Abstract
1. The psychophysical spectral sensitivity of cats was assessed using a two-choice visual discrimination task by determining increment thresholds and critical flicker frequency on white and chromatic backgrounds. 2. For large increments, on 0.0, 0.3 and 3.0 cd/m2 white backgrounds, the cats were most sensitive to 497 nm indicating that these backgrounds are scotopic. On 30 and 300 cd/m2 white backgrounds, the cats were most sensitive to about 454 and 561 nm indicating that these backgrounds are photopic. Sensitivity to intermediate wave-lengths indicated independent action of 'blue' and 'green' cones. 3. For large increments, thresholds on photopic yellow and magenta backgrounds indicated the additive influence of 'blue' and 'green' cones. 4. Spectral sensitivity functions obtained with a critical flicker frequency criterion of 10 Hz on a 30 cd/m2 white background reflected only the activity of the 'green' cone while at 20 Hz the function reflected an additive contribution of both 'blue' and 'green' cones. 5. For small increments, on a 30 cd/m2 white or 96 cd/m2 orange background, sensitivity reflected only the activity of the 'green' cone. 6. The cat's photopic spectral sensitivity is influenced by the psychophysical test upon which it is based in a manner that is similar to what has been found for other vertebrates. No evidence was found for a 500 nm mechanism active at photopic levels.
Full text
PDF


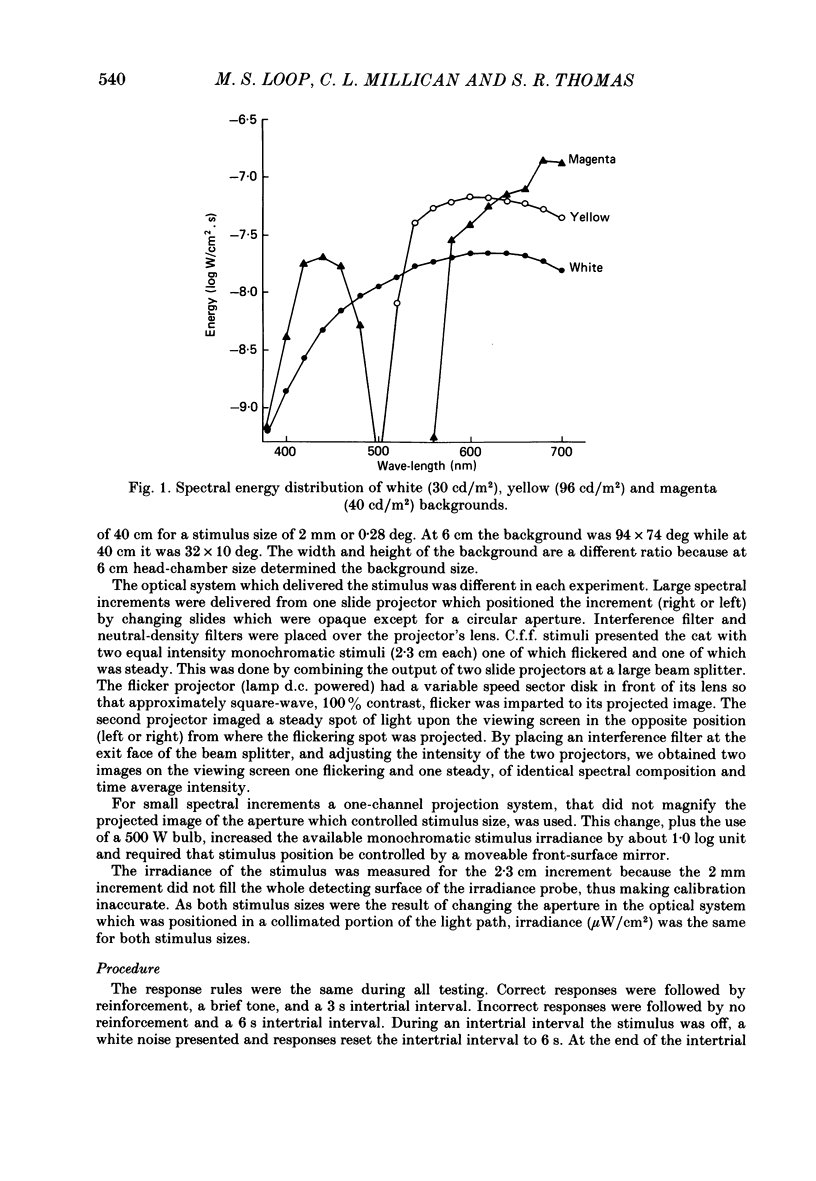

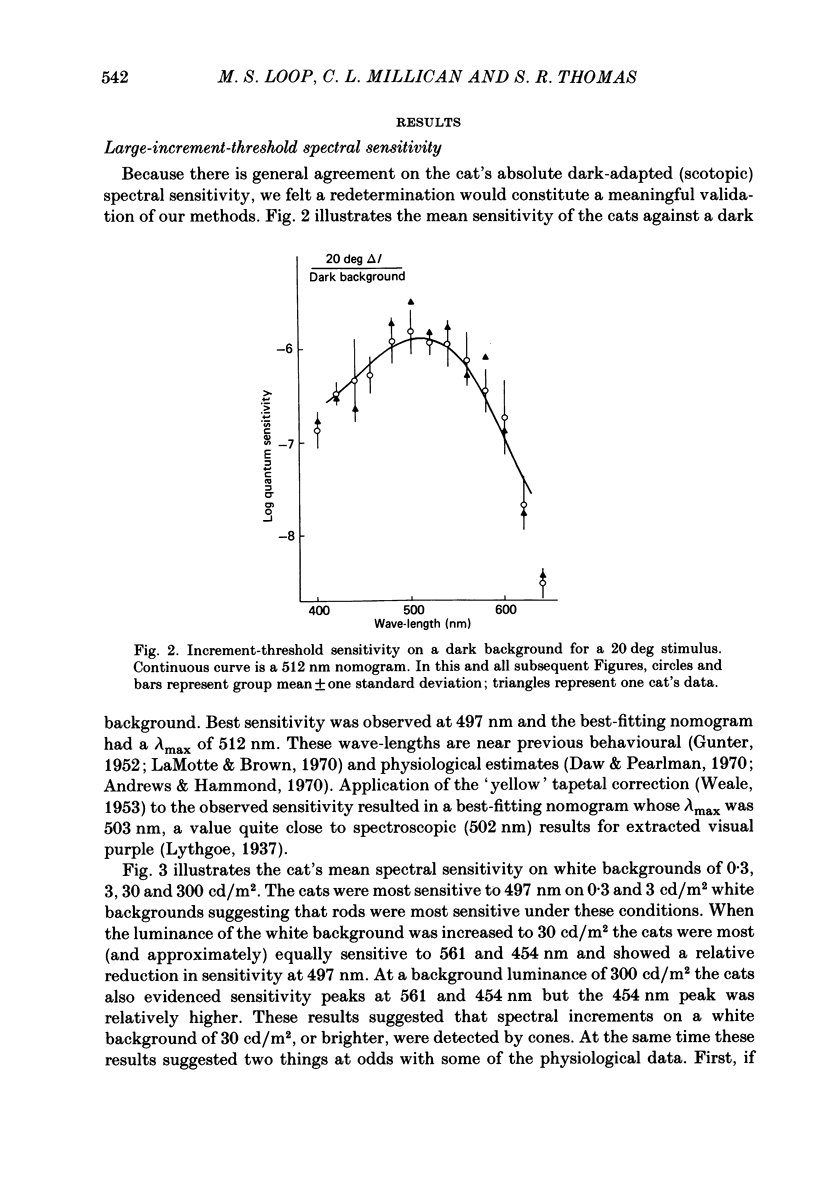

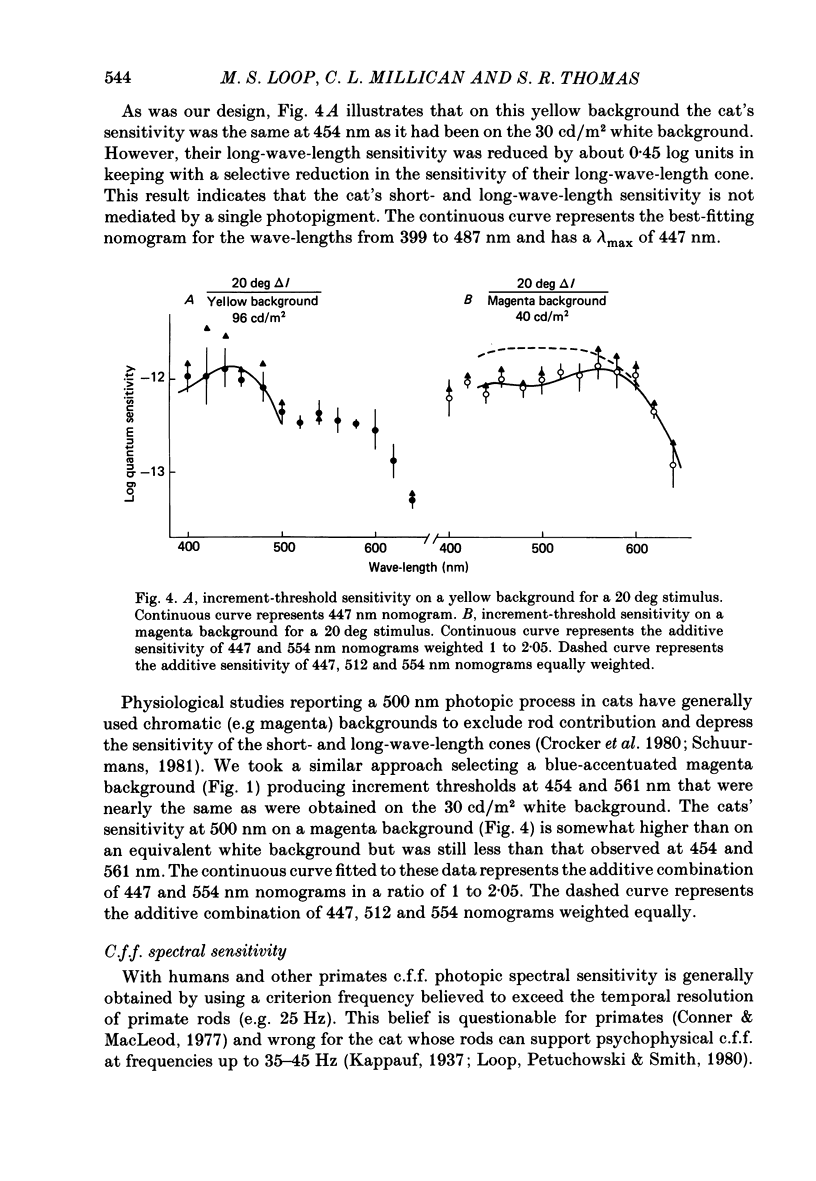

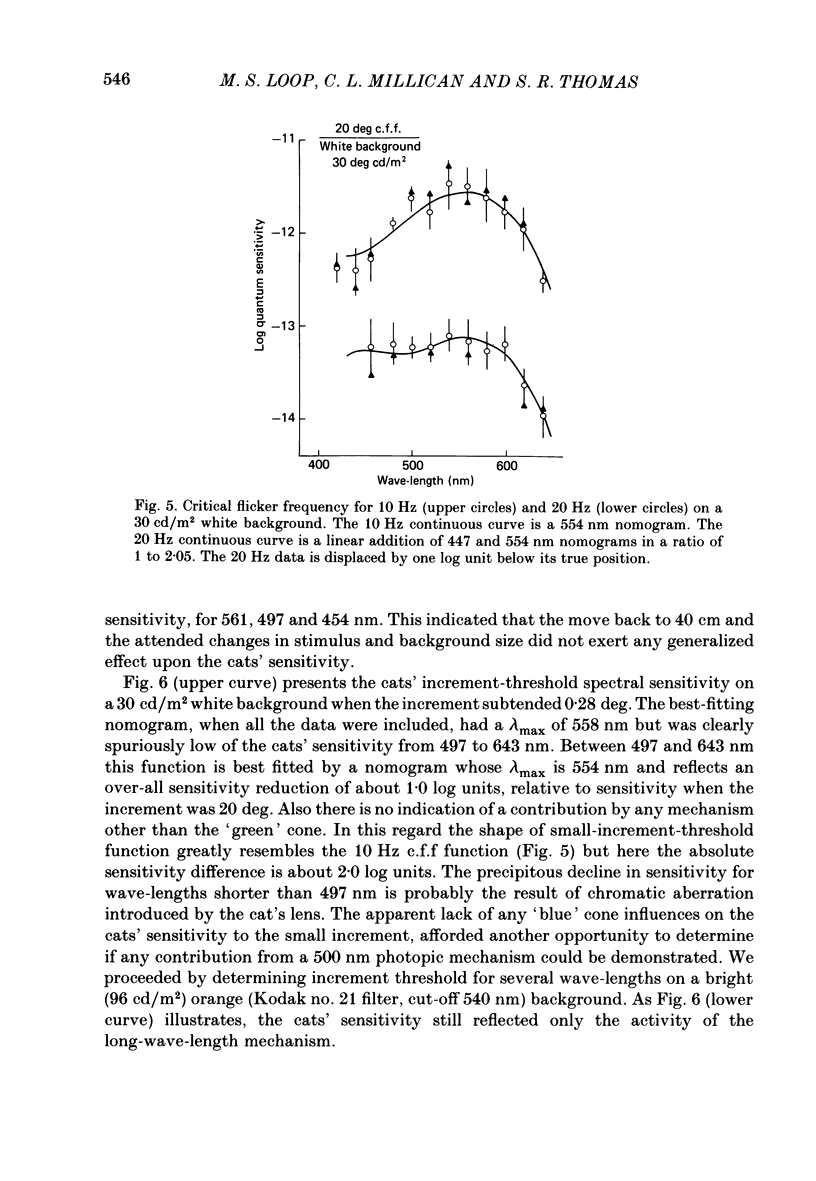






