Abstract
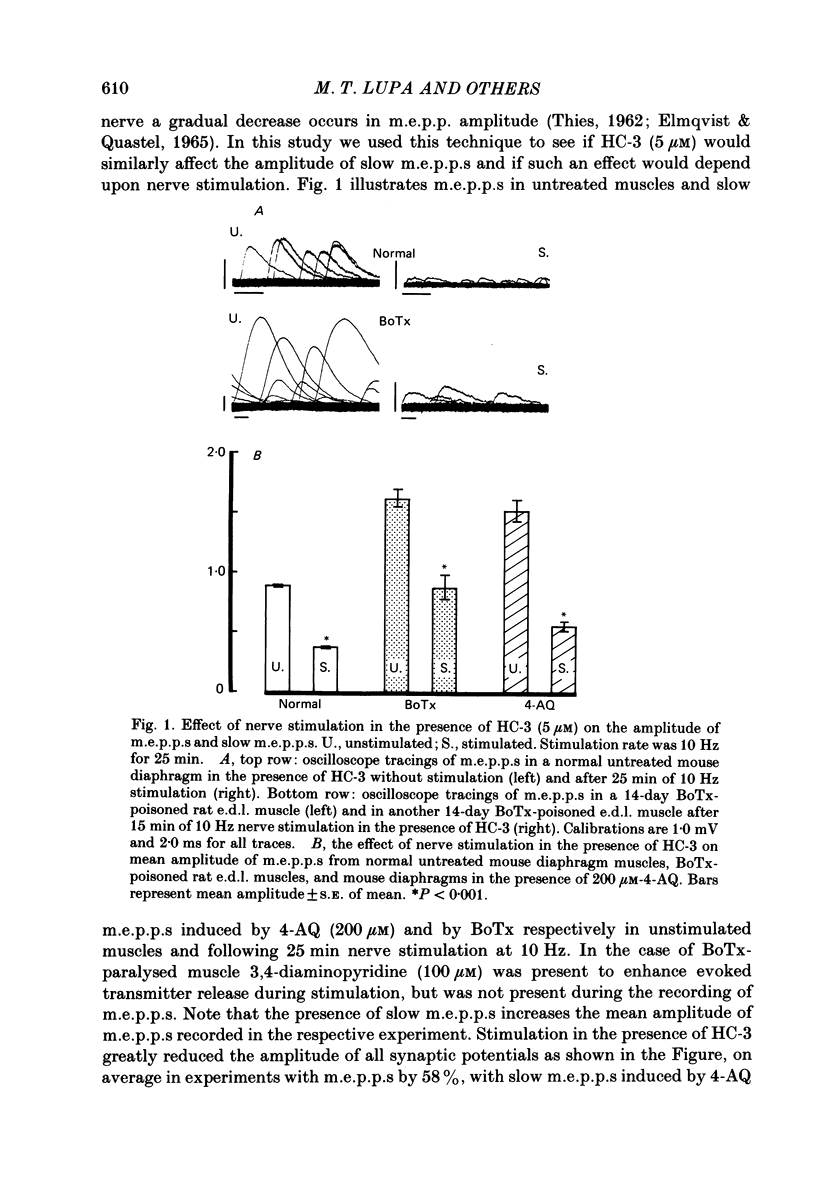
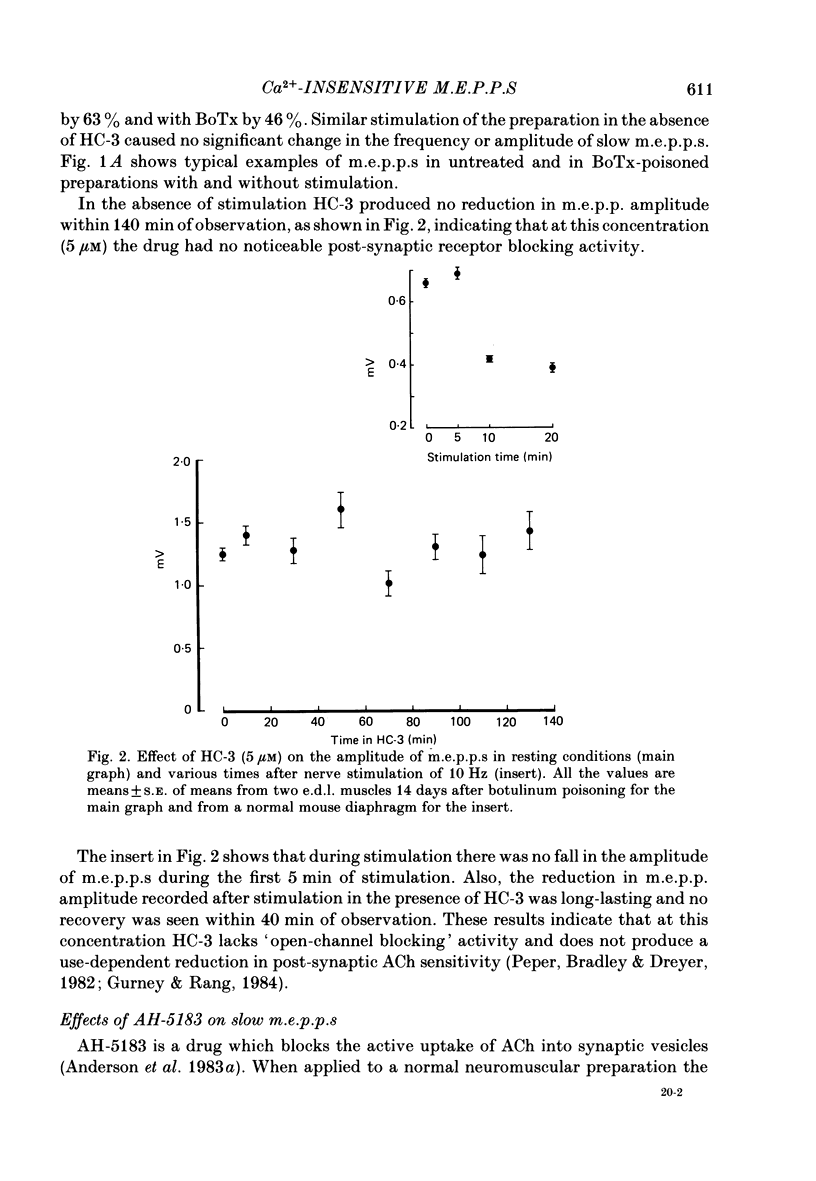
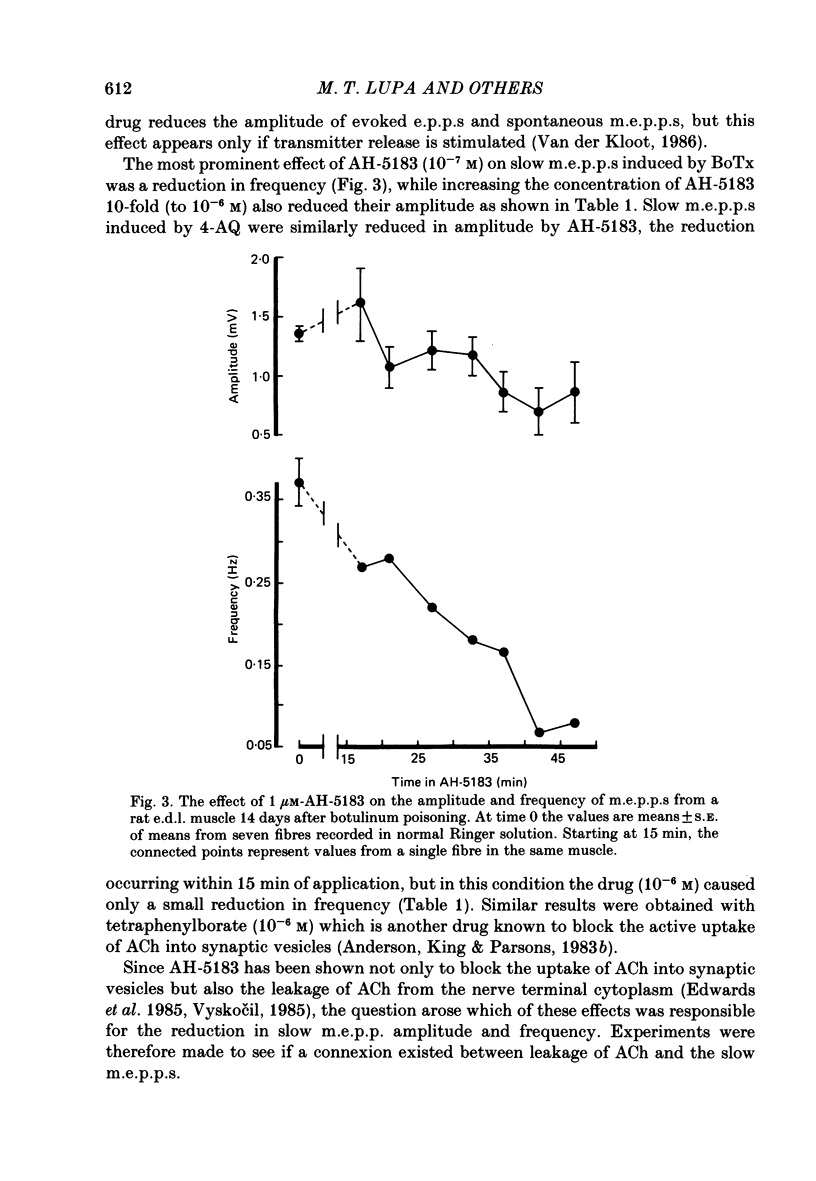
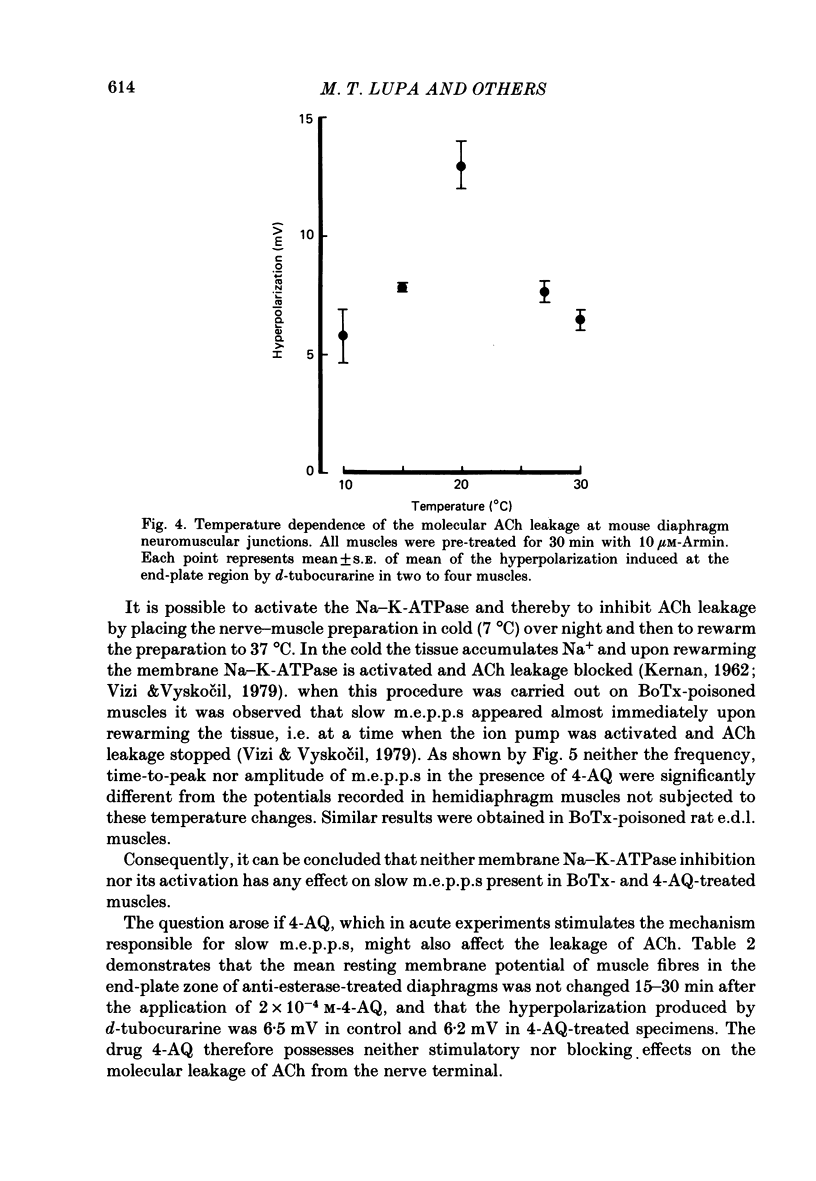
1. To study the nature and origin of slow-rising, Ca2+-insensitive miniature end-plate potentials (m.e.p.p.s) in mammalian muscle we used intracellular recording techniques and drugs which block acetylcholine (ACh) synthesis or the uptake of ACh into synaptic vesicles. Slow m.e.p.p.s were induced in vivo by paralysing the extensor digitorum longus muscle of the rat with botulinum toxin type A or in vitro by the application of 4-aminoquinoline to the mouse diaphragm nerve-muscle preparation. 2. Hemicholinium-3, which blocks ACh synthesis, reduced the amplitude of all synaptic potentials including slow m.e.p.p.s, but only if the nerve was stimulated. 3. 2(4-phenylpiperidino)cyclohexanol (AH-5183), which blocks the active uptake of ACh into synaptic vesicles, reduced both the frequency and the amplitude of slow m.e.p.p.s and did so without requiring nerve stimulation. 4. No correlation was observed between the molecular leakage of ACh from the motor nerve and the frequency and amplitude of slow m.e.p.p.s. 5. We conclude that slow m.e.p.p.s are caused by the release of ACh from the nerve terminal, possibly from a small pool of synaptic vesicle-like structures.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., King S. C., Parsons S. M. Inhibition of [3H]acetylcholine active transport by tetraphenylborate and other anions. Mol Pharmacol. 1983 Jul;24(1):55–59. [PubMed] [Google Scholar]
