Abstract
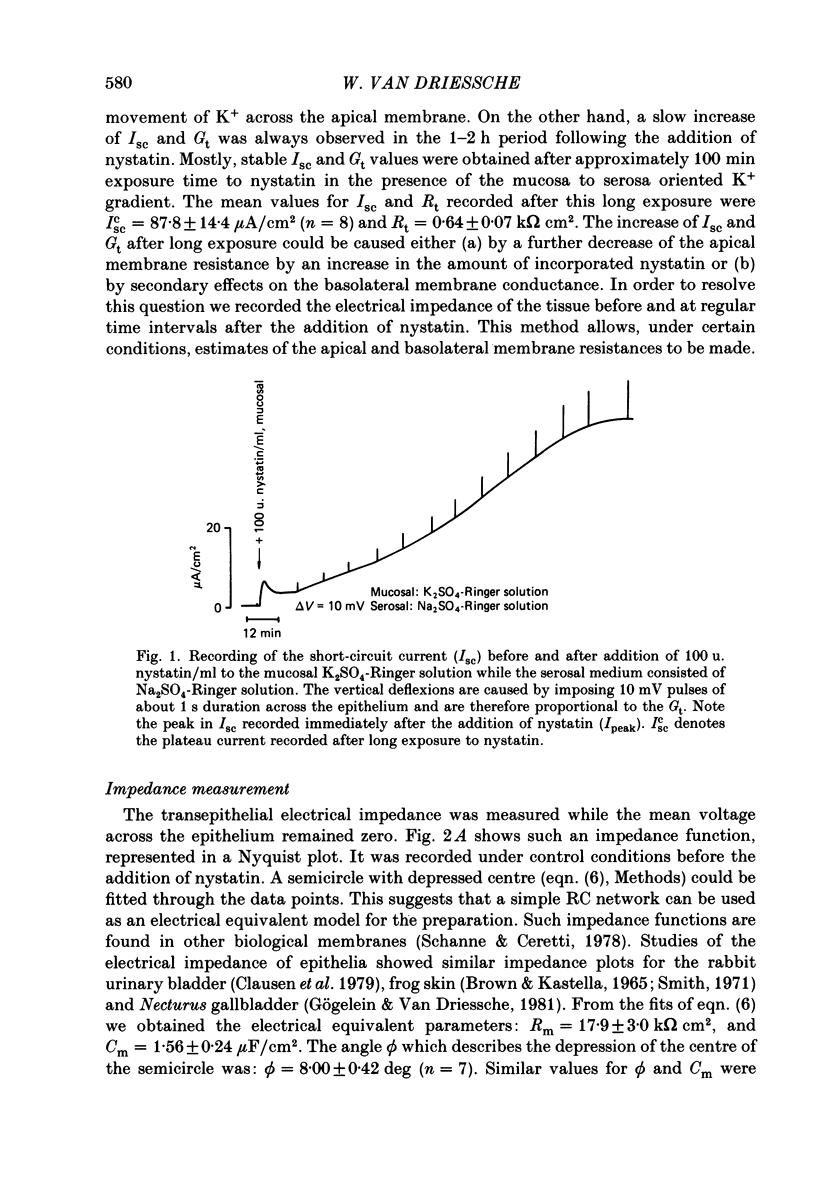
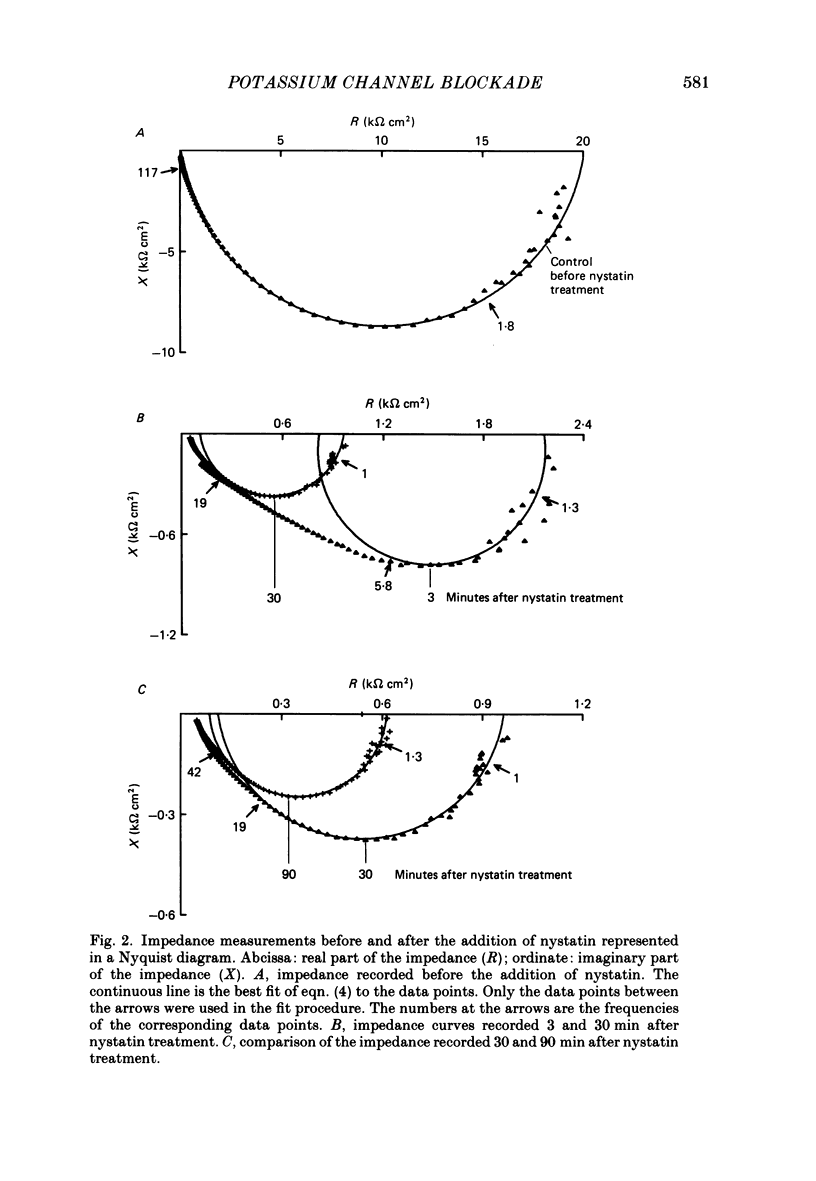
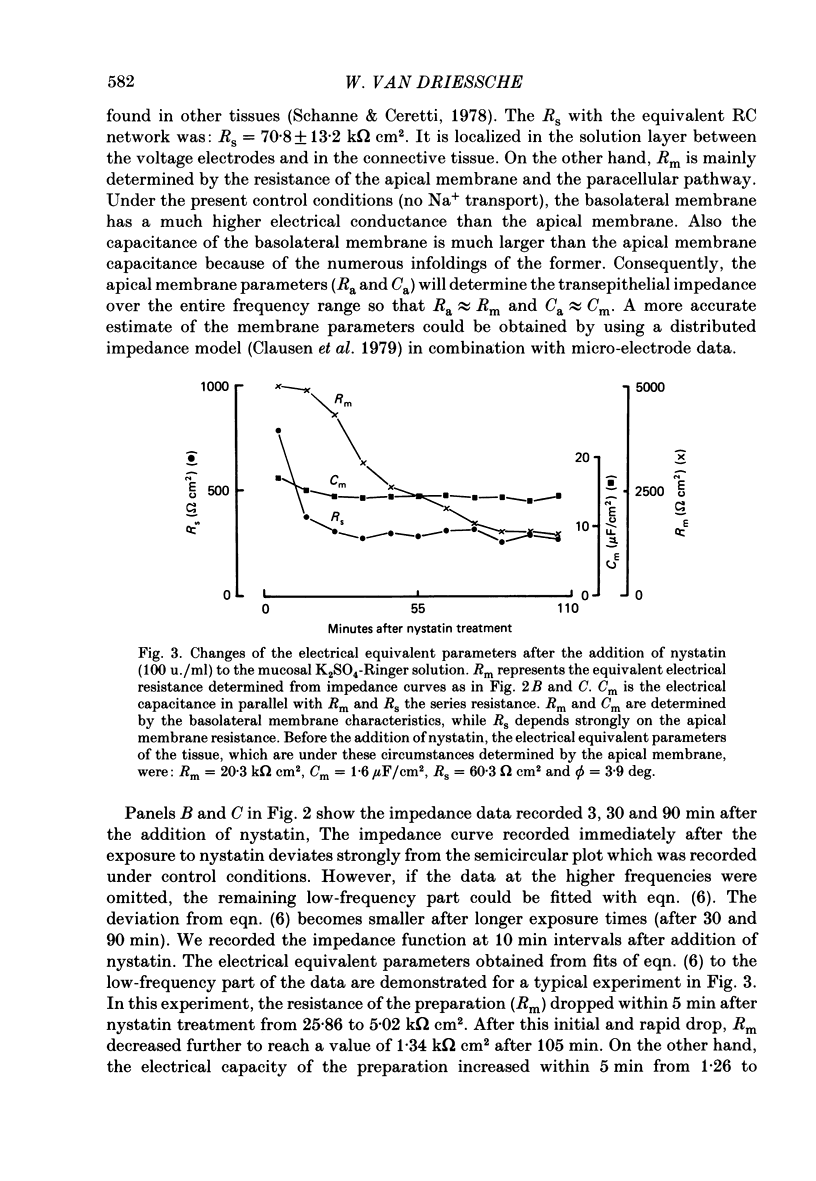
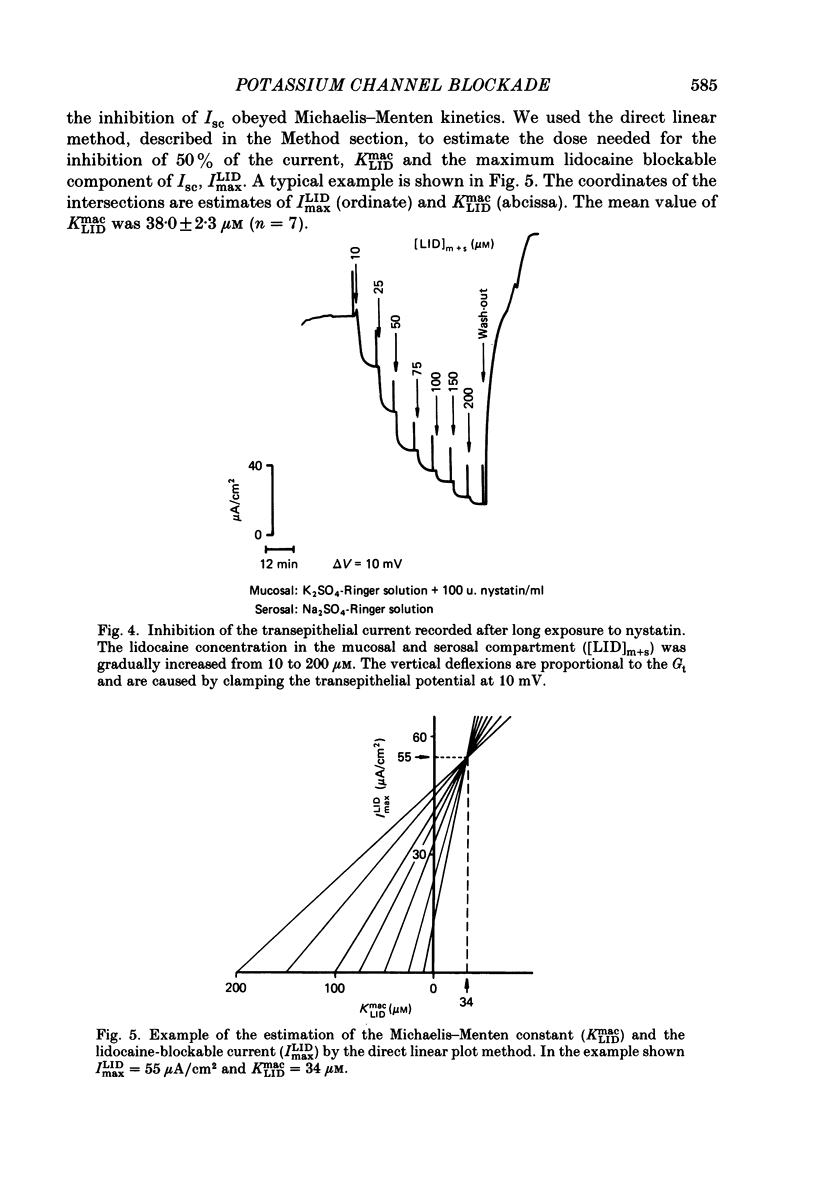
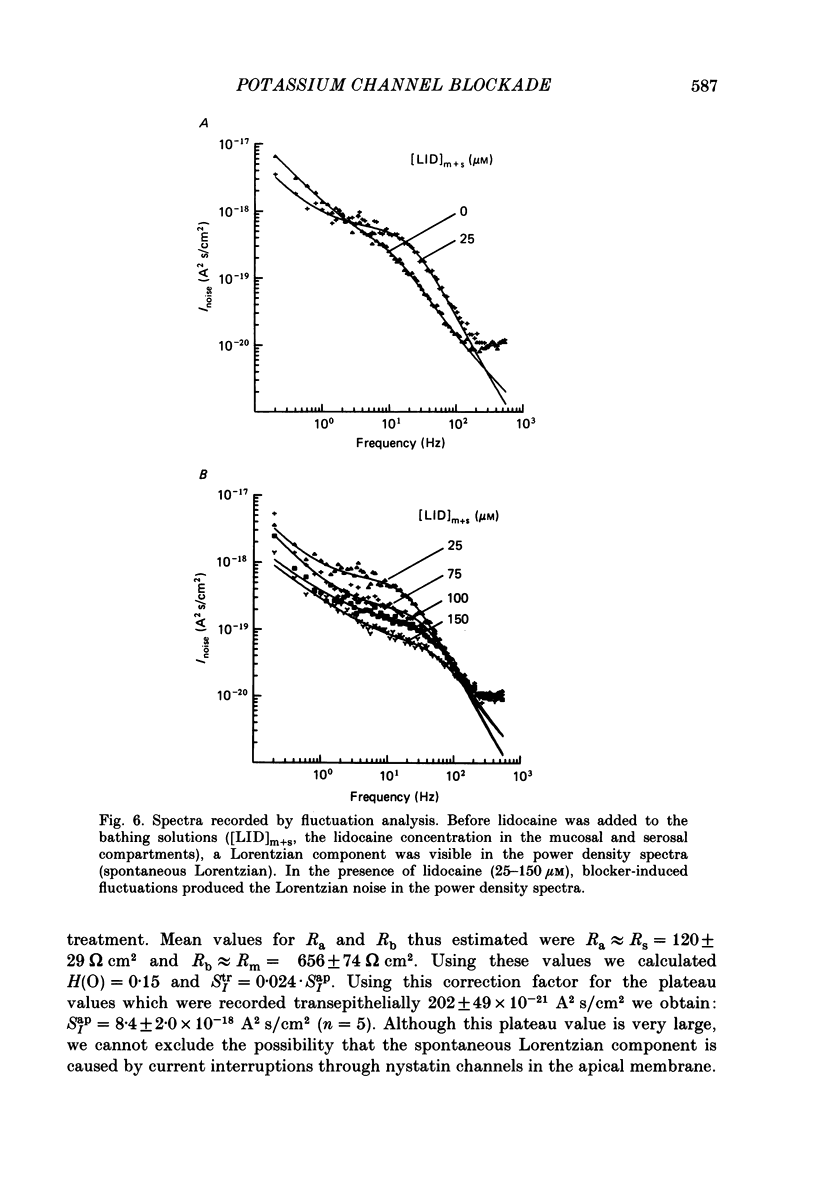
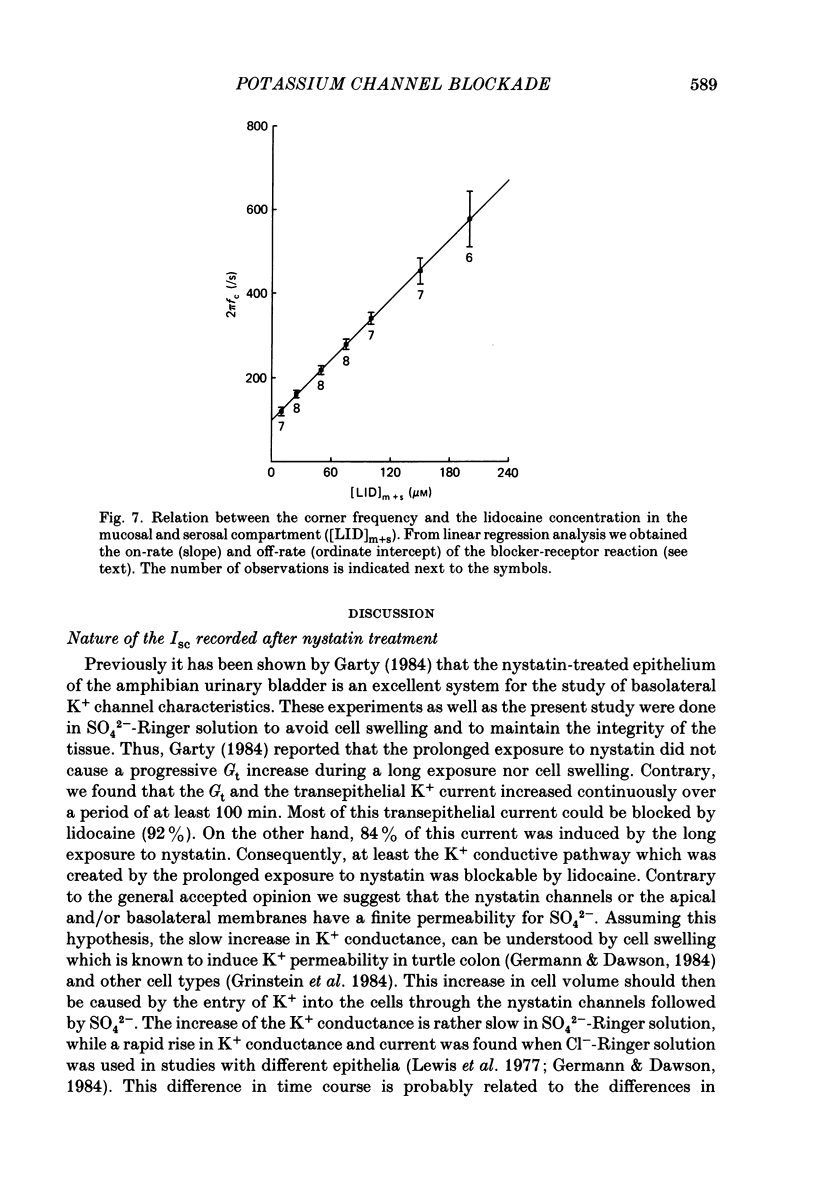
1. Basolateral membranes of the frog urinary bladder were investigated after increasing the cationic conductance of the apical membrane by the incorporation of nystatin. 2. K+ currents were recorded in the presence of a mucosa to serosa oriented K+ gradient (SO4(2-) Ringer solution). Nystatin caused a rapid rise of the short-circuit current (Isc) followed by a slow increase over a period of 1-2 h. 3. Impedance analysis showed that the apical membrane resistance was drastically reduced by nystatin. The slow increase in Isc was accompanied by a progressive increase in basolateral conductance. 4. The transepithelial current and conductance recorded in the presence of nystatin could be depressed with lidocaine added to the mucosal and serosal solution. The effects of lidocaine were completely reversible. 