Abstract
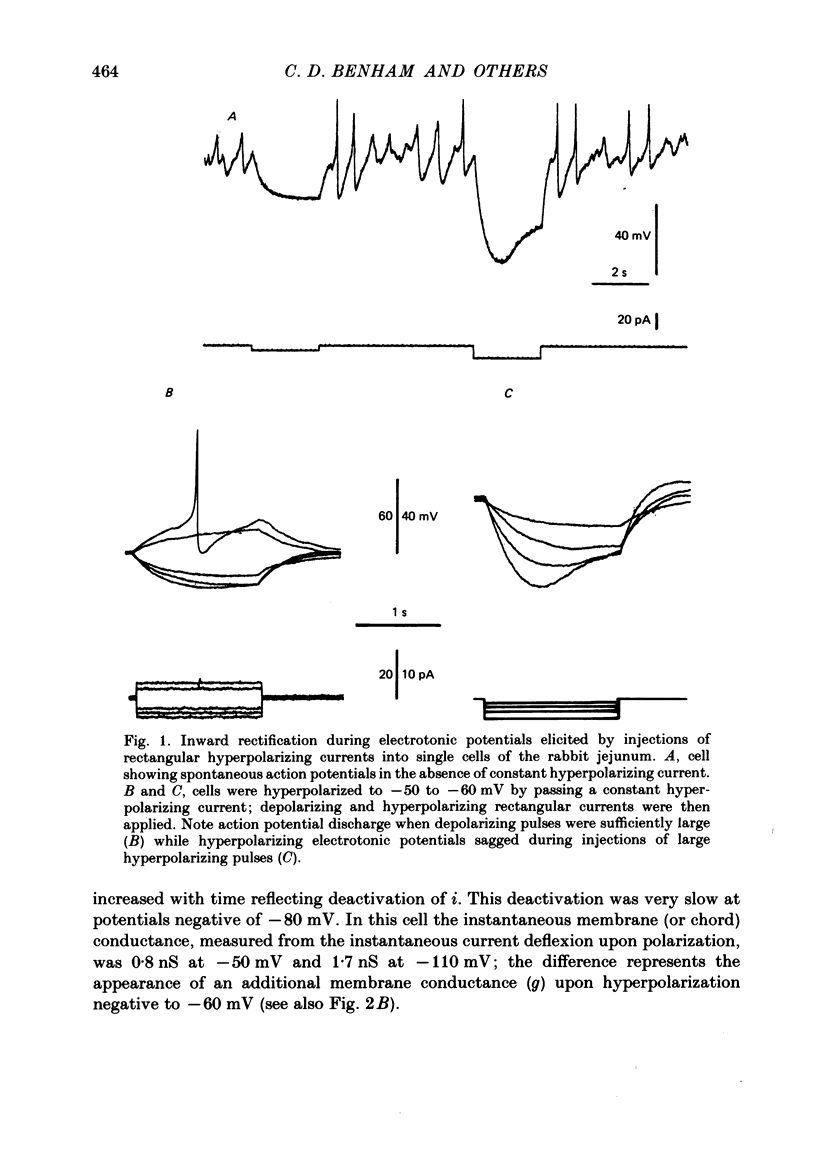