- Anderson D. C., King S. C., Parsons S. M. Pharmacological characterization of the acetylcholine transport system in purified Torpedo electric organ synaptic vesicles. Mol Pharmacol. 1983 Jul;24(1):48–54. [PubMed] [Google Scholar]
- Colméus C., Gomez S., Molgó J., Thesleff S. Discrepancies between spontaneous and evoked synaptic potentials at normal, regenerating and botulinum toxin poisoned mammalian neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1982 Apr 22;215(1198):63–74. doi: 10.1098/rspb.1982.0028. [DOI] [PubMed] [Google Scholar]
- Dolezal V., Vyskocil F., Tucek S. Decrease of the spontaneous non-quantal release of acetylcholine from the phrenic nerve in botulinum-poisoned rat diaphragm. Pflugers Arch. 1983 Jun 1;397(4):319–322. doi: 10.1007/BF00580268. [DOI] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C., Dolezal V., Tucek S., Zemková H., Vyskocil F. Is an acetylcholine transport system responsible for nonquantal release of acetylcholine at the rodent myoneural junction? Proc Natl Acad Sci U S A. 1985 May;82(10):3514–3518. doi: 10.1073/pnas.82.10.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Gurney A. M., Rang H. P. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br J Pharmacol. 1984 Jul;82(3):623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P., Lefresne P., Rossier J., Beaujouan J. C., Glowinski J. Inhibition by hemicholinium-3 of (14C)acetylcholine synthesis and (3H)choline high-affinity uptake in rat striatal synaptosomes. Mol Pharmacol. 1973 Sep;9(5):630–639. [PubMed] [Google Scholar]