5. Noise analysis showed that lidocaine induced additional fluctuations in Isc. The spectrum of these fluctuations was of the Lorentzian type. This noise component is caused by the random interruption of the current through the basolateral K+ channels. The Lorentzian parameters were used to calculate the microscopic parameters of the basolateral K+ channels.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramcheck F. J., Van Driessche W., Helman S. I. Autoregulation of apical membrane Na+ permeability of tight epithelia. Noise analysis with amiloride and CGS 4270. J Gen Physiol. 1985 Apr;85(4):555–582. doi: 10.1085/jgp.85.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Brown A. C., Kastella K. G. The AC impedance of frog skin and its relation to active transport. Biophys J. 1965 Jul;5(4):591–606. doi: 10.1016/S0006-3495(65)86736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen C., Lewis S. A., Diamond J. M. Impedance analysis of a tight epithelium using a distributed resistance model. Biophys J. 1979 May;26(2):291–317. doi: 10.1016/S0006-3495(79)85250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H. Current-voltage relations of the basolateral membrane in tight amphibian epithelia: use of nystatin to depolarize the apical membrane. J Membr Biol. 1984;77(3):213–222. doi: 10.1007/BF01870570. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Van Driessche W. Capacitive and inductive low frequency impedances of Necturus gallbladder epithelium. Pflugers Arch. 1981 Jan;389(2):105–113. doi: 10.1007/BF00582099. [DOI] [PubMed] [Google Scholar]
- Helman S. I., Fisher R. S. Microelectrode studies of the active Na transport pathway of frog skin. J Gen Physiol. 1977 May;69(5):571–604. doi: 10.1085/jgp.69.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Dawson D. C. Basolateral potassium channel in turtle colon. Evidence for single-file ion flow. J Gen Physiol. 1983 Sep;82(3):297–313. doi: 10.1085/jgp.82.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHTENSTEIN N. S., LEAF A. EFFECT OF AMPHOTERICIN B ON THE PERMEABILITY OF THE TOAD BLADDER. J Clin Invest. 1965 Aug;44:1328–1342. doi: 10.1172/JCI105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Eaton D. C., Clausen C., Diamond J. M. Nystatin as a probe for investigating the electrical properties of a tight epithelium. J Gen Physiol. 1977 Oct;70(4):427–440. doi: 10.1085/jgp.70.