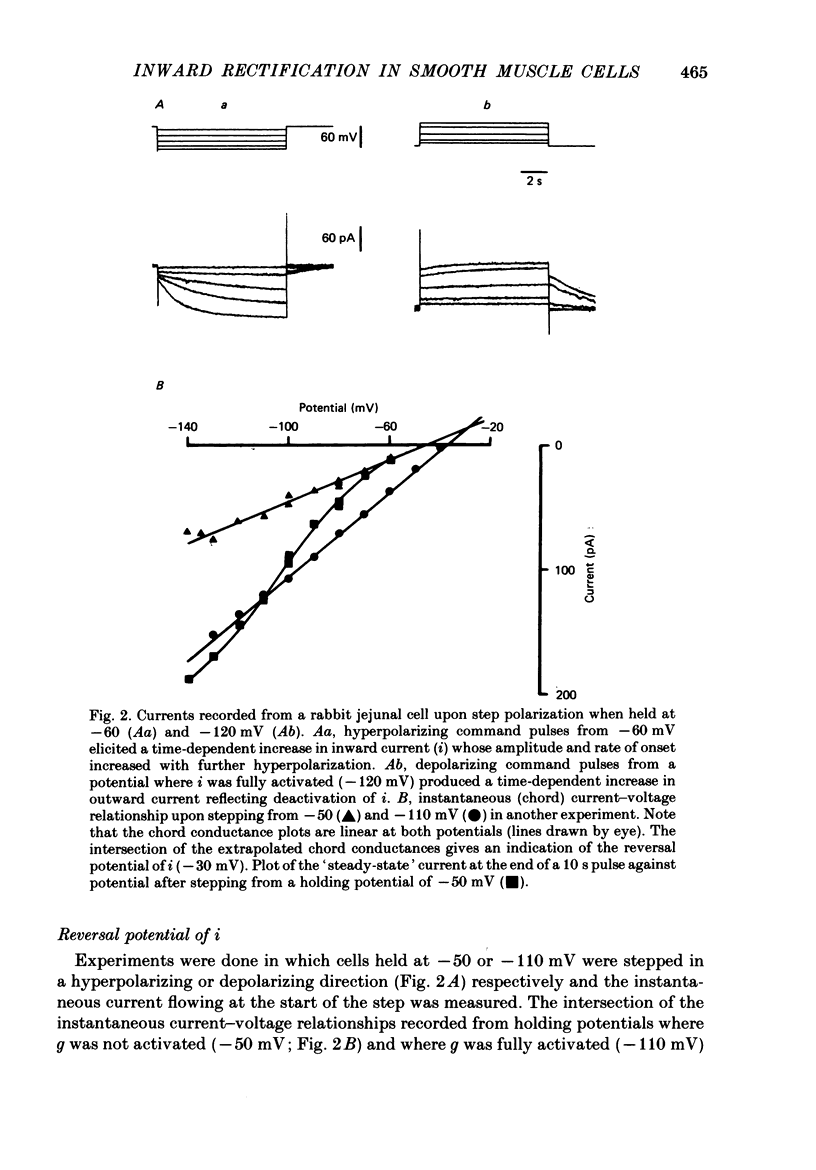
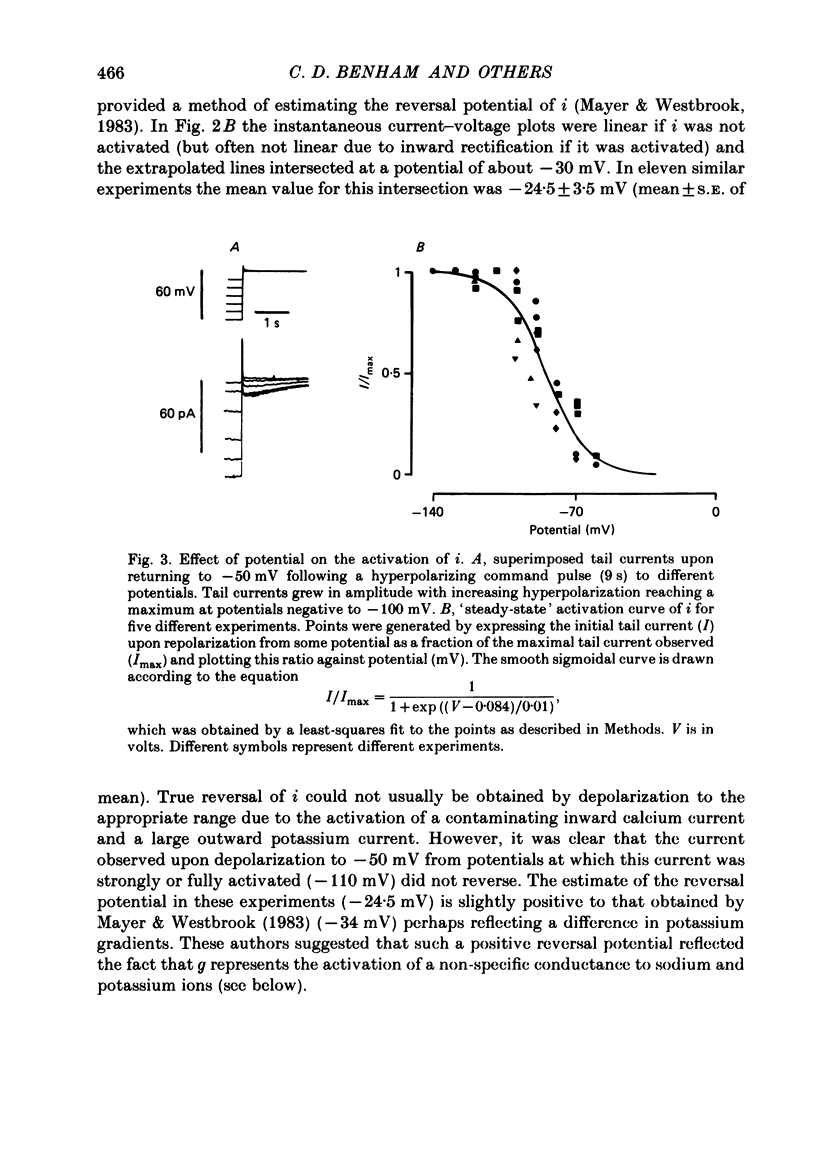
