Abstract
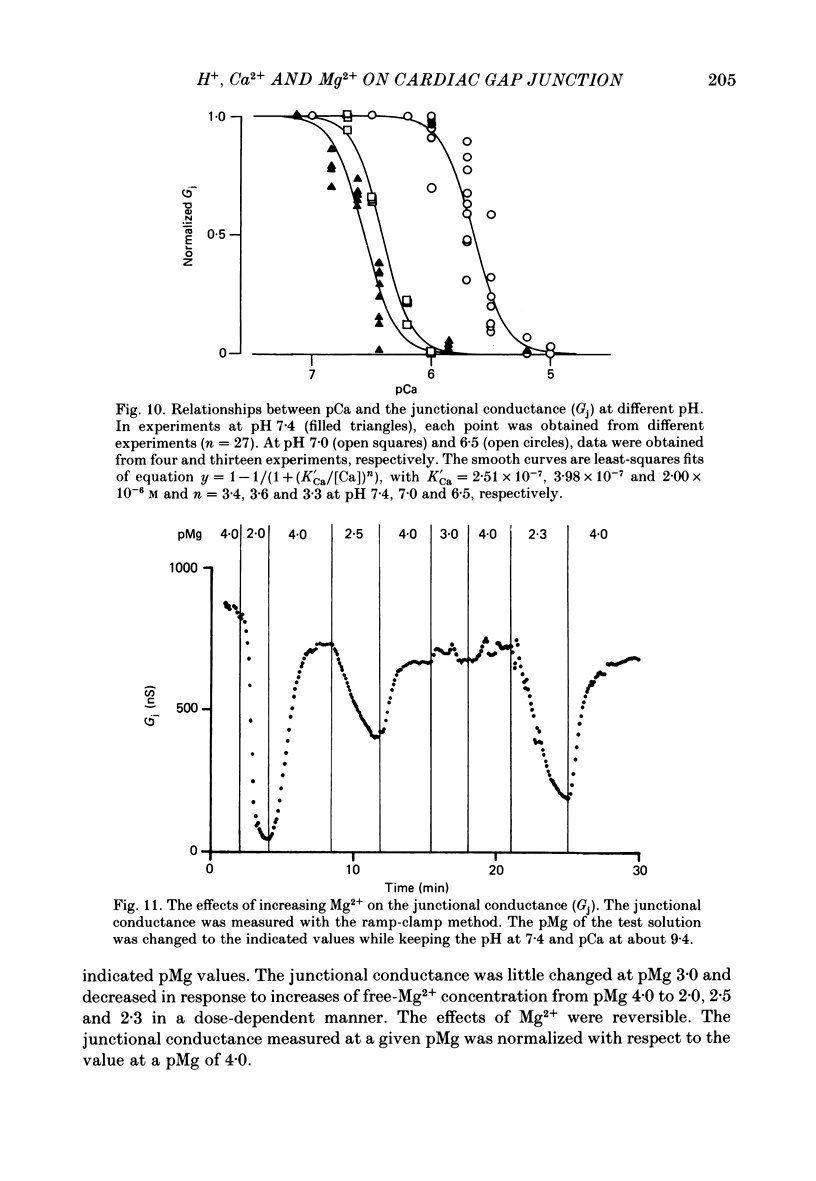
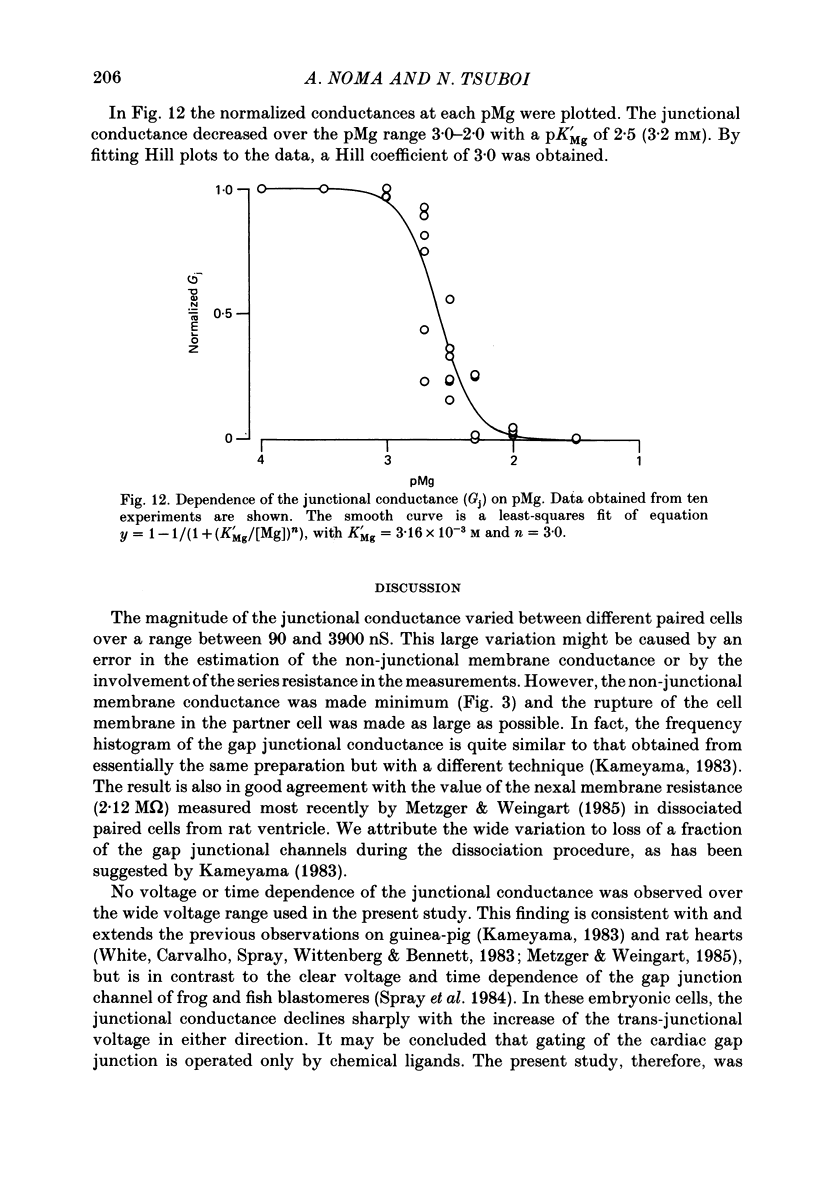
1. The dependence of gap junctional conductance on the intracellular concentrations of H+, Ca2+ and Mg2+ was studied in paired myocytes dissociated enzymatically from guinea-pig ventricle. To apply an internal solution buffered to specific H+, Ca2+ or Mg2+ concentration directly to one aspect of the gap junction, the non-junctional membrane of one of the pair was mechanically ruptured. The junctional conductance was measured by clamping the membrane potential of the other cell using a two-pipette voltage-clamp method. 2. The conductance of the non-junctional membrane was kept low in comparison with that of the junctional membrane (less than 1/50) by replacing both external and internal K+ with Cs+. 3. The current-voltage (I-V) relation of the junctional conductance was linear over the potential range examined (from -100 to +100 mV). No voltage or time dependence was detected. 4. The conductance of the gap junction between the paired cells ranged from 90 to 3900 nS with a peak distribution at 1000 nS. 5. The effect of H+ was examined over the pH range 7.4-5.4, while keeping the free-Ca2+ concentration at zero, or pCa 6.3 or 7.0 using 2-10 mM-EGTA. The junctional conductance was almost