Abstract
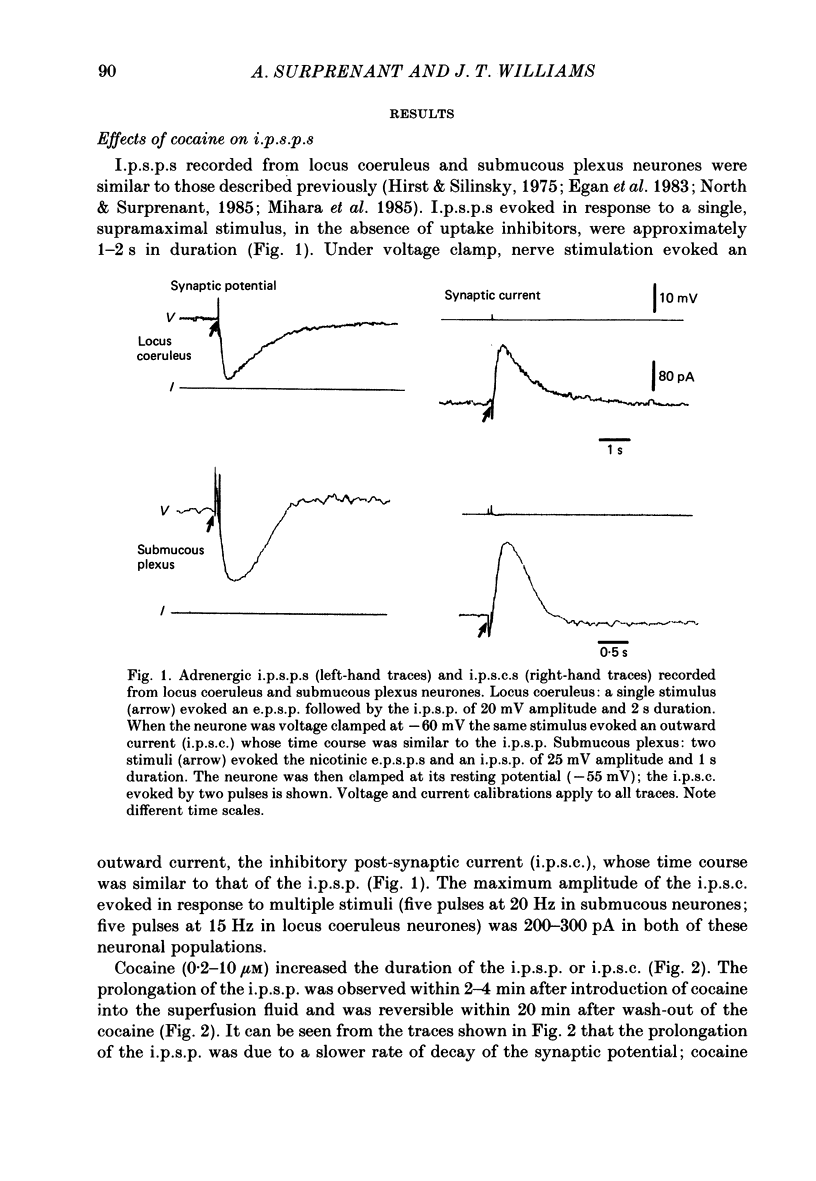
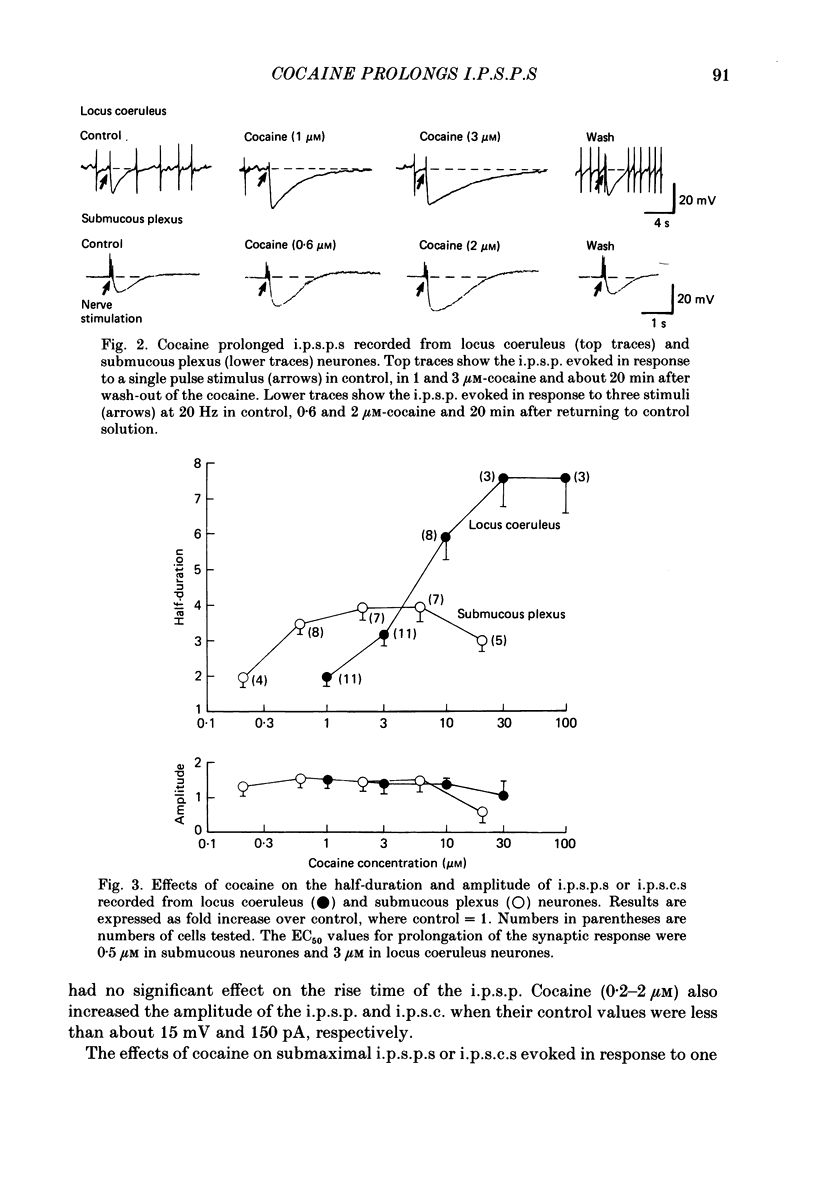
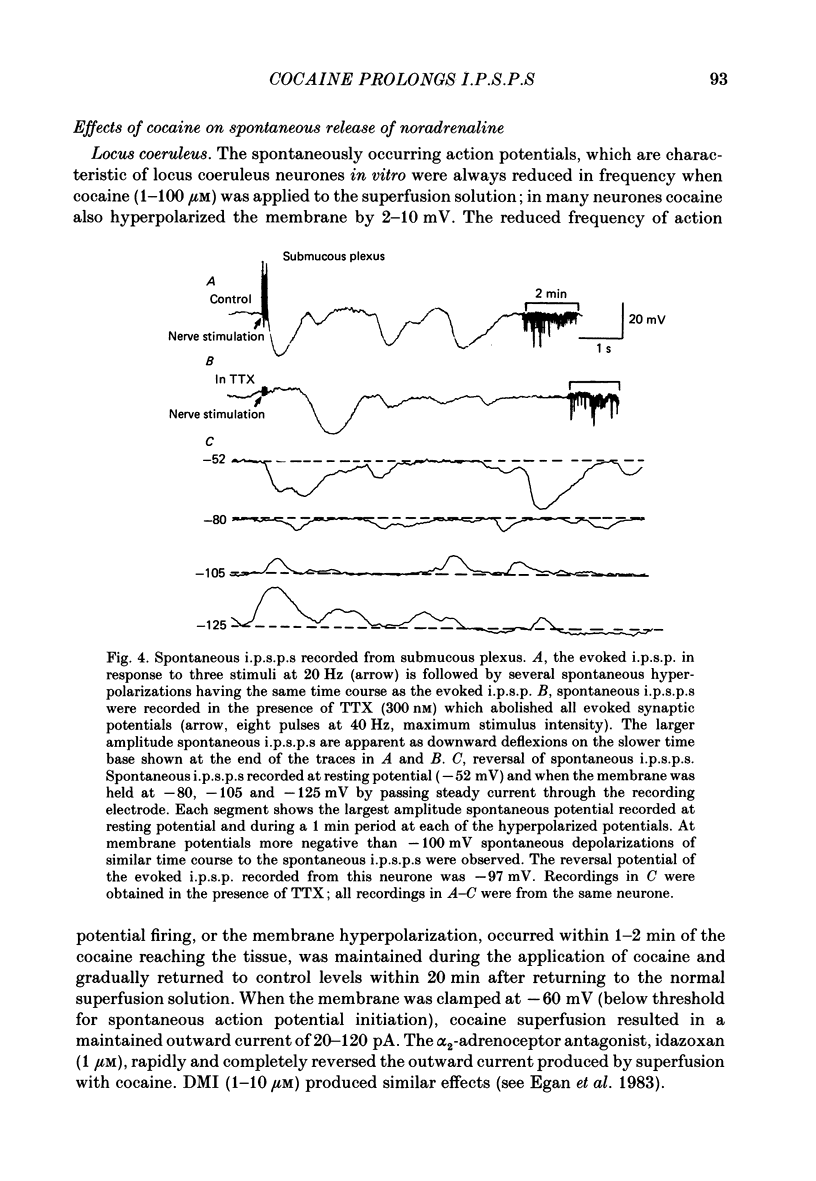
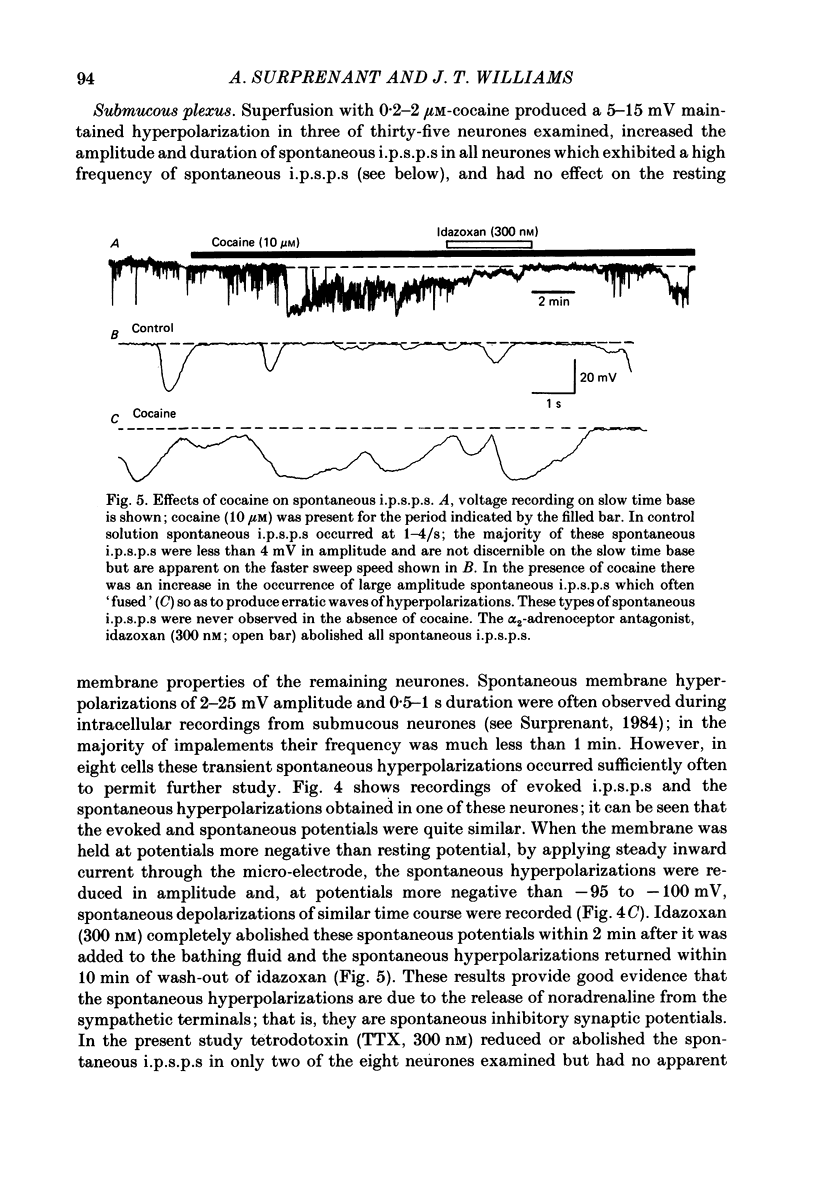
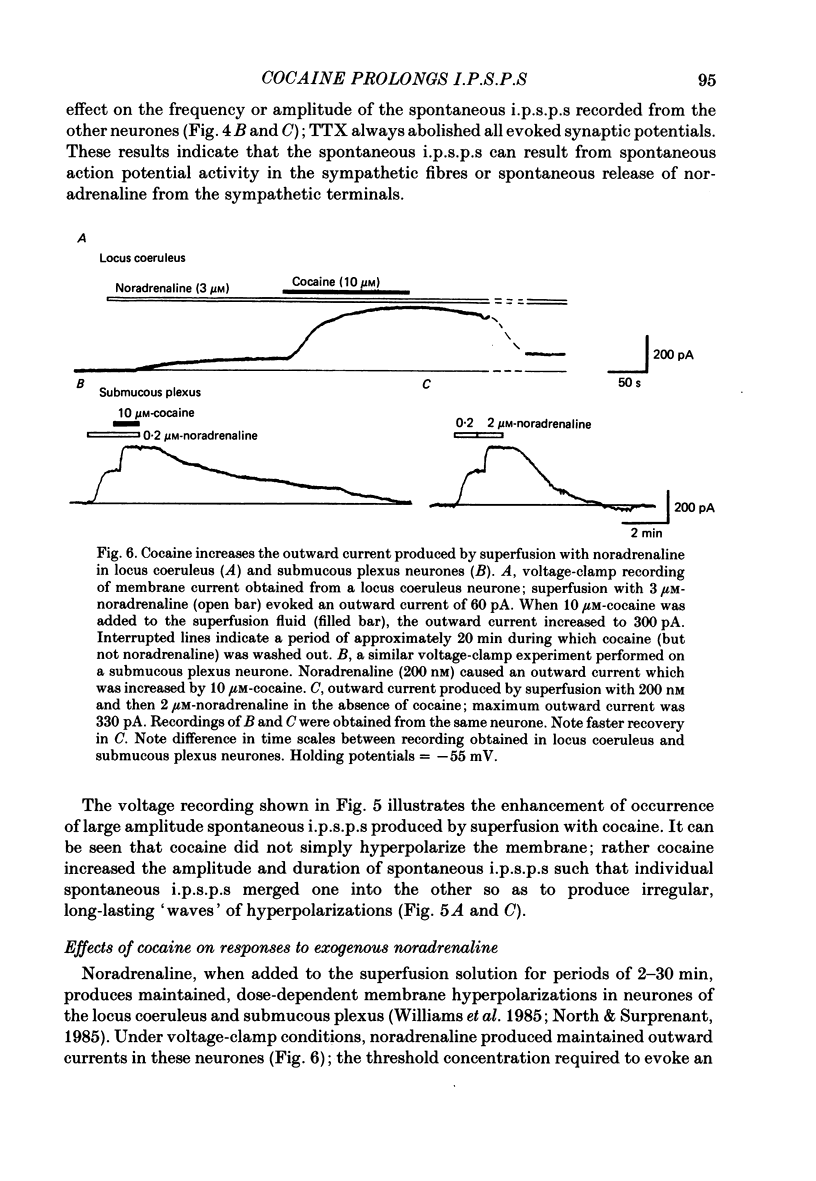
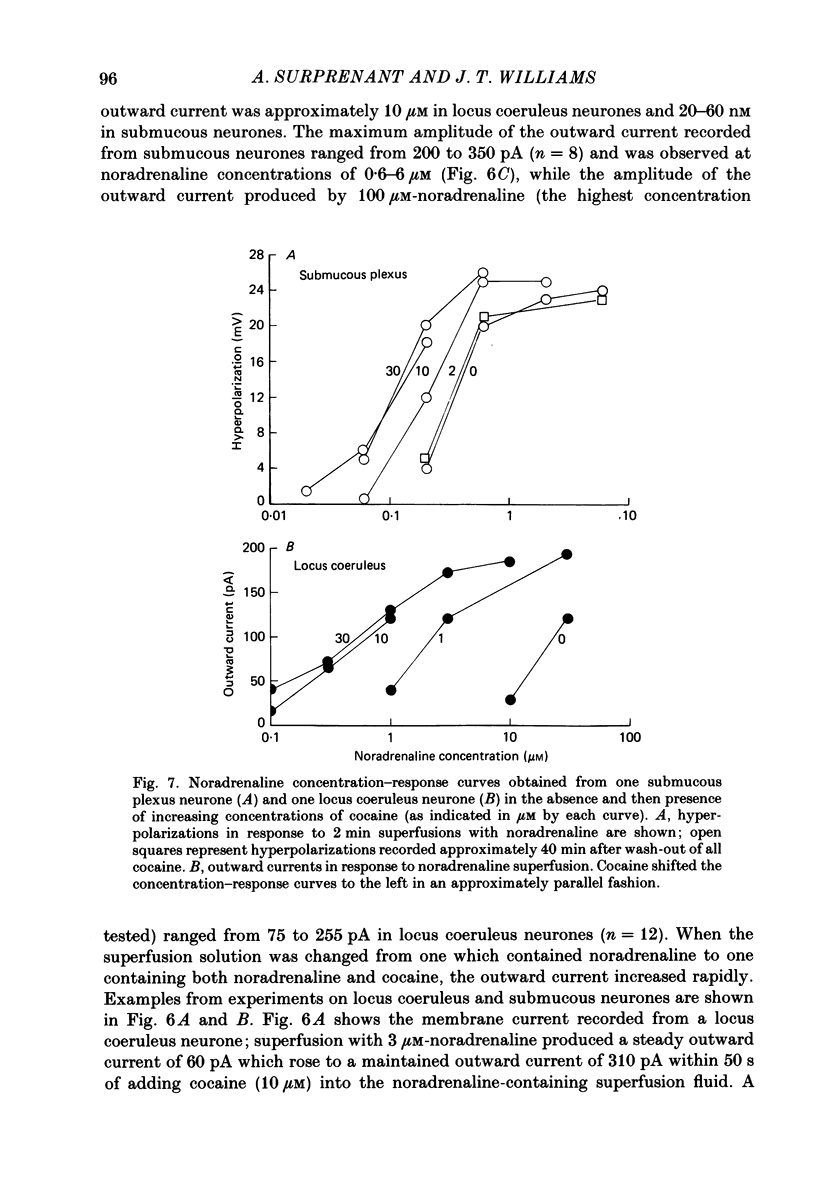
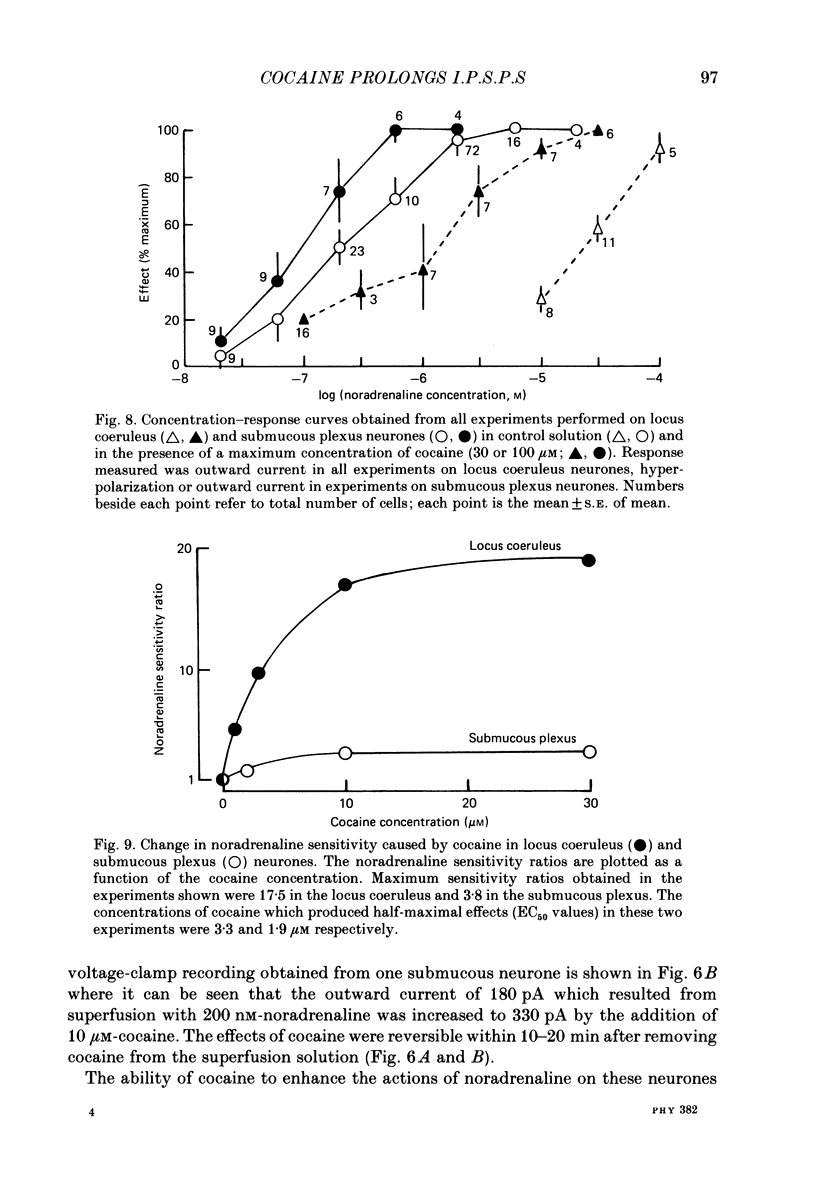
1. Intracellular recordings of membrane potential and membrane current were made from neurones of the rat nucleus locus coeruleus and the guinea-pig submucous plexus. These neurones exhibit inhibitory post-synaptic potentials (i.p.s.p.s) which result from noradrenaline acting on alpha 2-adrenoceptors to cause an increase in potassium conductance. 2. Cocaine (0.2-30 microM) reversibly increased the duration of the i.p.s.p. or inhibitory post-synaptic current (i.p.s.c.) in locus coeruleus neurones and submucous plexus neurones by approximately 750% and 350% respectively. The concentrations of cocaine causing half-maximal prolongation of the synaptic current were 3 microM in locus coeruleus and 0.5 microM in submucous plexus. The prolongation was due entirely to a slower rate of decay of the synaptic response. 