Abstract
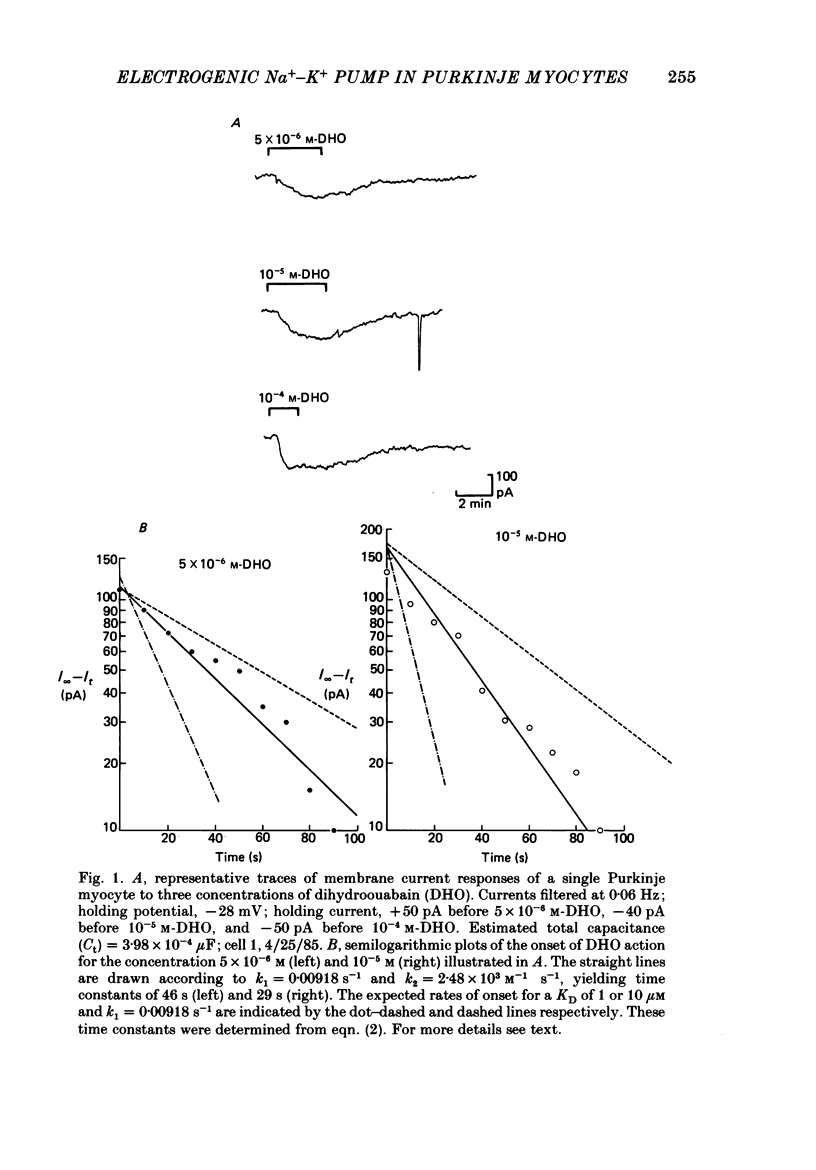
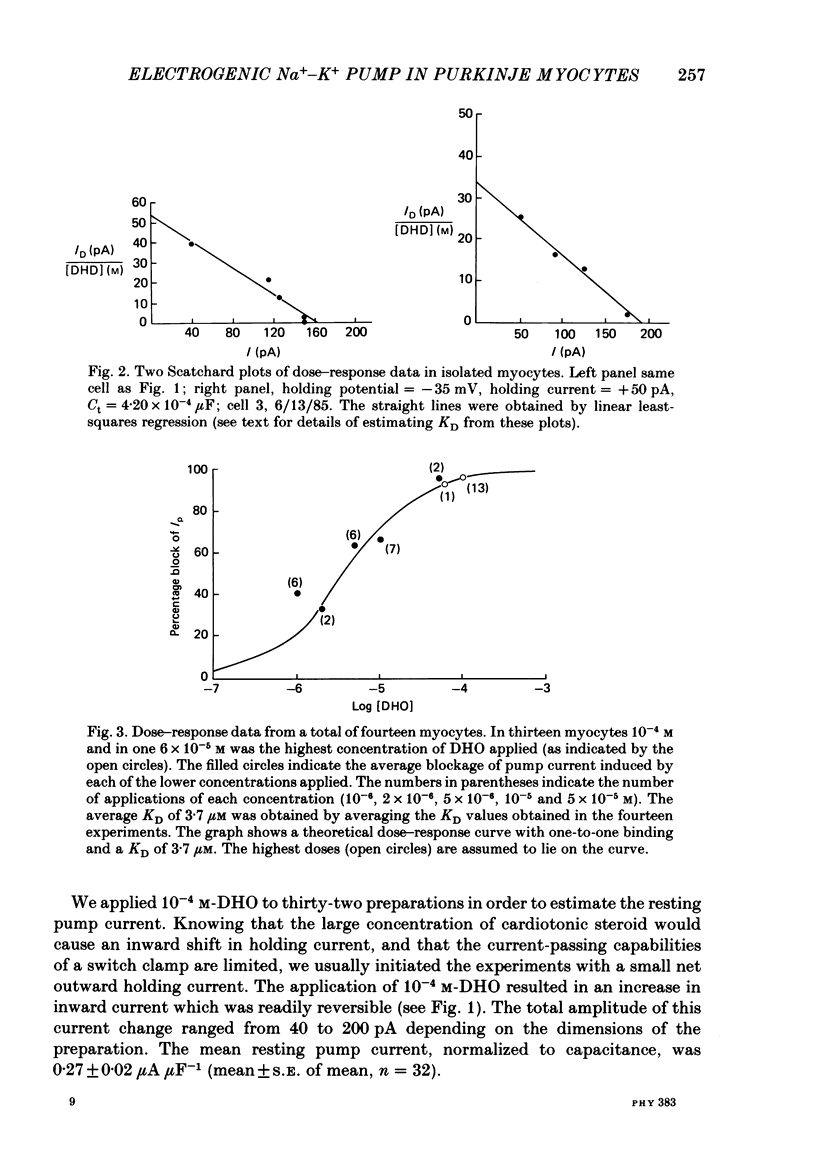
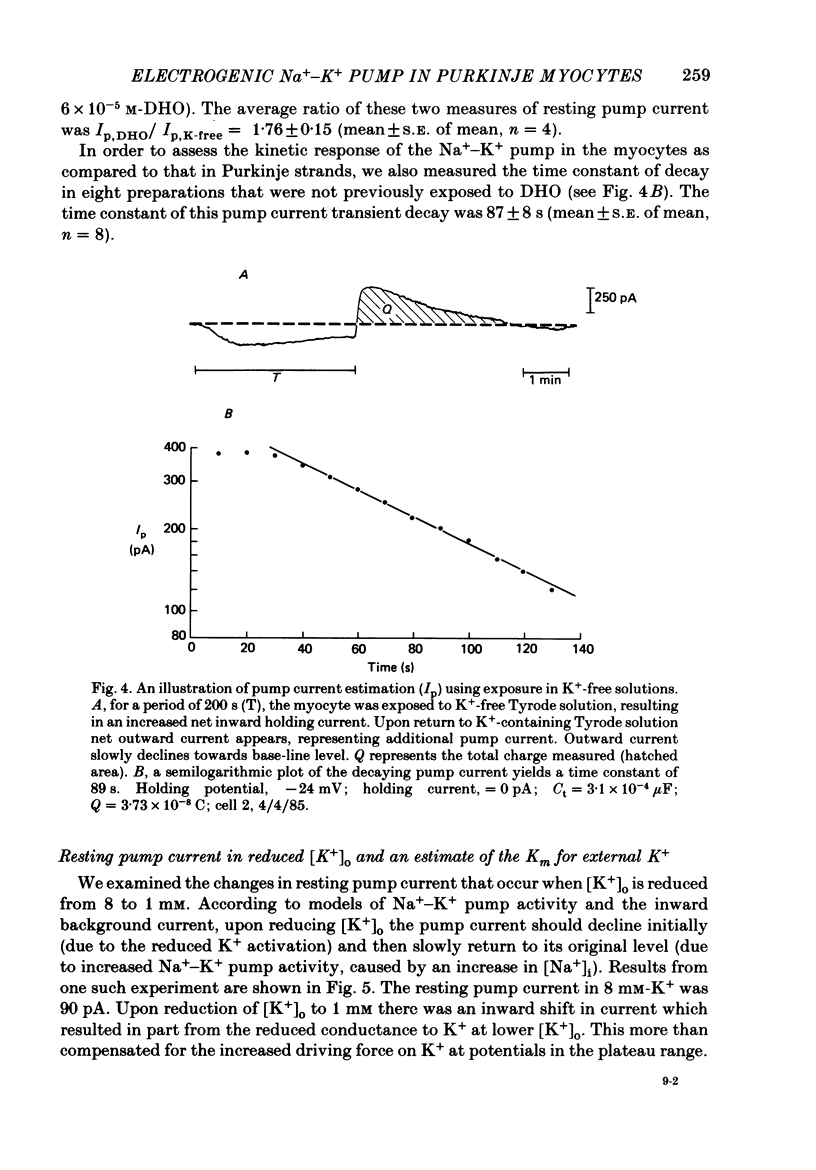
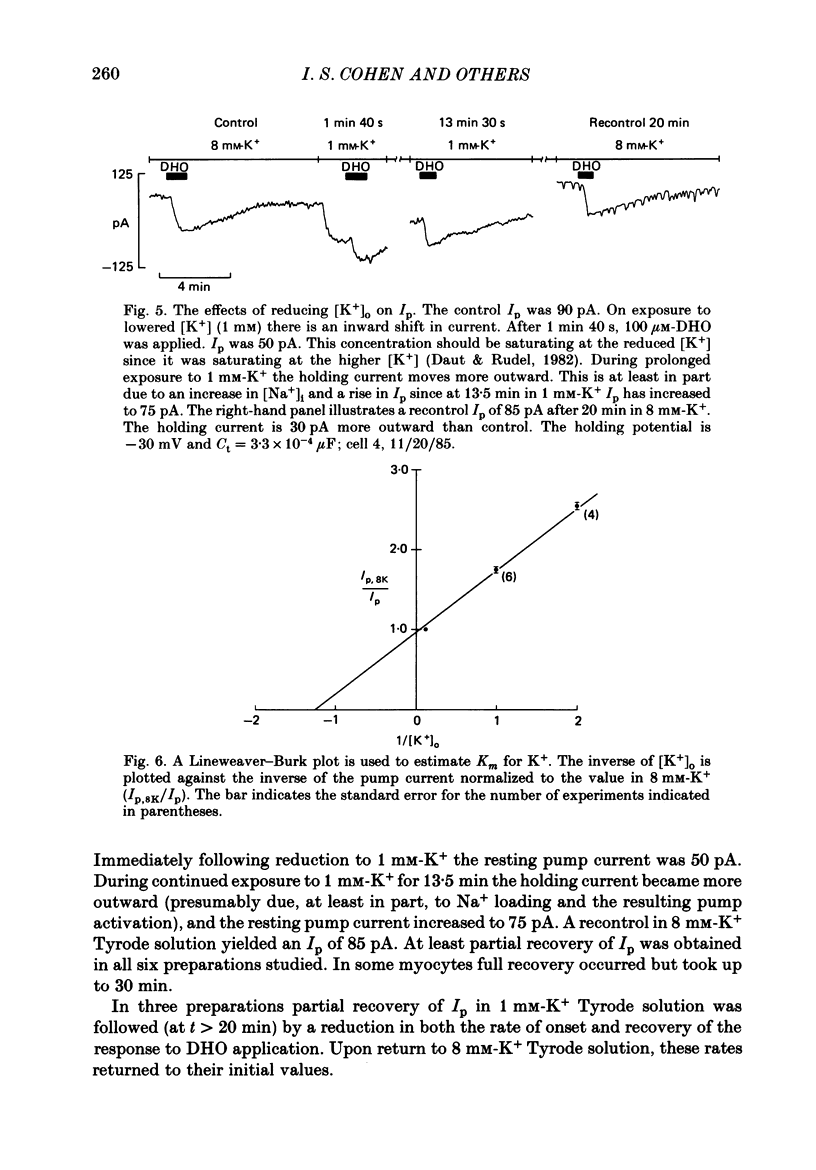
1. Purkinje myocytes were isolated from canine Purkinje strands by collagenase exposure and gentle trituration. The myocytes were studied by a switched single-micro-electrode voltage-clamp technique at 37 degrees C in Tyrode solution containing 8 mM-K+ and 2 mM-Ca2+. 2. The dose-response relation for the cardiotonic steroid dihydroouabain (DHO) was obtained by measuring the change in membrane current caused by application of concentrations of 1-100 microM. The KD obtained in fourteen experiments was 3.7 +/- 1.1 microM (mean +/- S.E. of mean). 3. We employed 100 microM-DHO (a concentration more than 25-fold greater than the KD) to estimate the resting pump current (Ip) in the isolated myocytes. A value of 0.27 +/- 0.02 microA microF-1 (mean +/- S.E. of mean, n = 32) was obtained. 4. Myocytes were also exposed to K+-free solution for a period of 200 s. On return to K+-containing Tyrode solution there was a slowly decaying outward current. The time constant of decay of this pump current transient was 87 +/- 8 s (mean +/- S.E. of mean, n = 8). The integral beneath this transient was used to obtain a second estimate of the resting pump current. In four preparations where exposures in DHO and in K+-free solutions were employed the ratio Ip, DHO/Ip, K-free was 1.76 +/- 0.15 (mean +/- S.E. of mean). 5. From the magnitude of resting pump current, in the presence of total pump blockade the Na+ activity should rise at a rate of 1.3 mM min-1. 