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews D. P., Hammond P. Mesopic increment threshold spectral sensitivity of single optic tract fibres in the cat: cone-rod interaction. J Physiol. 1970 Jul;209(1):65–81. doi: 10.1113/jphysiol.1970.sp009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R., Camisa J. M. Temporal aspects of spatial vision in the cat. Exp Brain Res. 1977 Jun 27;28(3-4):325–333. doi: 10.1007/BF00235714. [DOI] [PubMed] [Google Scholar]
- Conner J. D., MacLeod D. I. Rod photoreceptors detect rapid flicker. Science. 1977 Feb 18;195(4279):698–699. doi: 10.1126/science.841308. [DOI] [PubMed] [Google Scholar]
- Crocker R. A., Ringo J., Wolbarsht M. L., Wagner H. G. Cone contributions to cat retinal ganglion cell receptive fields. J Gen Physiol. 1980 Dec;76(6):763–785. doi: 10.1085/jgp.76.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: evidence for more than one cone process. J Physiol. 1970 Nov;211(1):125–137. doi: 10.1113/jphysiol.1970.sp009270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw N. W., Pearlman A. L. Cat colour vision: one cone process or several? J Physiol. 1969 May;201(3):745–764. doi: 10.1113/jphysiol.1969.sp008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawis S. M. Polynomial expressions of pigment nomograms. Vision Res. 1981;21(9):1427–1430. doi: 10.1016/0042-6989(81)90250-9. [DOI] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Abramov I., Jacobs G. H. Analysis of response patterns of LGN cells. J Opt Soc Am. 1966 Jul;56(7):966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz G., Lennie P. Cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):273–296. doi: 10.1113/jphysiol.1977.sp011902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein M. A., Hood D. C. Opponent-color cells can influence detection of small, brief lights. Vision Res. 1982;22(1):89–95. doi: 10.1016/0042-6989(82)90170-5. [DOI] [PubMed] [Google Scholar]