3. Cocaine (10 microM) produced a maintained hyperpolarization (2-10 mV) or outward current (20-120 pA) in locus coeruleus neurones; in submucous plexus neurones cocaine increased the amplitude and duration of spontaneous i.p.s.p.s. 4. Outward currents produced by superfusion with noradrenaline were increased by cocaine with maximum effects being observed at 10-30 microM-cocaine. The maximum leftward shift in the relation between outward current or membrane hyperpolarization and noradrenaline concentration was 18- to 100-fold in locus coeruleus neurones and 4-fold in submucous plexus neurones. The concentrations of cocaine which caused a half-maximal increase in sensitivity to superfused noradrenaline were similar in both tissues, being 4 microM in locus coeruleus and 2 microM in submucous plexus. 5. These results show that neuronal uptake of noradrenaline released from adrenergic nerves plays a significant role in determining the time course of synaptic potentials mediated by noradrenaline.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biegon A., Rainbow T. C. Localization and characterization of [3H]desmethylimipramine binding sites in rat brain by quantitative autoradiography. J Neurosci. 1983 May;3(5):1069–1076. doi: 10.1523/JNEUROSCI.03-05-01069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein J. C. The effects of physostigmine on synaptic transmission in the inferior mesenteric ganglion of guinea-pigs. J Physiol. 1974 Sep;241(2):309–325. doi: 10.1113/jphysiol.1974.sp010658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Henderson G., North R. A., Williams J. T. Noradrenaline-mediated synaptic inhibition in rat locus coeruleus neurones. J Physiol. 1983 Dec;345:477–488. doi: 10.1113/jphysiol.1983.sp014990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- GLOWINSKI J., KOPIN I. J., AXELROD J. METABOLISM OF (H3)NOREPINEPHRINE IN THE RAT BRAIN. J Neurochem. 