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Wills N. K. Electrical properties of the rabbit urinary bladder assessed using gramicidin D. J Membr Biol. 1982;67(1):45–53. doi: 10.1007/BF01868646. [DOI] [PubMed] [Google Scholar]
- Lindemann B., Van Driessche W. Sodium-specific membrane channels of frog skin are pores: current fluctuations reveal high turnover. Science. 1977 Jan 21;195(4275):292–294. doi: 10.1126/science.299785. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., DiBona D. R., Leaf A. Sodium transport across toad urinary bladder: a model "tight" epithelium. Physiol Rev. 1980 Jul;60(3):615–715. doi: 10.1152/physrev.1980.60.3.615. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. Epithelial transport of potassium. Kidney Int. 1977 Jun;11(6):391–414. doi: 10.1038/ki.1977.59. [DOI] [PubMed] [Google Scholar]
- Nagel W., Essig A. Relationship of transepithelial electrical potential to membrane potentials and conductance ratios in frog skin. J Membr Biol. 1982;69(2):125–136. doi: 10.1007/BF01872272. [DOI] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. Effect of the polyene antibiotic filipin on the permeability of the inward- and the outward-facing membranes of the isolated from skin (Rana temporaria). Acta Physiol Scand. 1977 Apr;99(4):399–411. doi: 10.1111/j.1748-1716.1977.tb10393.x. [DOI] [PubMed] [Google Scholar]
