Abstract
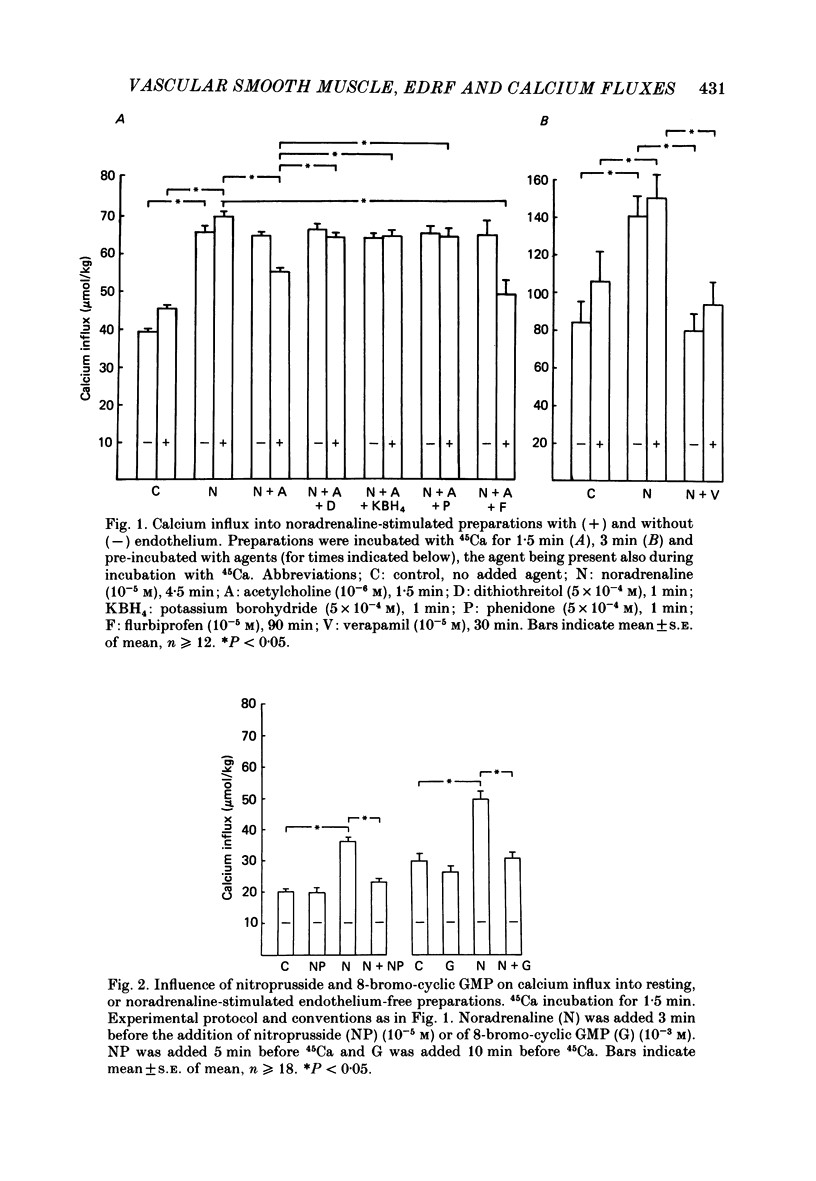
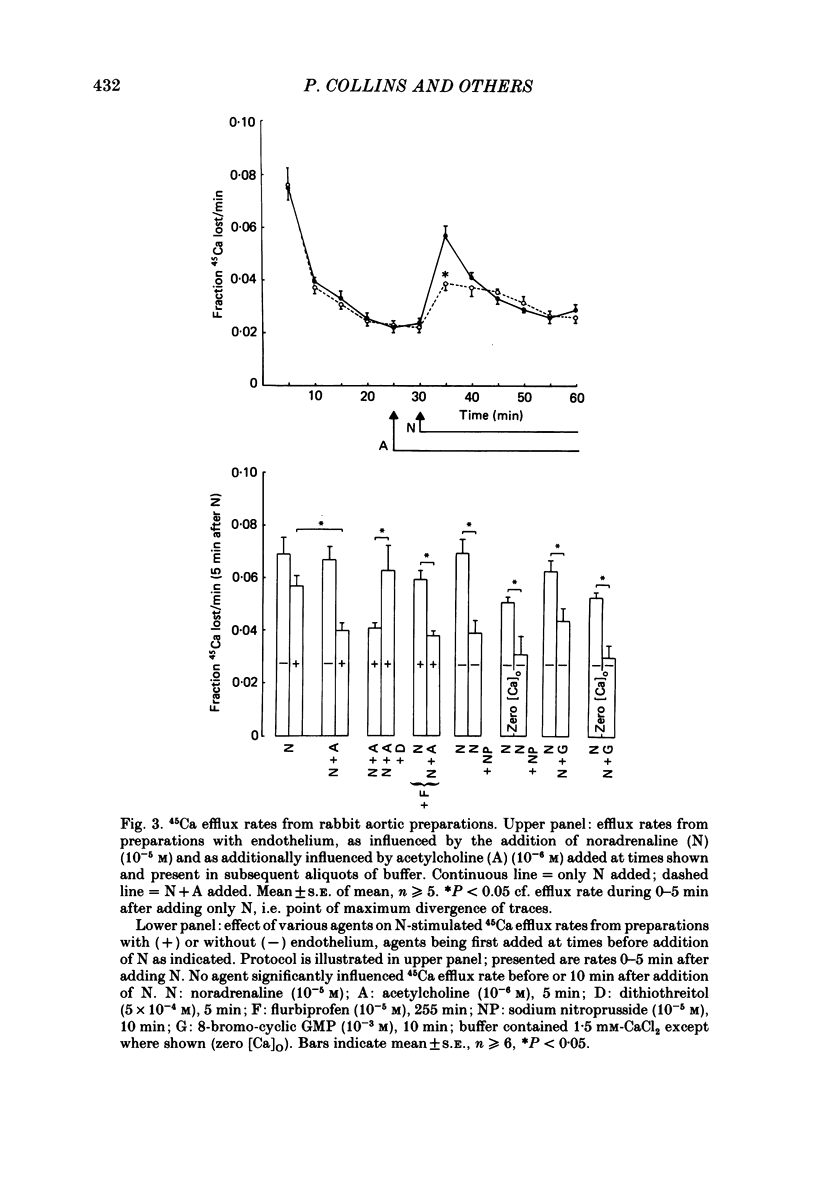
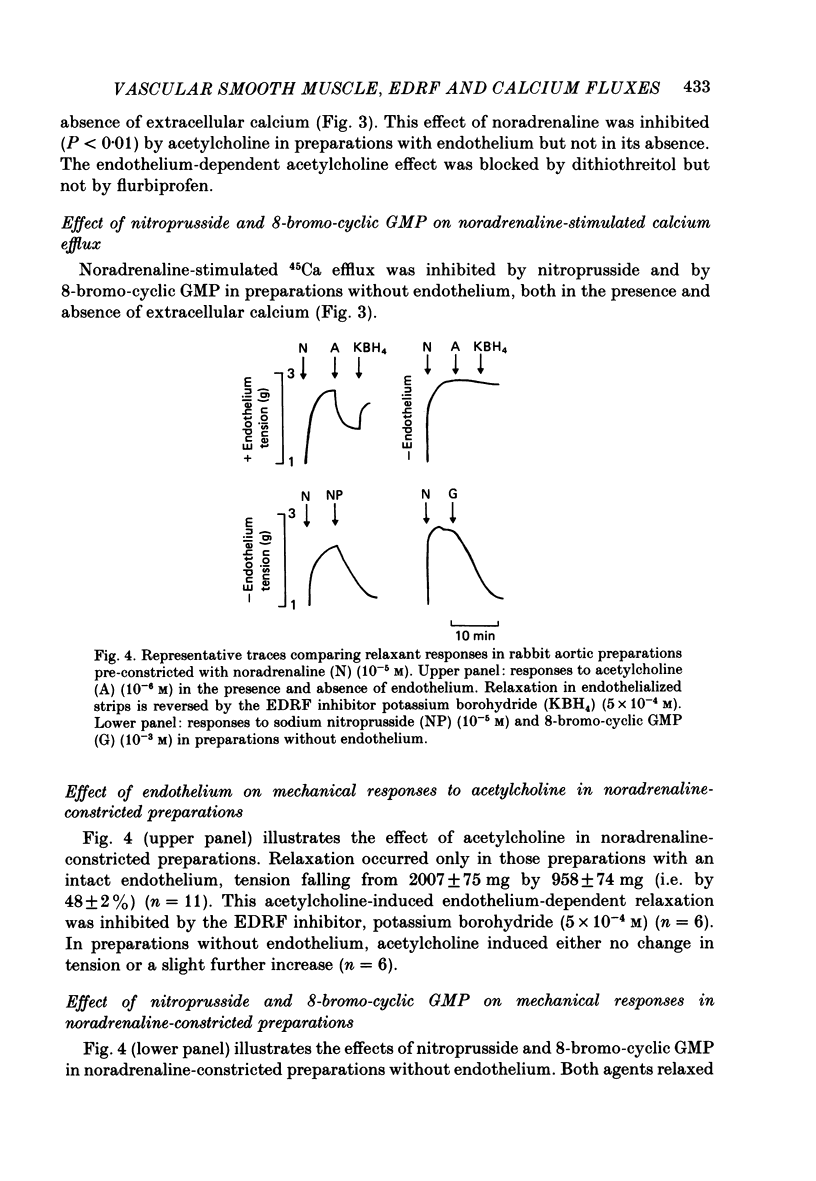
1. Measurement of tension and 45Ca influx and efflux were used to study the effects of endothelium-derived relaxing factor (EDRF), sodium nitroprusside and 8-bromo-cyclic guanosine monophosphate (GMP) on contractile responses and calcium movements in aortic ring preparations of the rabbit. 2. EDRF activity, induced by stimulating endothelium-containing rings with acetylcholine, was associated with relaxation of noradrenaline-constricted rings and with a marked reduction of noradrenaline-stimulated increase in calcium influx. Sodium nitroprusside and 8-bromo-cyclic GMP had a similar effect in de-endothelialized preparations. 3. EDRF also inhibited noradrenaline-stimulated calcium efflux. Sodium nitroprusside and 8-bromo-cyclic GMP had a similar effect in de-endothelialized preparations, both in the presence and absence of extracellular calcium. 4. The vascular smooth muscle relaxant effect of EDRF and of nitrovasodilators may be effected by a cyclic GMP-mediated reduction of cytosolic calcium, through both inhibition of calcium influx and reduction of intracellular calcium release.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P., van Breemen C. Effects of sodium gradient manipulation upon cellular calcium, 45Ca fluxes and cellular sodium in the guinea-pig taenia coli. J Physiol. 1981;319:443–461. doi: 10.1113/jphysiol.1981.sp013920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R., Trogisch G., Bassenge E. The role of endothelium in the control of vascular tone. Basic Res Cardiol. 1985 Sep-Oct;80(5):475–490. doi: 10.1007/BF01907912. [DOI] [PubMed] [Google Scholar]
- Deth R. C., Lynch C. J. Mobilization of a common source of smooth muscle Ca2+ by norepinephrine and methylxanthines. Am J Physiol. 1981 May;240(5):C239–C247. doi: 10.1152/ajpcell.1981.240.5.C239. [DOI] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Relative contributions of Ca2+ influx and cellular Ca2+ release during drug induced activation of the rabbit aorta. Pflugers Arch. 1974 Apr 4;348(1):13–22. doi: 10.1007/BF00587735. [DOI] [PubMed] [Google Scholar]