constant from pH 7.4 to 6.5 and decreased in a dose-dependent manner with further acidification. There was no difference in the pH-conductance relationships at various Ca2+ concentrations. The Hill coefficient was approximately 2.4 and the half-maximum concentration (pK'H) was 6.1. 6. The closing effect of Ca2+ on the gap junction channel was examined over the concentration range from pCa 7 to 5, while keeping the pH at 7.4, 7.0 or 6.5. At each pH, increasing Ca2+ decreased the junctional conductance with similar Hill coefficients of about 3.4. The pCa-conductance relationship shifted toward a higher Ca2+ concentration range as the pH was lowered (pK'Ca = 6.6, 6.4 and 5.6, at pH 7.4, 7.0 and 6.5, respectively). 7. Increasing Mg2+ also caused a fall in the junctional conductance over the pMg range 3.0-2.0 with a pK'Mg of 2.5 (3.2 mM), and a Hill coefficient of 3.0. 8. These results suggest that there are two respective binding sites for divalent cations and H+, and that the gap junctional conductance is regulated reversibly by the ligand-receptor reactions. Comparing the threshold concentrations of Ca2+ and H+ for electrical uncoupling, it was concluded that Ca2+ plays a more important role in regulating the gap junctional conductance of cardiac cells under physiological conditions.