- Schifferdecker E., Frömter E. The AC impedance of Necturus gallbladder epithelium. Pflugers Arch. 1978 Nov 14;377(2):125–133. doi: 10.1007/BF00582842. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Coggins C. H., Lichtenstein N. S., Leaf A. Evidence for a mucosal effect of aldosterone on sodium transport in the toad bladder. J Clin Invest. 1966 Oct;45(10):1640–1647. doi: 10.1172/JCI105471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. G. The low-frequency electrical impedance of the isolated frog skin. Acta Physiol Scand. 1971 Mar;81(3):355–366. doi: 10.1111/j.1748-1716.1971.tb04910.x. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Erlij D. Noise analysis of inward and outward Na+ currents across the apical border of ouabain-treated frog skin. Pflugers Arch. 1983 Aug;398(3):179–188. doi: 10.1007/BF00657149. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Gögelein H. Attenuation of current and voltage noise signals recorded from epithelia. J Theor Biol. 1980 Oct 21;86(4):629–648. doi: 10.1016/0022-5193(80)90303-3. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Lindemann B. Low-noise amplification of voltage and current fluctuations arising in epithelia. Rev Sci Instrum. 1978 Jan;49(1):52–52. doi: 10.1063/1.1135251. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Zeiske W. Ba2+-induced conductance fluctuations of spontaneously fluctuating K+ channels in the apical membrane of frog skin (Rana temporaria). J Membr Biol. 1980 Aug 21;56(1):31–42. doi: 10.1007/BF01869349. [DOI] [PubMed] [Google Scholar]
- Wills N. K. Antibiotics as tools for studying the electrical properties of tight epithelia. Fed Proc. 1981 Jun;40(8):2202–2205. [PubMed] [Google Scholar]
- Wills N. K., Eaton D. C., Lewis S. A., Ifshin M. S. Current-voltage relationship of the basolateral membrane of a tight epithelium. Biochim Biophys Acta. 1979 Aug 23;555(3):519–523. doi: 10.1016/0005-2736(79)90405-x. [DOI] [PubMed] [Google Scholar]
- Wills N. K., Lewis S. A., Eaton D. C. Active and passive properties of rabbit descending colon: a microelectrode and nystatin study. J Membr Biol. 1979 Mar 28;45(1-2):81–108. doi: 10.1007/BF01869296. [DOI] [PubMed] [Google Scholar]