- Fiscus R. R., Rapoport R. M., Murad F. Endothelium-dependent and nitrovasodilator-induced activation of cyclic GMP-dependent protein kinase in rat aorta. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):415–425. [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gleason M. M., Ratz P. H., Flaim S. F. Measurement of calcium influx in tethered rings of rabbit aorta under tension. Am J Physiol. 1985 Sep;249(3 Pt 2):H470–H476. doi: 10.1152/ajpheart.1985.249.3.H470. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Collins P., Lewis M. J., Henderson A. H. Endothelium derived relaxant factor. J R Coll Physicians Lond. 1985 Apr;19(2):74–79. [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Henderson A. H. Evidence that cyclic guanosine monophosphate (cGMP) mediates endothelium-dependent relaxation. Eur J Pharmacol. 1985 Jun 7;112(2):195–202. doi: 10.1016/0014-2999(85)90496-0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Henderson A. H., Edwards D. H., Lewis M. J. Isolated perfused rabbit coronary artery and aortic strip preparations: the role of endothelium-derived relaxant factor. J Physiol. 1984 Jun;351:13–24. doi: 10.1113/jphysiol.1984.sp015228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway D. R., Konicki M. V., Coolican S. A. Phosphorylation of myosin light chain kinase from vascular smooth muscle by cAMP- and cGMP-dependent protein kinases. J Mol Cell Cardiol. 1985 Sep;17(9):841–850. doi: 10.1016/s0022-2828(85)80098-5. [DOI] [PubMed] [Google Scholar]
- Hester R. K. Effects of 2-nicotinamidoethyl nitrate on agonist-sensitive Ca++ release and Ca++ entry in rabbit aorta. J Pharmacol Exp Ther. 1985 Apr;233(1):100–111. [PubMed] [Google Scholar]
