Abstract
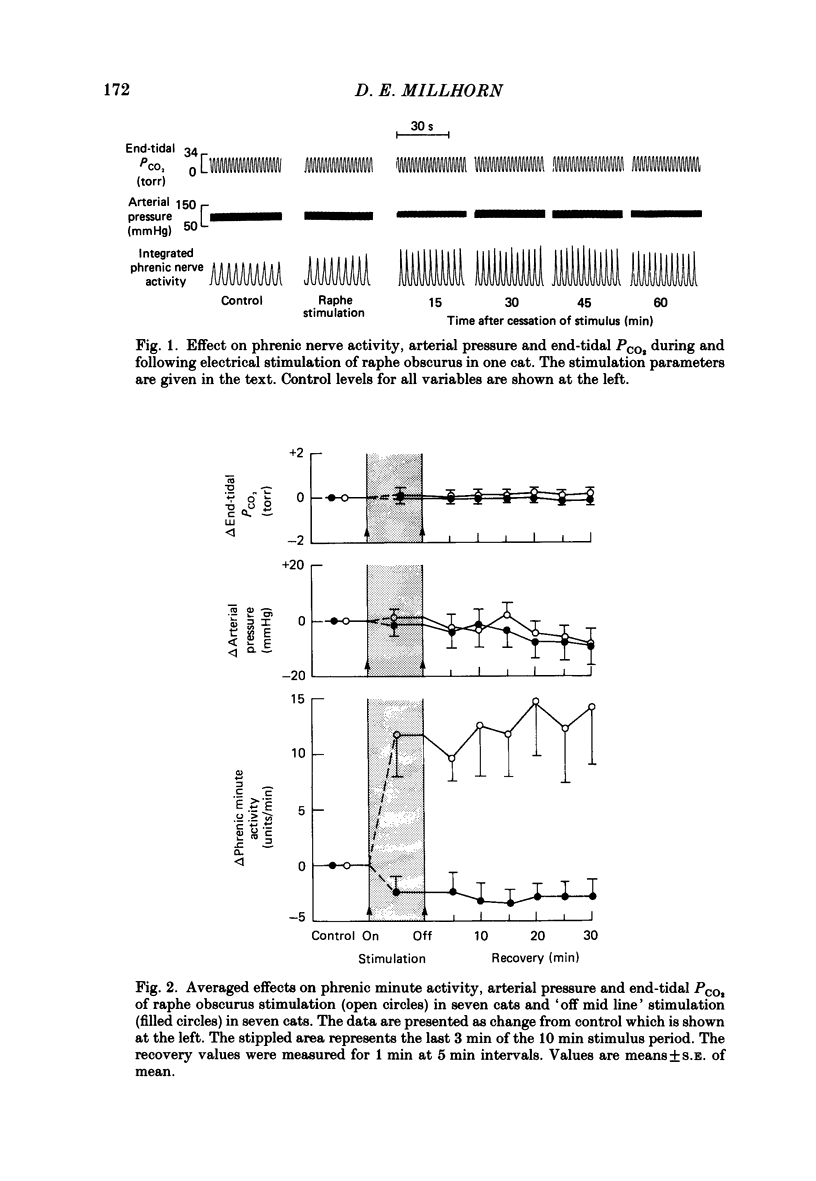
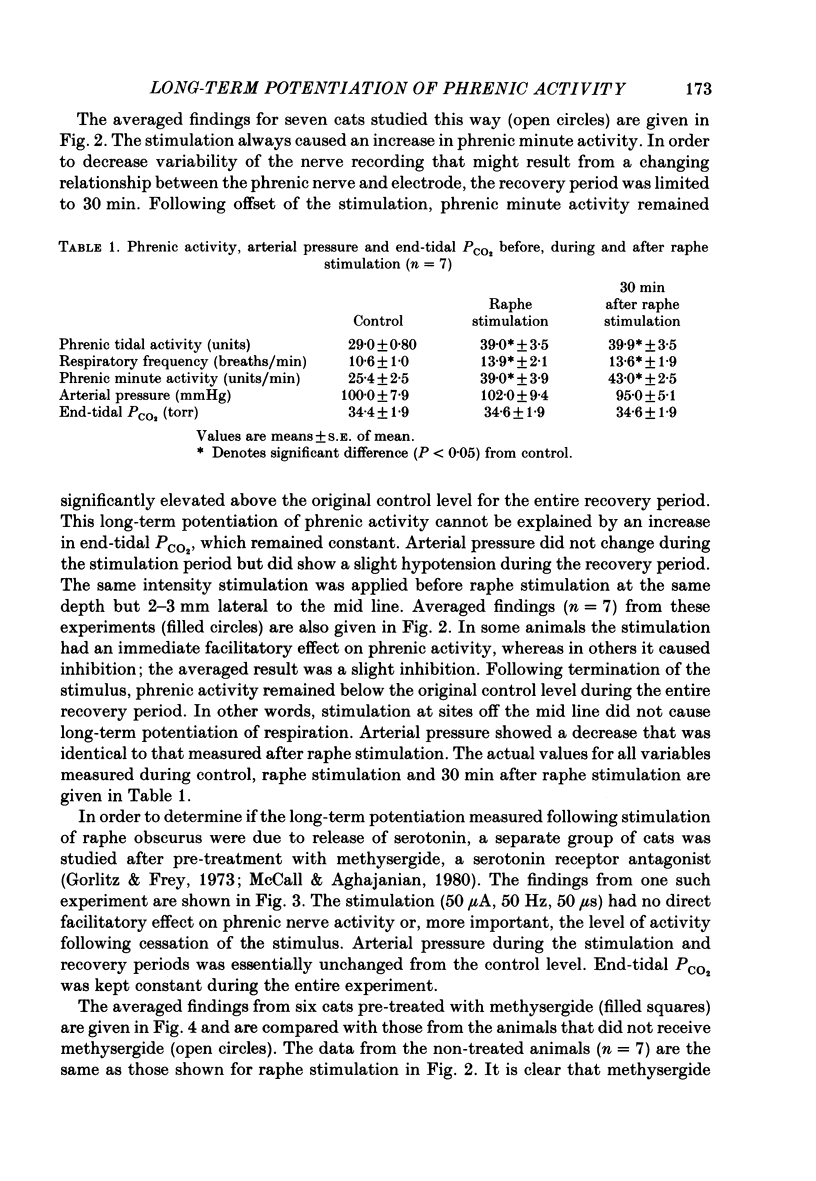
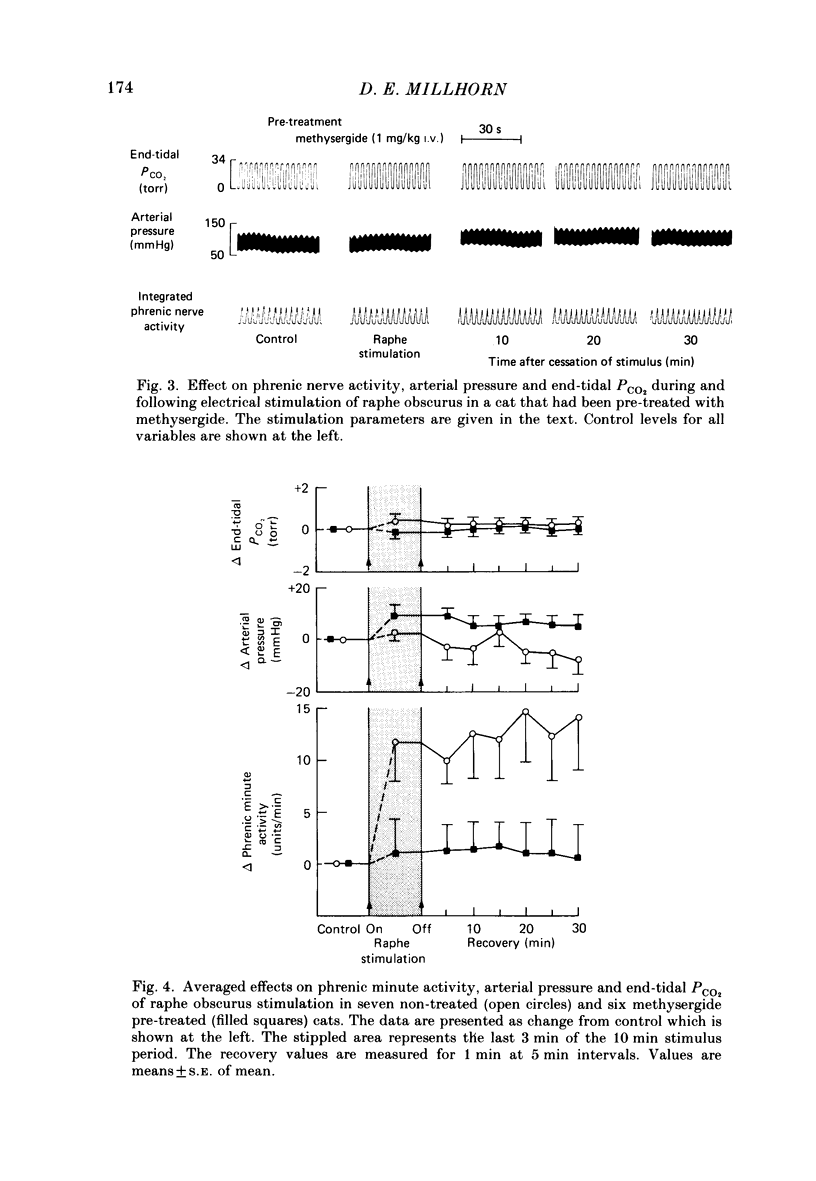
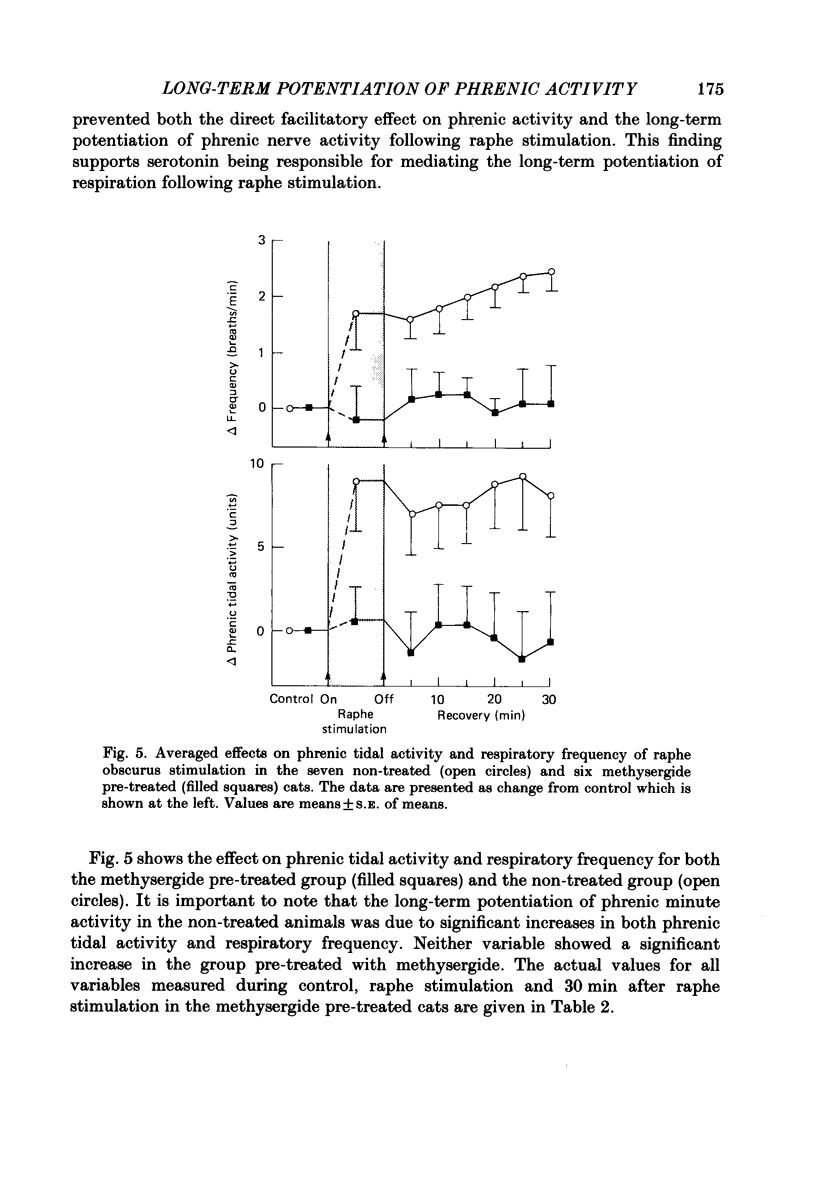
1. The respiratory response, measured as integrated phrenic nerve activity, during and for up to an hour following 10 min of continuous electrical stimulation of raphe obscurus was quantitated in anaesthetized, artificially ventilated cats whose carotid sinus nerves and vagus nerves had been cut. End-tidal PCO2 and body temperature were kept constant with servocontrollers. 2. Stimulation of raphe obscurus caused a significant increase in both phrenic tidal activity and respiratory frequency that persisted following cessation of the stimulus. This persistent facilitation is referred to as 'long-term potentiation' of respiration. 3. Control stimulations in the parenchyma of the medulla oblongata failed to stimulate respiration and cause the long-term potentiation. 4. Both the direct facilitatory effects of raphe obscurus stimulation on phrenic nerve activity and the long-term potentiation of respiration following the stimulus were prevented by pre-treating cats with methysergide, a serotonin receptor antagonist. 5. The results are discussed in terms of the raphe obscurus being the potential source of the long-term potentiation of respiration that occurs following stimulation of carotid body afferents (Millhorn, Eldridge & Waldrop, 1980a, b).