6. Reducing [K+]o from 8 to 1 mM reduced Ip by more than 40% initially. Ip then slowly increased over the next 30 min. These results suggest that the steady-state inward background current is not greatly altered by changes in [K+]o, and that [Na+]i rises to a new level. The changes in Ip obtained at early times following reduction of [K+]o to 1 or 0.5 mM (t less than 1.75 min) were used to estimate the Km for external K+; a value of 0.8 mM was obtained. 7. The results suggest that the properties of the Na+-K+ pump in isolated canine Purkinje myocytes are similar to those in canine Purkinje strands. This argues against major distortions of measured pump properties in the canine Purkinje strand and for the physiological state of the Na+-K+ pump in the isolated Purkinje myocyte.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwell D., Eisner D., Cohen I. Voltage clamp and tracer flux data: effects of a restricted extra-cellular space. Q Rev Biophys. 1979 Aug;12(3):213–261. doi: 10.1017/s0033583500005448. [DOI] [PubMed] [Google Scholar]
- Bachmaier A., Ebner F., Reiter M. Potassium changes the relationship between receptor occupancy and the inotropic effect of cardiac glycosides in guinea-pig myocardium. Br J Pharmacol. 1985 Aug;85(4):755–765. doi: 10.1111/j.1476-5381.1985.tb11073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Hug E., Wagner G., Erdmann E. Comparison of ouabain receptors in sheep myocardium and Purkinje fibres. Biochem Pharmacol. 1985 Oct 15;34(20):3701–3710. doi: 10.1016/0006-2952(85)90234-5. [DOI] [PubMed] [Google Scholar]
- Callewaert G., Carmeliet E., Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. J Physiol. 1984 Apr;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. S., Falk R. T., Kline R. P. Voltage-clamp studies on the canine Purkinje strand. Proc R Soc Lond B Biol Sci. 1983 Jan 22;217(1207):215–236. doi: 10.1098/rspb.1983.0007. [DOI] [PubMed] [Google Scholar]
