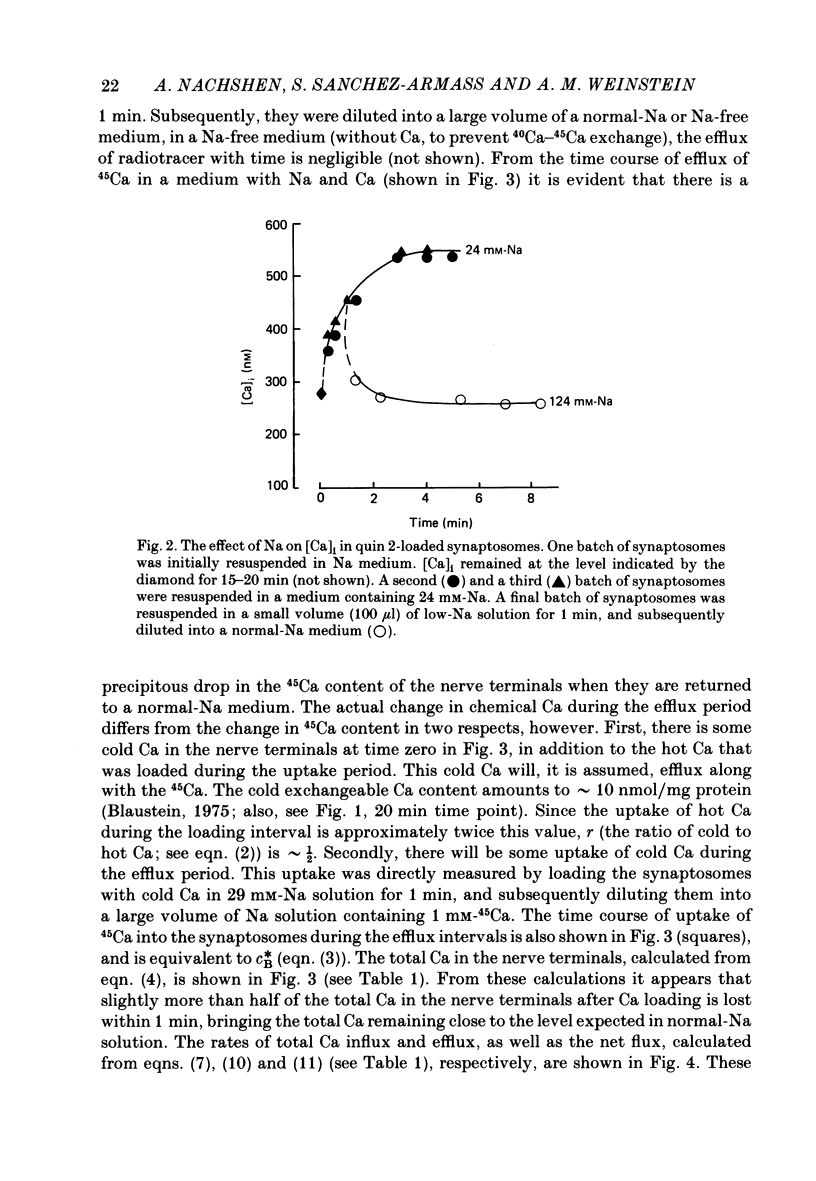
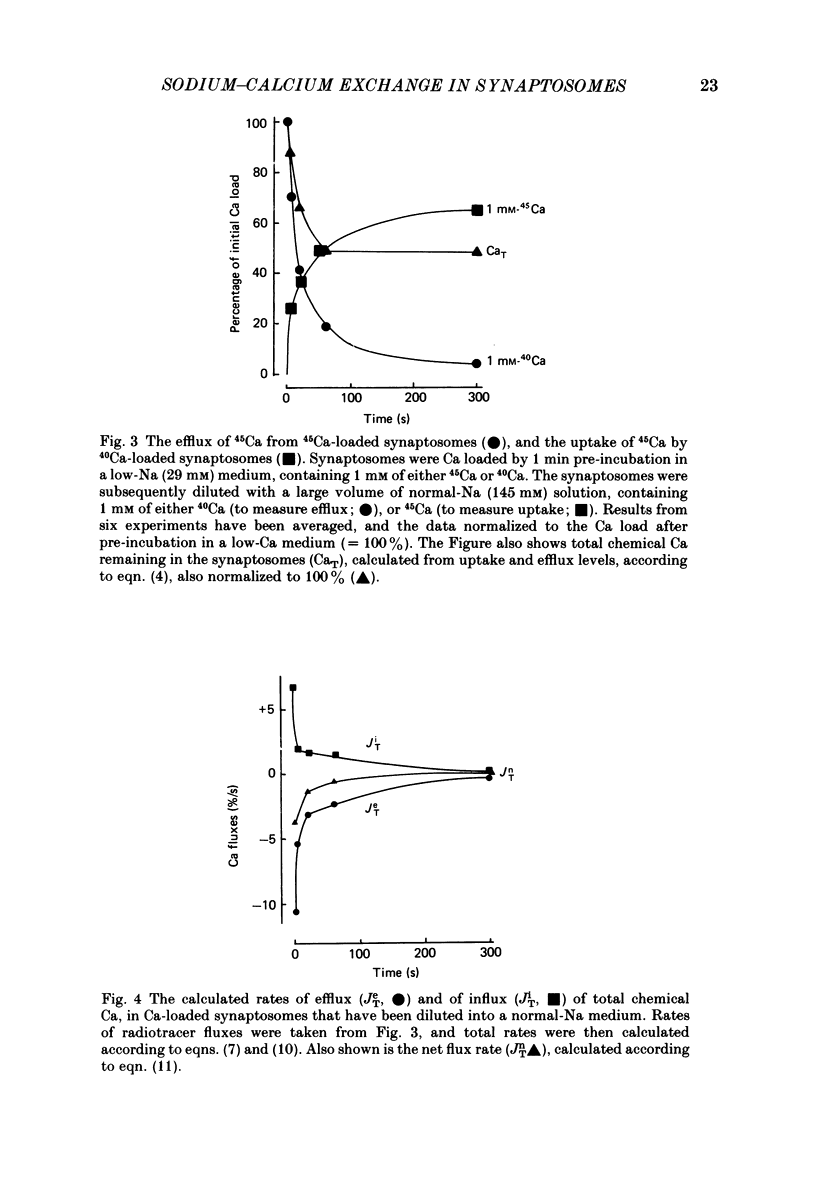
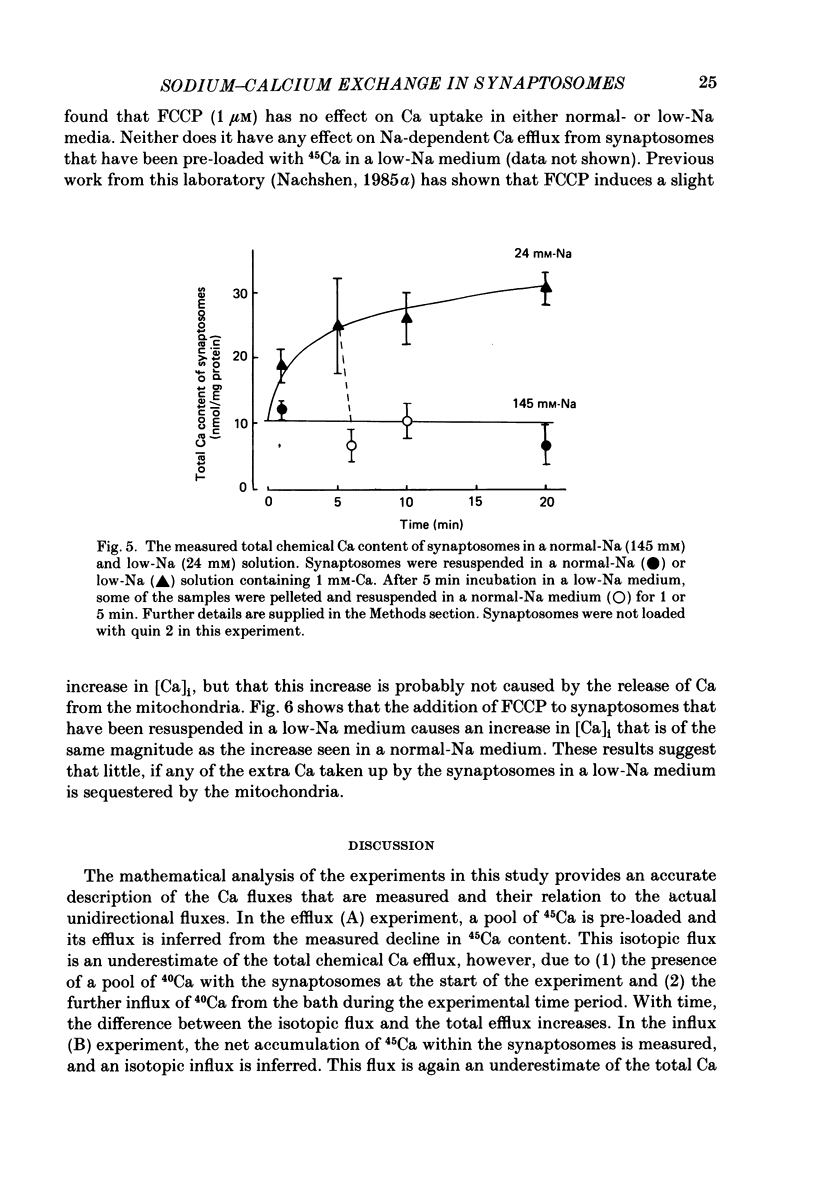
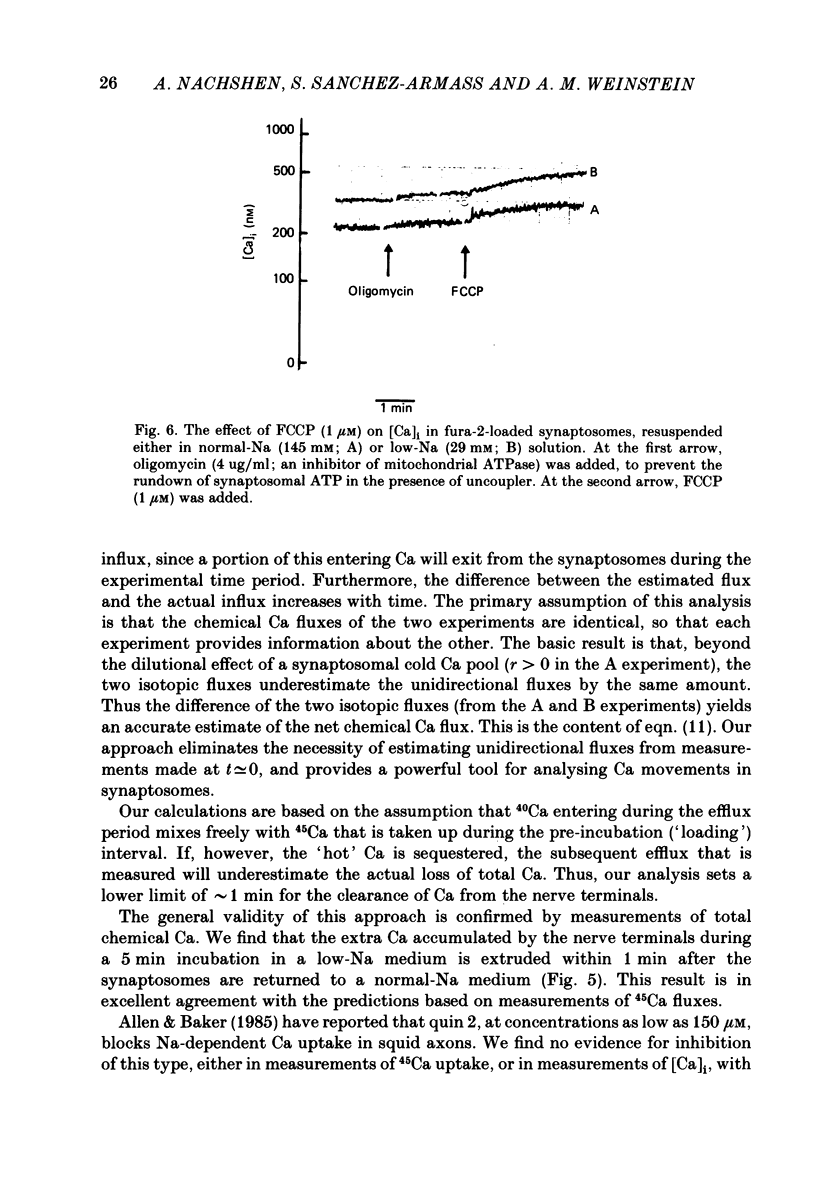
1. When pinched-off presynaptic nerve endings (synaptosomes) isolated from rat brain are incubated in a low-Na (24-36 mM) medium, they take up 45Ca in a time-dependent manner. In a medium containing 1 mM-Ca, this Na-dependent 45Ca uptake amounts to approximately 10 nmol/mg protein at 1 min, and to approximately 40 nmol/mg protein at 20 min. The Na-dependent Ca uptake is not reduced when the synaptosomes are loaded with concentrations of quin 2 as high as 2 mM. 2. The increase in 45Ca uptake is paralleled by an increase in the free cytosolic Ca concentration [Ca]i, as monitored with the fluorescent Ca indicators quin 2 or fura 2. [Ca]i increases from the value of approximately 200 to approximately 500 nM within 3-5 min, and thereafter, remains at this elevated level. 3. When synaptosomes that have been loaded with 45Ca (for 1 min, in a low-Na medium) are diluted into an Na-containing medium, there is a rapid efflux of the Ca load. After correcting for Ca that is taken up during the efflux period, calculations show that the total Ca in the synaptosomes returns to the control level within 1 min. Measurements of