1965 Jan;12:25–30. doi: 10.1111/j.1471-4159.1965.tb10247.x. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silinsky E. M. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Oct;251(3):817–832. doi: 10.1113/jphysiol.1975.sp011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol. 1971 Apr;41(4):571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. T. The pharmacology of cocaine. NIDA Res Monogr. 1984;50:34–53. [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. P. Errors in the measurement of agonist potency-ratios produced by uptake processes: a general model applied to beta-adrenoceptor agonists. Br J Pharmacol. 1980;71(2):407–417. doi: 10.1111/j.1476-5381.1980.tb10953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. P. The classification of drugs and drug receptors in isolated tissues. Pharmacol Rev. 1984 Sep;36(3):165–222. [PubMed] [Google Scholar]
- Koe B. K. Molecular geometry of inhibitors of the uptake of catecholamines and serotonin in synaptosomal preparations of rat brain. J Pharmacol Exp Ther. 1976 Dec;199(3):649–661. [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. M., Snyder S. H. Norepinephrine neuronal uptake binding sites in rat brain membranes labeled with [3H]desipramine. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5250–5254. doi: 10.1073/pnas.78.8.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara S., Katayama Y., Nishi S. Slow postsynaptic potentials in neurones of submucous plexus of guinea-pig caecum and their mimicry by noradrenaline and various peptides. Neuroscience. 1985 Dec;16(4):1057–1068. doi: 10.1016/0306-4522(85)90116-2. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986 Jun;88(2):315–322. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C., Hounsgaard J. Diffusion in the slice microenvironment and implications for physiological studies. Fed Proc. 1983 Sep;42(12):2865–2868. [PubMed] [Google Scholar]