- Hester R. K., Weiss G. B., Fry W. J. Differing actions of nitroprusside and D-600 on tension and 45Ca fluxes in canine renal arteries. J Pharmacol Exp Ther. 1979 Jan;208(1):155–160. [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Ueno H. Mechanisms of the nitroglycerine-induced vasodilation in vascular smooth muscles of the rabbit and pig. J Physiol. 1983 Oct;343:233–252. doi: 10.1113/jphysiol.1983.sp014890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Lincoln T. M. Effects of nitroprusside, glyceryl trinitrate, and 8-bromo cyclic GMP on phosphorylase a formation and myosin light chain phosphorylation in rat aorta. Mol Pharmacol. 1985 Mar;27(3):333–342. [PubMed] [Google Scholar]
- Karaki H., Hester R. K., Weiss G. B. Cellular basis of nitroprusside-induced relaxation of graded responses to norepinephrine and potassium in canine renal arteries. Arch Int Pharmacodyn Ther. 1980 Jun;245(2):198–210. [PubMed] [Google Scholar]
- Karaki H., Nakagawa H., Urakawa N. Comparative effects of verapamil and sodium nitroprusside on contraction and 45Ca uptake in the smooth muscle of rabbit aorta, rat aorta and guinea-pig taenia coli. Br J Pharmacol. 1984 Feb;81(2):393–400. doi: 10.1111/j.1476-5381.1984.tb10091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Kreye V. A., Baron G. D., Lüth J. B., Schmidt-Gayk H. Mode of action of sodium nitroprusside on vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1975;288(4):381–402. doi: 10.1007/BF00501284. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M. Effects of nitroprusside and 8-bromo-cyclic GMP on the contractile activity of the rat aorta. J Pharmacol Exp Ther. 1983 Jan;224(1):100–107. [PubMed] [Google Scholar]
- Long C. J., Stone T. W. The release of endothelium-derived relaxant factor is calcium dependent. Blood Vessels. 1985;22(4):205–208. doi: 10.1159/000158602. [DOI] [PubMed] [Google Scholar]
- Loutzenhiser R., van Breemen C. Mechanism of activation of isolated rabbit aorta by PGH2 analogue U-44069. Am J Physiol. 1981 Nov;241(5):C243–C249. doi: 10.1152/ajpcell.1981.241.5.C243. [DOI] [PubMed] [Google Scholar]