total chemical Ca parallel the measurements made with radiotracer Ca, and confirm that the Ca loaded into the nerve terminals during a 5 min incubation in a low-Na medium is extruded from the nerve terminals within 1 min in a normal-Na medium. 4. The efflux of Ca from the synaptosomes is paralleled by a drop of [Ca]i to its basal level, also within 1 min. 5. The mitochondrial uncoupler, carbonyl cyanide p-trifluoromethyloxy-phenyl-hydrazone (FCCP, 1 microM), has no effect on either Na-dependent Ca uptake or efflux in synaptosomes. FCCP causes a slight (100-200 nM) increase in [Ca]i in synaptosomes resuspended in either a Na or a low-Na medium. This indicates that little of the Ca that is taken up by the synaptosomes in a low-Na medium is sequestered by the mitochondria. 6. These results suggest that Na-dependent Ca efflux (probably Na-Ca exchange) plays an important role in allowing nerve terminals to recover rapidly from a Ca load.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. J., Baker P. F. Intracellular Ca indicator Quin-2 inhibits Ca2+ inflow via Na/Ca exchange in squid axon. 1985 Jun 27-Jul 3Nature. 315(6022):755–756. doi: 10.1038/315755a0. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Carrier-mediated sodium-dependent and calcium-dependent calcium efflux from pinched-off presynaptic nerve terminals (synaptosomes) in vitro. Biochim Biophys Acta. 1976 Jan 21;419(2):295–308. doi: 10.1016/0005-2736(76)90355-2. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. Effects of potassium, veratridine, and scorpion venom on calcium accumulation and transmitter release by nerve terminals in vitro. J Physiol. 1975 Jun;247(3):617–655. doi: 10.1113/jphysiol.1975.sp010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Oborn C. J. The influence of sodium on calcium fluxes in pinched-off nerve terminals in vitro. J Physiol. 1975 Jun;247(3):657–686. doi: 10.1113/jphysiol.1975.sp010951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. The energetics and kinetics of sodium-calcium exchange in barnacle muscles, squid axons, and mammalian heart: the role of ATP. Soc Gen Physiol Ser. 1984;38:129–147. [PubMed] [Google Scholar]
- Gill D. L., Grollman E. F., Kohn L. D. Calcium transport mechanisms in membrane vesicles from guinea pig brain synaptosomes. J Biol Chem. 1981 Jan 10;256(1):184–192. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hajós F. An improved method for the preparation of synaptosomal fractions in high purity. Brain Res. 1975 Aug 15;93(3):485–489. doi: 10.1016/0006-8993(75)90186-9. [DOI] [PubMed] [Google Scholar]