- North R. A., Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985 Jan;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman R., Sette M., Pimoule C., Briley M., Langer S. Z. High-affinity [3H]desipramine binding in the peripheral and central nervous system: a specific site associated with the neuronal uptake of noradrenaline. Eur J Pharmacol. 1982 Mar 12;78(3):345–351. doi: 10.1016/0014-2999(82)90036-x. [DOI] [PubMed] [Google Scholar]
- Rehavi M., Skolnick P., Brownstein M. J., Paul S. M. High-affinity binding of [3H]desipramine to rat brain: a presynaptic marker for noradrenergic uptake sites. J Neurochem. 1982 Apr;38(4):889–895. doi: 10.1111/j.1471-4159.1982.tb05326.x. [DOI] [PubMed] [Google Scholar]
- Reith M. E., Sershen H., Allen D. L., Lajtha A. A portion of [3H]cocaine binding in brain is associated with serotonergic neurons. Mol Pharmacol. 1983 May;23(3):600–606. [PubMed] [Google Scholar]
- Snyder S. H., Coyle J. T. Regional differences in H3-norepinephrine and H3-dopamine uptake into rat brain homogenates. J Pharmacol Exp Ther. 1969 Jan;165(1):78–86. [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984 Jun;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg U. The effect of cocaine on the pacemaker of isolated guinea-pig atria. J Pharmacol Exp Ther. 1968 Jun;161(2):222–231. [PubMed] [Google Scholar]
- Williams J. T., Henderson G., North R. A. Characterization of alpha 2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. Neuroscience. 1985 Jan;14(1):95–101. doi: 10.1016/0306-4522(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Williams J. T., North R. A., Shefner S. A., Nishi S., Egan T. M. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984 Sep;13(1):137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Wilson A. J., Furness J. B., Costa M. The fine structure of the submucous plexus of the guinea-pig ileum. I. The ganglia, neurons, Schwann cells and neuropil. J Neurocytol. 1981 Oct;10(5):759–784. doi: 10.1007/BF01262652. [DOI] [PubMed] [Google Scholar]
- Wise R. A. Neural mechanisms of the reinforcing action of cocaine. NIDA Res Monogr. 1984;50:15–33. [PubMed] [Google Scholar]