- Matlib M. A., Dubé G. P., Millard R. W., Lathrop D. A., Baik Y. H., Sakai K., DiSalvo J., Schwartz A. Studies on the mode of action of isosorbide dinitrate: a physiologic and biochemical approach. Am Heart J. 1985 Jul;110(1 Pt 2):204–212. doi: 10.1016/0002-8703(85)90488-0. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Palmer R. F., Van Breemen C. The effects of amrinone on contractility, Ca2+ uptake and cAMP in smooth muscle. Eur J Pharmacol. 1980 Jan 25;61(2):159–165. doi: 10.1016/0014-2999(80)90158-2. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Alteration of cytoplasmic ionized calcium levels in smooth muscle by vasodilators in the ferret. J Physiol. 1984 Dec;357:539–551. doi: 10.1113/jphysiol.1984.sp015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Shibata S., Kitano H., Matsumoto P., Ishida Y. A comparative study of the relaxing effect of nitroprusside and verapamil on human umbilical vessels. Blood Vessels. 1981;18(6):321–329. doi: 10.1159/000158365. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., SANDERS A. G., FLOREY H. W. The regeneration of aortic endothelium. J Pathol Bacteriol. 1958 Jan;75(1):133–143. doi: 10.1002/path.1700750116. [DOI] [PubMed] [Google Scholar]
- Popescu L. M., Panoiu C., Hinescu M., Nutu O. The mechanism of cGMP-induced relaxation in vascular smooth muscle. Eur J Pharmacol. 1985 Jan 8;107(3):393–394. doi: 10.1016/0014-2999(85)90269-9. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Kuriyama H. Effects of cAMP- and cGMP-dependent protein kinases, and calmodulin on Ca2+ uptake by highly purified sarcolemmal vesicles of vascular smooth muscle. Biochim Biophys Acta. 1984 Jun 13;773(1):83–90. doi: 10.1016/0005-2736(84)90552-2. [DOI] [PubMed] [Google Scholar]
- Zsotér T. T., Henein N. F., Wolchinsky C. The effect of sodium nitroprusside on the uptake and efflux of 45Ca from rabbit and rat vessels. Eur J Pharmacol. 1977 Sep 1;45(1):7–12. doi: 10.1016/0014-2999(77)90052-8. [DOI] [PubMed] [Google Scholar]