- GUNTER R., HARDING H. G. W., STILES W. S. Spectral reflexion factor of the cat's tapetum. Nature. 1951 Aug 18;168(4268):293–294. doi: 10.1038/168293a0. [DOI] [PubMed] [Google Scholar]
- GUNTER R. The spectral sensitivity of dark-adapted cats. J Physiol. 1952 Nov;118(3):395–404. doi: 10.1113/jphysiol.1952.sp004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTER R. The spectral sensitivity of light-adapted cats. J Physiol. 1954 Feb 26;123(2):409–415. doi: 10.1113/jphysiol.1954.sp005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. G., Siegel I. M. Double branched flicker fusion curves from the all-rod skate retina. Science. 1975 Jun 13;188(4193):1120–1122. doi: 10.1126/science.1215989. [DOI] [PubMed] [Google Scholar]
- Hammond P. The neural basis for colour discrimination in the domestic cat. Vision Res. 1978;18(2):233–235. doi: 10.1016/0042-6989(78)90193-1. [DOI] [PubMed] [Google Scholar]
- JACOBS G. H. Spectral sensitivity and colo vision of the squirrel moneky. J Comp Physiol Psychol. 1963 Jun;56:616–621. doi: 10.1037/h0045322. [DOI] [PubMed] [Google Scholar]
- Jacobs G. H., Neitz J. Spectral sensitivity of cat cones to rapid flicker. Exp Brain Res. 1986;62(2):446–448. doi: 10.1007/BF00238865. [DOI] [PubMed] [Google Scholar]
- King-Smith P. E., Carden D. Luminance and opponent-color contributions to visual detection and adaptation and to temporal and spatial integration. J Opt Soc Am. 1976 Jul;66(7):709–717. doi: 10.1364/josa.66.000709. [DOI] [PubMed] [Google Scholar]
- LENNOX M. A. Geniculate and cortical responses to colored light flash in cat. J Neurophysiol. 1956 May;19(3):271–279. doi: 10.1152/jn.1956.19.3.271. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Brown J. L. Dark adaptation and spectral sensitivity in the cat. Vision Res. 1970 Aug;10(8):703–716. doi: 10.1016/0042-6989(70)90017-9. [DOI] [PubMed] [Google Scholar]