- Datyner N. B., Gintant G. A., Cohen I. S. Microprocessor controlled trituration device for the dissociation of cardiac and other tissues. Pflugers Arch. 1985 Jan;403(1):105–108. doi: 10.1007/BF00583289. [DOI] [PubMed] [Google Scholar]
- Datyner N. B., Gintant G. A., Cohen I. S. Versatile temperature controlled tissue bath for studies of isolated cells using an inverted microscope. Pflugers Arch. 1985 Mar;403(3):318–323. doi: 10.1007/BF00583607. [DOI] [PubMed] [Google Scholar]
- Daut J. Inhibition of the sodium pump in guinea-pig ventricular muscle by dihydro-ouabain: effects of external potassium and sodium. J Physiol. 1983 Jun;339:643–662. doi: 10.1113/jphysiol.1983.sp014740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Rüdel R. The electrogenic sodium pump in guinea-pig ventricular muscle: inhibition of pump current by cardiac glycosides. J Physiol. 1982 Sep;330:243–264. doi: 10.1113/jphysiol.1982.sp014339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. The passive electrical properties of guinea-pig ventricular muscle as examined with a voltage-clamp technique. J Physiol. 1982 Sep;330:221–242. doi: 10.1113/jphysiol.1982.sp014338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Noble D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos Trans R Soc Lond B Biol Sci. 1985 Jan 10;307(1133):353–398. doi: 10.1098/rstb.1985.0001. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The effects of rubidium ions and membrane potentials on the intracellular sodium activity of sheep Purkinje fibres. J Physiol. 1981 Aug;317:189–205. doi: 10.1113/jphysiol.1981.sp013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann E., Werdan K., Brown L. Evidence for two kinetically and functionally different types of cardiac glycoside receptors in the heart. Eur Heart J. 1984 Dec;5 (Suppl F):297–302. doi: 10.1093/eurheartj/5.suppl_f.297. [DOI] [PubMed] [Google Scholar]