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair J. R., Hamilton B. L., Scappaticci K. A., Helke C. J., Gillis R. A. Cardiovascular responses to electrical stimulation of the medullary raphe area of the cat. Brain Res. 1977 Jun 3;128(1):141–145. doi: 10.1016/0006-8993(77)90241-4. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Jonsson G., Palay S. L. Serotonin and substance P coexist i, neurons of the rat's central nervous system. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1582–1586. doi: 10.1073/pnas.75.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P., Millhorn D. E. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981 Feb;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L. Posthyperventilation breathing: different effects of active and passive hyperventilation. J Appl Physiol. 1973 Apr;34(4):422–430. doi: 10.1152/jappl.1973.34.4.422. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975 Oct;39(4):567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Görlitz B. D., Frey H. H. Letter: Comparison of the blocking effects of antagonists of adrenaline and 5-hydroxytryptamine on their mutual receptors. J Pharm Pharmacol. 1973 Aug;25(8):651–653. doi: 10.1111/j.2042-7158.1973.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Anastasi N. C., Norman W. P., Dretchen K. L. Effect of electrical and chemical stimulation of the raphe obscurus on phrenic nerve activity in the cat. Brain Res. 1986 Jan 8;362(2):214–220. doi: 10.1016/0006-8993(86)90446-4. [DOI] [PubMed] [Google Scholar]
- Holtman J. R., Jr, Dick T. E., Berger A. J. Involvement of serotonin in the excitation of phrenic motoneurons evoked by stimulation of the raphe obscurus. J Neurosci. 1986 Apr;6(4):1185–1193. doi: 10.1523/JNEUROSCI.06-04-01185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Goldstein M. Chemical anatomy of the brain. Science. 1984 Sep 21;225(4668):1326–1334. doi: 10.1126/science.6147896. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A., Steinbusch H., Verhofstad A., Nilsson G., Brodin E., Pernow B., Goldstein M. Immunohistochemical evidence of substance P-like immunoreactivity in some 5-hydroxytryptamine-containing neurons in the rat central nervous system. Neuroscience. 1978;3(6):517–538. doi: 10.1016/0306-4522(78)90017-9. [DOI] [PubMed] [Google Scholar]
- Lipski J., Kubin L., Jodkowski J. Synaptic action of R beta neurons on phrenic motoneurons studied with spike-triggered averaging. Brain Res. 1983 Dec 12;288(1-2):105–118. doi: 10.1016/0006-8993(83)90085-9. [DOI] [PubMed] [Google Scholar]