- HEBB C. O., LING G. M., MCGEER E. G., MCGEER P. L., PERKINS D. EFFECT OF LOCALLY APPLIED HEMICHOLINIUM ON THE ACETYLCHOLINE CONTENT OF THE CAUDATE NUCLEUS. Nature. 1964 Dec 26;204:1309–1311. doi: 10.1038/2041309a0. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Van Essen D. Proceedings: A population of miniature end-plate potentials not evoked by nerve stimulation. J Physiol. 1976 Jun;258(2):103P–104P. [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERNAN R. P. Membrane potential changes during sodium transport in frog sartorius muscle. Nature. 1962 Mar 10;193:986–987. doi: 10.1038/193986a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Does the motor nerve impulse evoke 'non-quantal' transmitter release? Proc R Soc Lond B Biol Sci. 1981 May 7;212(1186):131–137. doi: 10.1098/rspb.1981.0029. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Lømo T., Lupa M. T., Thesleff S. Miniature end-plate potentials in rat skeletal muscle poisoned with botulinum toxin. J Physiol. 1984 Nov;356:587–599. doi: 10.1113/jphysiol.1984.sp015484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall I. G. A comparison between the blocking actions of 2-(4-phenylpiperidino) cyclohexanol (AH 5183) and its N-methyl quaternary analogue (AH 5954). Br J Pharmacol. 