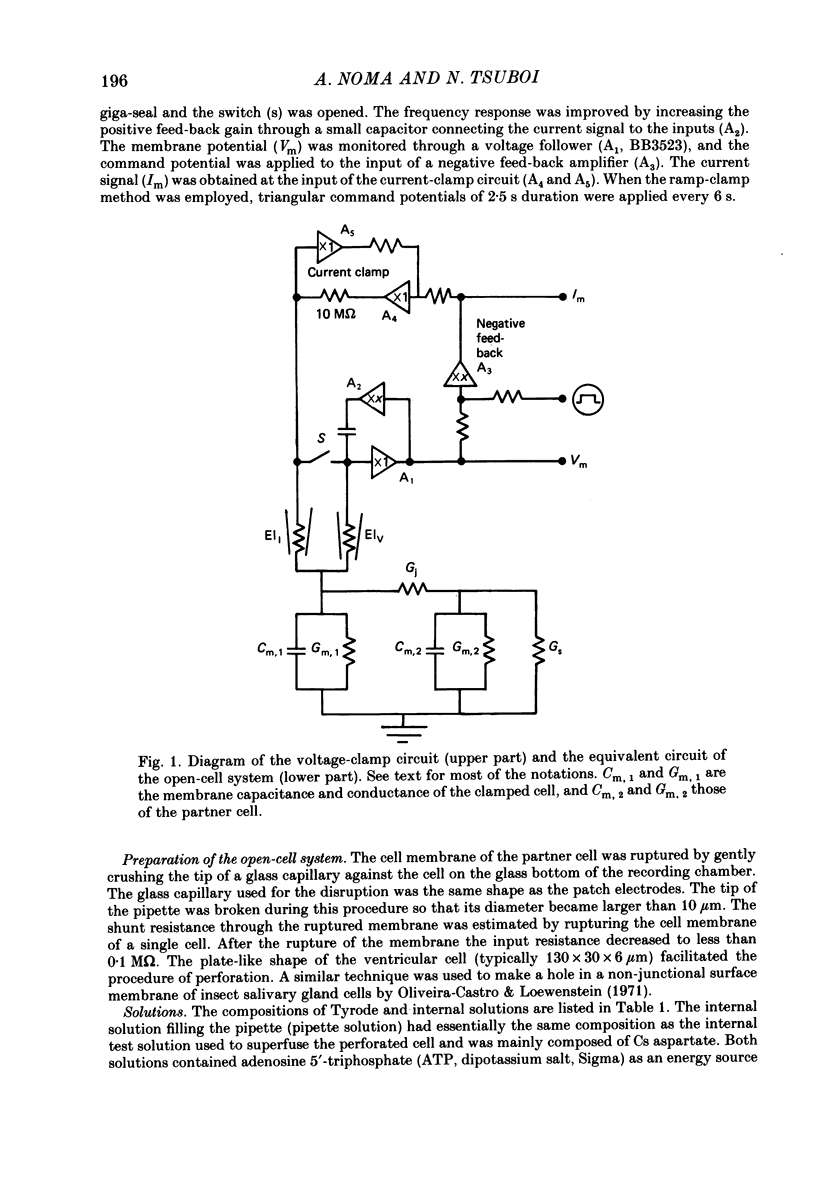
Full text
PDF




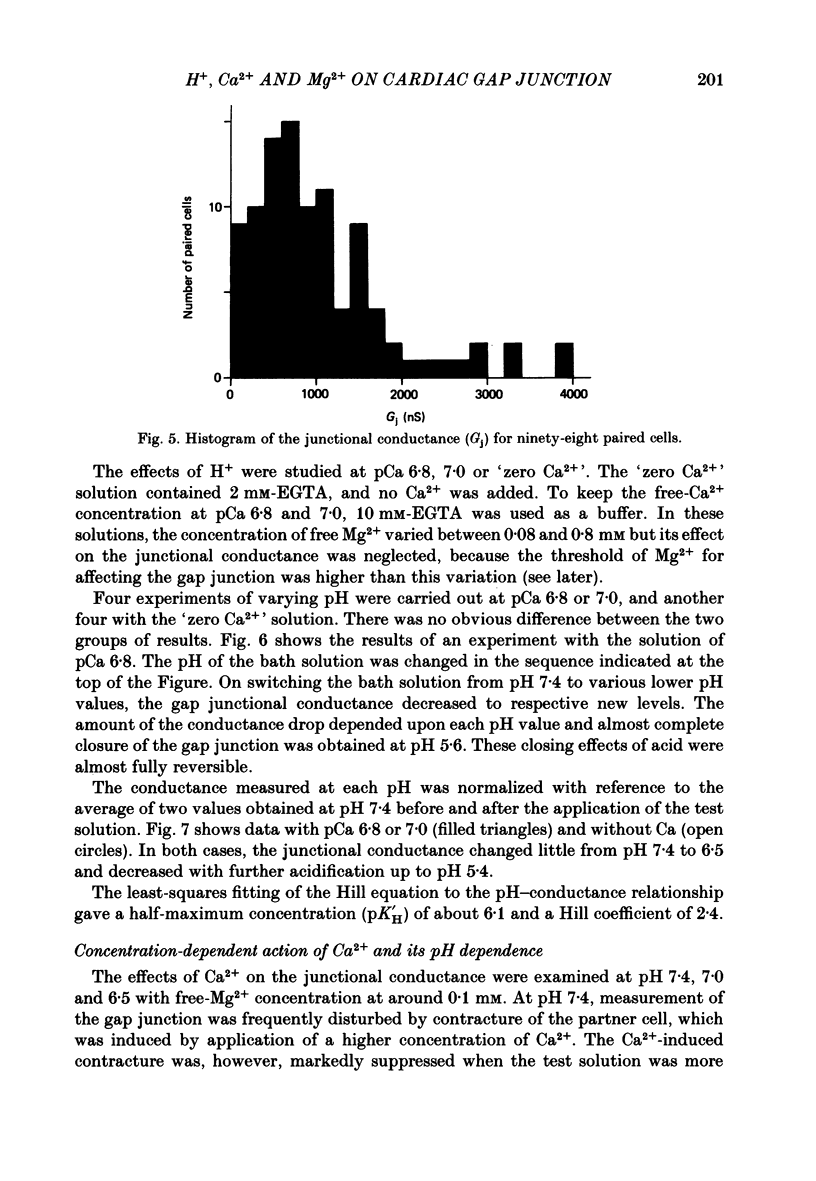





Images in this article
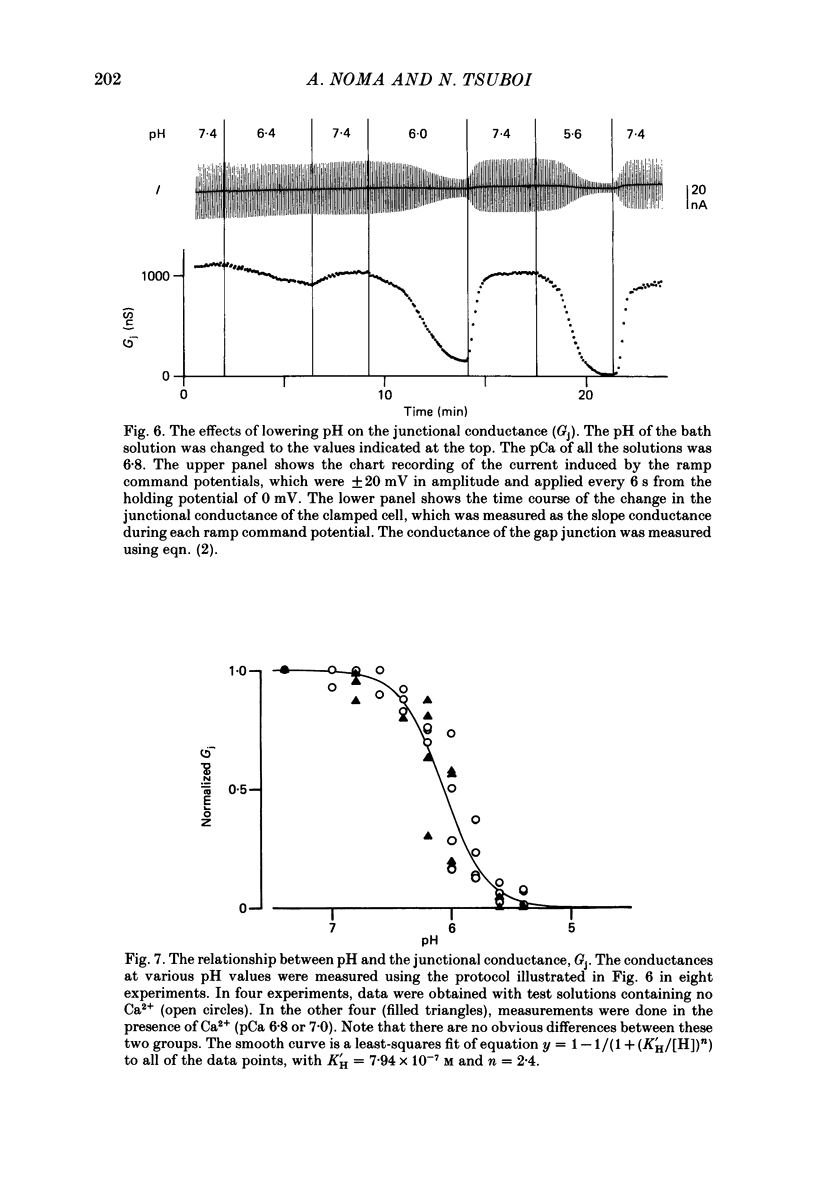
Selected References
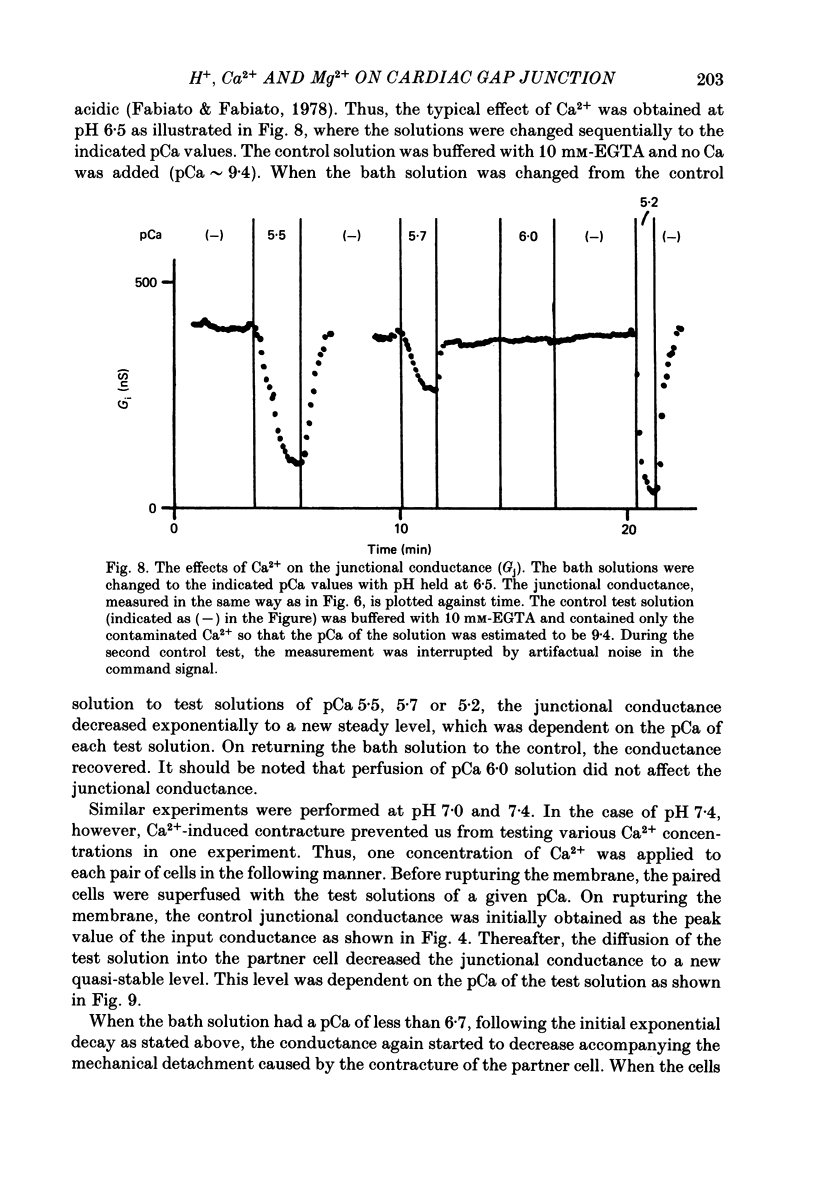
These references are in PubMed. This may not be the complete list of references from this article.
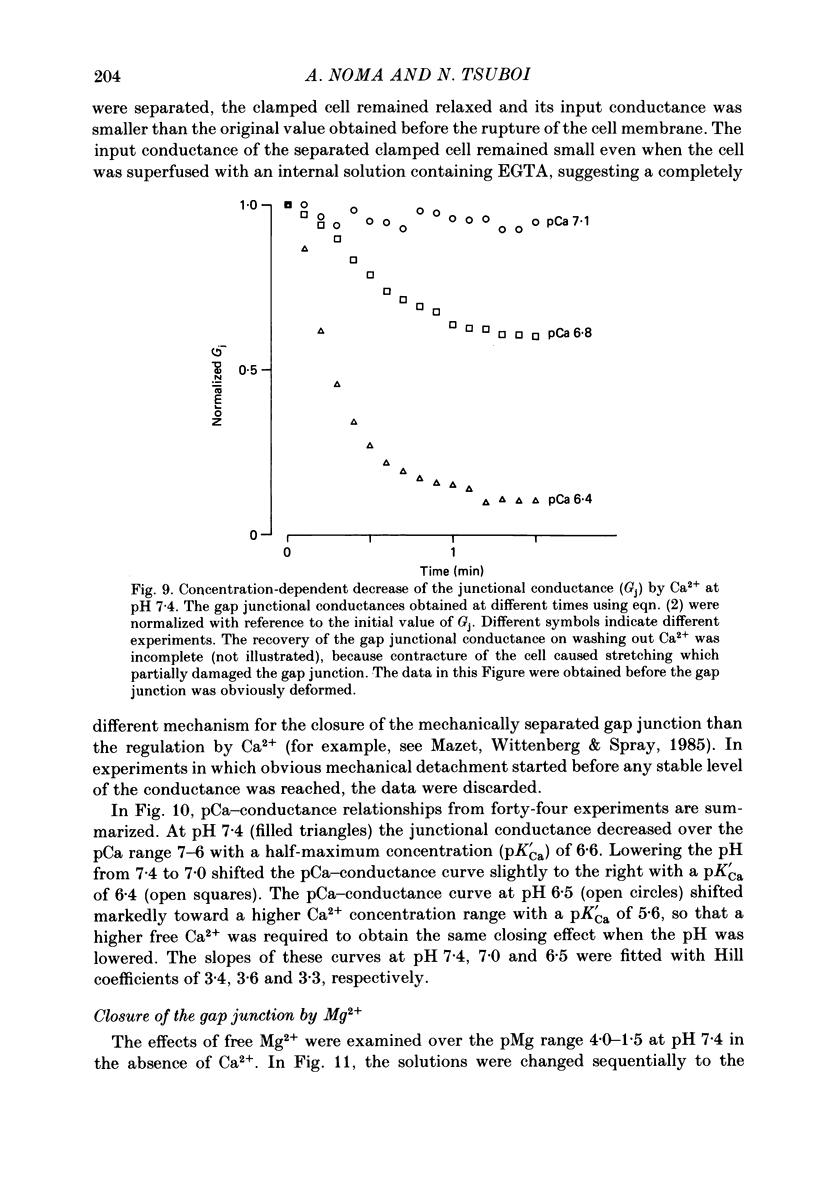
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Blatter L. A., McGuigan J. A. Free intracellular magnesium concentration in ferret ventricular muscle measured with ion selective micro-electrodes. Q J Exp Physiol. 1986 Jul;71(3):467–473. doi: 10.1113/expphysiol.1986.sp003005. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Crompton M. The regulation of intracellular calcium by mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:269–284. doi: 10.1111/j.1749-6632.1978.tb41957.x. [DOI] [PubMed] [Google Scholar]
- Dahl G., Isenberg G. Decoupling of heart muscle cells: correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J Membr Biol. 1980 Mar 31;53(1):63–75. doi: 10.1007/BF01871173. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Effect of intracellular injection of calcium and strontium on cell communication in heart. J Physiol. 1975 Sep;250(2):231–245. doi: 10.1113/jphysiol.1975.sp011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Influence of intracellular injection of H+ on the electrical coupling in cardiac Purkinje fibres. Cell Biol Int Rep. 1980 Jan;4(1):51–58. doi: 10.1016/0309-1651(80)90009-0. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Influence of the sodium pump on intercellular communication in heart fibres: effect of intracellular injection of sodium ion on electrical coupling. J Physiol. 1976 Dec;263(2):171–197. doi: 10.1113/jphysiol.1976.sp011627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Intercellular communication in cardiac muscle. Circ Res. 1982 Jul;51(1):1–9. doi: 10.1161/01.res.51.1.1. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. The influence of pH on the healing-over of mammalian cardiac muscle. J Physiol. 1983 Jun;339:299–307. doi: 10.1113/jphysiol.1983.sp014717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A. K., Kwan C. Y., Daniel E. E. High-affinity pH-dependent passive Ca binding by myometrial plasma membrane vesicles. Am J Physiol. 1983 Jan;244(1):C61–C67. doi: 10.1152/ajpcell.1983.244.1.C61. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Gupta P., Moore R. D. NMR studies of intracellular metal ions in intact cells and tissues. Annu Rev Biophys Bioeng. 1984;13:221–246. doi: 10.1146/annurev.bb.13.060184.001253. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kameyama M. Electrical coupling between ventricular paired cells isolated from guinea-pig heart. J Physiol. 1983 Mar;336:345–357. doi: 10.1113/jphysiol.1983.sp014585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea T. J., Ashley C. C. Increase in free Ca2+ in muscle after exposure to CO2. Nature. 1978 Sep 21;275(5677):236–238. doi: 10.1038/275236a0. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A. Isolation of calcium current and its sensitivity to monovalent cations in dialysed ventricular cells of guinea-pig. J Physiol. 