- Krueger B. K., Ratzlaff R. W., Strichartz G. R., Blaustein M. P. Saxitoxin binding to synaptosomes, membranes, and solubilized binding sites from rat brain. J Membr Biol. 1979 Nov 30;50(3-4):287–310. doi: 10.1007/BF01868894. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nachshen D. A. Regulation of cytosolic calcium concentration in presynaptic nerve endings isolated from rat brain. J Physiol. 1985 Jun;363:87–101. doi: 10.1113/jphysiol.1985.sp015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen D. A. The early time course of potassium-stimulated calcium uptake in presynaptic nerve terminals isolated from rat brain. J Physiol. 1985 Apr;361:251–268. doi: 10.1113/jphysiol.1985.sp015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer E. S., Blaustein M. P. Calcium buffering in presynaptic nerve terminals. Free calcium levels measured with arsenazo III. Biochim Biophys Acta. 1980 Aug 14;600(3):912–921. doi: 10.1016/0005-2736(80)90493-9. [DOI] [PubMed] [Google Scholar]
- Scott I. D., Akerman K. E., Nicholls D. G. Calcium-ion transport by intact synaptosomes. Intrasynaptosomal compartmentation and the role of the mitochondrial membrane potential. Biochem J. 1980 Dec 15;192(3):873–880. doi: 10.1042/bj1920873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]