1970 Sep;40(1):68–77. doi: 10.1111/j.1476-5381.1970.tb10611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgó J., Gomez S., Polak R. L., Thesleff S. Giant miniature endplate potentials induced by 4-aminoquinoline. Acta Physiol Scand. 1982 Jun;115(2):201–207. doi: 10.1111/j.1748-1716.1982.tb07066.x. [DOI] [PubMed] [Google Scholar]
- Molgó J., Thesleff S. 4-aminoquinoline-induced 'giant' miniature endplate potentials at mammalian neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1982 Jan 22;214(1195):229–244. doi: 10.1098/rspb.1982.0006. [DOI] [PubMed] [Google Scholar]
- Peper K., Bradley R. J., Dreyer F. The acetylcholine receptor at the neuromuscular junction. Physiol Rev. 1982 Oct;62(4 Pt 1):1271–1340. doi: 10.1152/physrev.1982.62.4.1271. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- Sun Y. A., Poo M. M. Non-quantal release of acetylcholine at a developing neuromuscular synapse in culture. J Neurosci. 1985 Mar;5(3):634–642. doi: 10.1523/JNEUROSCI.05-03-00634.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H., Kojima M., Nagata M., Kuromi H. On the site of action of hemicholinium-3 at the rat phrenic nerve-diaphragm preparation with special reference to its multiple presynaptic actions. Neuropharmacology. 1970 Jul;9(4):359–367. doi: 10.1016/0028-3908(70)90033-x. [DOI] [PubMed] [Google Scholar]
- Thesleff S., Molgó J. A new type of transmitter release at the neuromuscular junction. Neuroscience. 1983 May;9(1):1–8. doi: 10.1016/0306-4522(83)90041-6. [DOI] [PubMed] [Google Scholar]