- Falk R. T., Cohen I. S. Membrane current following activity in canine cardiac Purkinje fibers. J Gen Physiol. 1984 May;83(5):771–799. doi: 10.1085/jgp.83.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Beta-adrenoceptor agonists increase membrane K+ conductance in cardiac Purkinje fibres. Nature. 1983 Dec 15;306(5944):691–693. doi: 10.1038/306691a0. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Direct measurement of changes in sodium pump current in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1783–1787. doi: 10.1073/pnas.76.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. The Na/K pump of cardiac cells. Annu Rev Biophys Bioeng. 1984;13:373–398. doi: 10.1146/annurev.bb.13.060184.002105. [DOI] [PubMed] [Google Scholar]
- Gintant G. A., Datyner N. B., Cohen I. S. Gating of delayed rectification in acutely isolated canine cardiac Purkinje myocytes. Evidence for a single voltage-gated conductance. Biophys J. 1985 Dec;48(6):1059–1064. doi: 10.1016/S0006-3495(85)83869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Pusch H. Correlation between changes in membrane potential and intracellular sodium activity during K activated response in sheep Purkinje fibres. Pflugers Arch. 1980 Mar;384(2):189–191. doi: 10.1007/BF00584438. [DOI] [PubMed] [Google Scholar]
- Houser S. R., Freeman A. R. Volumetric properties of intracellular compartments in canine cardiac Purkinje cells. Am J Physiol. 1980 Apr;238(4):H561–H568. doi: 10.1152/ajpheart.1980.238.4.H561. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- January C. T., Fozzard H. A. The effects of membrane potential, extracellular potassium, and tetrodotoxin on the intracellular sodium ion activity of sheep cardiac muscle. Circ Res. 1984 Jun;54(6):652–665. doi: 10.1161/01.res.54.6.652. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Kazazoglou T., Renaud J. F., Rossi B. Digitalis receptors in cardiac cells and their relation with positive inotropic and cardiotoxic effects. Eur Heart J. 1984 Dec;5 (Suppl F):281–290. doi: 10.1093/eurheartj/5.suppl_f.281. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel F., Godfraind T. Heterogeneity of ouabain specific binding sites and (Na+ + K+)-ATPase inhibition in microsomes from rat heart. Biochem Pharmacol. 1984 Jan 1;33(1):47–53. doi: 10.1016/0006-2952(84)90369-1. [DOI] [PubMed] [Google Scholar]
- Repke K. R., Herrmann I., Portius H. J. Interaction of cardiac glycosides and Na,K-ATPase is regulated by effector-controlled equilibrium between two limit enzyme conformers. Biochem Pharmacol. 1984 Jul 1;33(13):2089–2099. doi: 10.1016/0006-2952(84)90578-1. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Somberg J. C., Barry W. H., Smith T. W. Differing sensitivities of Purkinje fibers and myocardium to inhibition of monovalent cation transport by digitalis. J Clin Invest. 1981 Jan;67(1):116–123. doi: 10.1172/JCI110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweadner K. J. Enzymatic properties of separated isozymes of the Na,K-ATPase. Substrate affinities, kinetic cooperativity, and ion transport stoichiometry. J Biol Chem. 1985 Sep 25;260(21):11508–11513. [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- VASSALLE M., KARIS J., HOFFMAN B. F. Toxic effects of ouabain on Purkinje fibers and ventricular muscle fibers. Am J Physiol. 1962 Sep;203:433–439. doi: 10.1152/ajplegacy.1962.203.3.433. [DOI] [PubMed] [Google Scholar]