- Loop M. S., Berkley M. A. Temporal modulation sensitivity of the cat. I. Behavioral measures. Vision Res. 1975 May;15(5):555–561. doi: 10.1016/0042-6989(75)90302-8. [DOI] [PubMed] [Google Scholar]
- Loop M. S., Millican C. L. Increment thresholds in normal and binocularly deprived cats. Behav Brain Res. 1983 Aug;9(2):143–150. doi: 10.1016/0166-4328(83)90124-9. [DOI] [PubMed] [Google Scholar]
- Loop M. S., Petuchowski S., Smith D. C. Critical flicker fusion in normal and binocularly deprived cats. Vision Res. 1980;20(1):49–57. doi: 10.1016/0042-6989(80)90141-8. [DOI] [PubMed] [Google Scholar]
- Lythgoe R. J. The absorption spectra of visual purple and of indicator yellow. J Physiol. 1937 Jun 3;89(4):331–358. doi: 10.1113/jphysiol.1937.sp003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T., Merigan W. H. The luminance dependence of spatial vision in the cat. Vision Res. 1981;21(9):1333–1339. doi: 10.1016/0042-6989(81)90240-6. [DOI] [PubMed] [Google Scholar]
- Pearlman A. L., Daw N. W. Opponent color cells in the cat lateral geniculate nucleus. Science. 1970 Jan 2;167(3914):84–86. doi: 10.1126/science.167.3914.84. [DOI] [PubMed] [Google Scholar]
- Rabin A. R., Mehaffey L., 3rd, Berson E. L. Blue cone function in the retina of the cat. Vision Res. 1976;16(8):799–801. doi: 10.1016/0042-6989(76)90138-3. [DOI] [PubMed] [Google Scholar]
- Ringo J., Wolbarsht M. L., Wagner H. G., Crocker R., Amthor F. Trichromatic vision in the cat. Science. 1977 Nov 18;198(4318):753–755. doi: 10.1126/science.910161. [DOI] [PubMed] [Google Scholar]
- Saunders R. M. The spectral responsiveness and the temporal frequency response (TFR) of cat optic tract and lateral geniculate neurons: sinusoidal stimulation studies. Vision Res. 1977 Feb;17(2):285–292. doi: 10.1016/0042-6989(77)90092-x. [DOI] [PubMed] [Google Scholar]