- Thesleff S., Molgó J., Lundh H. Botulinum toxin and 4-aminoquinoline induce a similar abnormal type of spontaneous quantal transmitter release at the rat neuromuscular junction. Brain Res. 1983 Mar 28;264(1):89–97. doi: 10.1016/0006-8993(83)91123-x. [DOI] [PubMed] [Google Scholar]
- Van der Kloot W. 2-(4-phenylpiperidino) cyclohexanol (AH5183) decreases quantal size at the frog neuromuscular junction. Pflugers Arch. 1986 Jan;406(1):83–85. doi: 10.1007/BF00582958. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Na+-K+-activated adenosinetriphosphatase as a trigger in transmitter release. Neuroscience. 1978;3(4-5):367–384. doi: 10.1016/0306-4522(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Vizi E. S., Vyskocil F. Changes in total and quantal release of acetylcholine in the mouse diaphragm during activation and inhibition of membrane ATPase. J Physiol. 1979 Jan;286:1–14. doi: 10.1113/jphysiol.1979.sp012603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Electrophysiological examination of transmitter release in non-quantal form in the mouse diaphragm and the activity of membrane ATP-ase. Physiol Bohemoslov. 1978;27(5):449–455. [PubMed] [Google Scholar]
- Vyskocil F., Nikolsky E., Edwards C. An analysis of the mechanisms underlying the non-quantal release of acetylcholine at the mouse neuromuscular junction. Neuroscience. 1983 Jun;9(2):429–435. doi: 10.1016/0306-4522(83)90305-6. [DOI] [PubMed] [Google Scholar]