1984 Dec;357:553–573. doi: 10.1113/jphysiol.1984.sp015517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazet F., Wittenberg B. A., Spray D. C. Fate of intercellular junctions in isolated adult rat cardiac cells. Circ Res. 1985 Feb;56(2):195–204. doi: 10.1161/01.res.56.2.195. [DOI] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger P., Weingart R. Electric current flow in cell pairs isolated from adult rat hearts. J Physiol. 1985 Sep;366:177–195. doi: 10.1113/jphysiol.1985.sp015791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J., Trautmann A. Single-channel currents of an intercellular junction. 1985 Sep 26-Oct 2Nature. 317(6035):331–335. doi: 10.1038/317331a0. [DOI] [PubMed] [Google Scholar]
- Nishiye H., Mashima H., Ishida A. Ca binding of isolated cardiac nexus membranes related to intercellular uncoupling. Jpn J Physiol. 1980;30(1):131–136. doi: 10.2170/jjphysiol.30.131. [DOI] [PubMed] [Google Scholar]
- Peracchia C., Peracchia L. L. Gap junction dynamics: reversible effects of divalent cations. J Cell Biol. 1980 Dec;87(3 Pt 1):708–718. doi: 10.1083/jcb.87.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber W. R., Weingart R. Ungulate cardiac purkinje fibres: the influence of intracellular pH on the electrical cell-to-cell coupling. J Physiol. 1982 Jul;328:87–104. doi: 10.1113/jphysiol.1982.sp014254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Calcium ion distribution in cytoplasm visualised by aequorin: diffusion in cytosol restricted by energized sequestering. Science. 1975 Dec 19;190(4220):1204–1206. doi: 10.1126/science.1198106. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol. 1976 Aug 27;28(1):87–119. doi: 10.1007/BF01869692. [DOI] [PubMed] [Google Scholar]
- Rose B., Rick R. Intracellular pH, intracellular free Ca, and junctional cell-cell coupling. J Membr Biol. 1978 Dec 29;44(3-4):377–415. doi: 10.1007/BF01944230. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Stern J. H., Harris A. L., Bennett M. V. Gap junctional conductance: comparison of sensitivities to H and Ca ions. Proc Natl Acad Sci U S A. 1982 Jan;79(2):441–445. doi: 10.1073/pnas.79.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., White R. L., de Carvalho A. C., Harris A. L., Bennett M. V. Gating of gap junction channels. Biophys J. 1984 Jan;45(1):219–230. doi: 10.1016/S0006-3495(84)84150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi J., Kokubun S., Noma A., Irisawa H. Spontaneously active cells isolated from the sino-atrial and atrio-ventricular nodes of the rabbit heart. Jpn J Physiol. 1981;31(4):547–558. doi: 10.2170/jjphysiol.31.547. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970 Nov;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart R. The actions of ouabain on intercellular coupling and conduction velocity in mammalian ventricular muscle. J Physiol. 1977 Jan;264(2):341–365. doi: 10.1113/jphysiol.1977.sp011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtczak J. Contractures and increase in internal longitudianl resistance of cow ventricular muscle induced by hypoxia. Circ Res. 1979 Jan;44(1):88–95. doi: 10.1161/01.res.44.1.88. [DOI] [PubMed] [Google Scholar]