- Sperling H. G., Harwerth R. S. Red-green cone interactions in the increment-threshold spectral sensitivity of primates. Science. 1971 Apr 9;172(3979):180–184. doi: 10.1126/science.172.3979.180. [DOI] [PubMed] [Google Scholar]
- Sterling P. Microcircuitry of the cat retina. Annu Rev Neurosci. 1983;6:149–185. doi: 10.1146/annurev.ne.06.030183.001053. [DOI] [PubMed] [Google Scholar]
- Thornton J. E., Pugh E. N., Jr Red/Green color opponency at detection threshold. Science. 1983 Jan 14;219(4581):191–193. doi: 10.1126/science.6849131. [DOI] [PubMed] [Google Scholar]
- WEALE R. A. Light absorption in the crystalline lens of the cat. Nature. 1954 May 29;173(4413):1049–1050. doi: 10.1038/1731049a0. [DOI] [PubMed] [Google Scholar]
- WEALE R. A. The spectral reflectivity of the cat's tapetum measured in situ. J Physiol. 1953 Jan;119(1):30–42. doi: 10.1113/jphysiol.1953.sp004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G., Boynton R. M. Comparison of four methods of heterochromatic photometry. J Opt Soc Am. 1972 Dec;62(12):1508–1515. doi: 10.1364/josa.62.001508. [DOI] [PubMed] [Google Scholar]
- Wooten B. R., Butler T. W. Possible rod-cone interaction in dark adaptation. J Opt Soc Am. 1976 Dec;66(12):1429–1430. doi: 10.1364/josa.66.001429. [DOI] [PubMed] [Google Scholar]
- Wooten B. R., Fuld K., Spillmann L. Photopic spectral sensitivity of the peripheral retina. J Opt Soc Am. 1975 Mar;65(3):334–342. doi: 10.1364/josa.65.000334. [DOI] [PubMed] [Google Scholar]
- Zrenner E., Gouras P. Blue-sensitive cones of the cat produce a rodlike electroretinogram. Invest Ophthalmol Vis Sci. 1979 Oct;18(10):1076–1081. [PubMed] [Google Scholar]