- McCall R. B., Aghajanian G. K. Pharmacological characterization of serotonin receptors in the facial motor nucleus: a microiontophoretic study. Eur J Pharmacol. 1980 Jul 25;65(2-3):175–183. doi: 10.1016/0014-2999(80)90390-8. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Waldrop T. G. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980 Jul;41(1):87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn D. E., Eldridge F. L., Waldrop T. G. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980 Dec;42(3):171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Miura M., Reis D. J. Termination and secondary projections of carotid sinus nerve in the cat brain stem. Am J Physiol. 1969 Jul;217(1):142–153. doi: 10.1152/ajplegacy.1969.217.1.142. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Brownstein M., Saavedra J. M. Serotonin content of the brain stem nuclei in the rat. Brain Res. 1974 Nov 15;80(2):237–249. doi: 10.1016/0006-8993(74)90688-x. [DOI] [PubMed] [Google Scholar]
- Poitras D., Parent A. Atlas of the distribution of monoamine-containing nerve cell bodies in the brain stem of the cat. J Comp Neurol. 1978 Jun 15;179(4):699–717. doi: 10.1002/cne.901790402. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Mercer R. R., Eldridge F. L. Servo control of end-tidal CO2 in paralyzed animals. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):133–136. doi: 10.1152/jappl.1978.45.1.133. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]