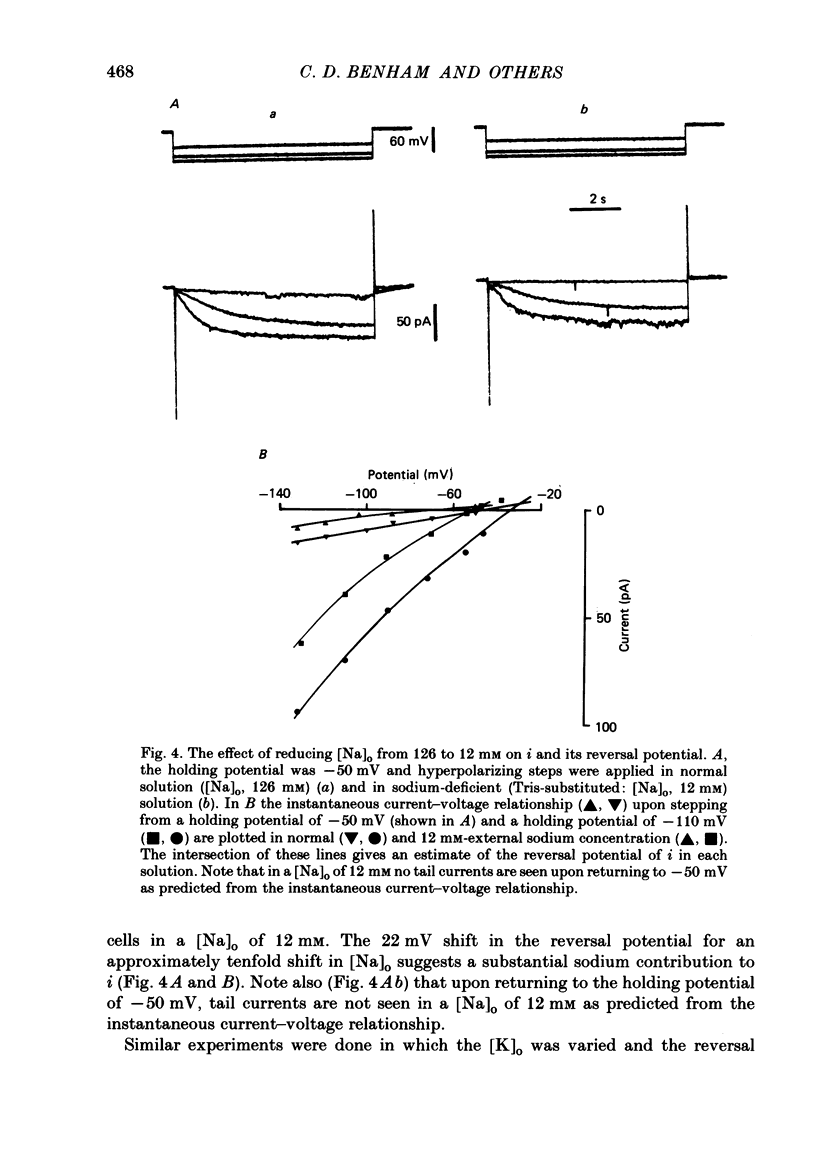
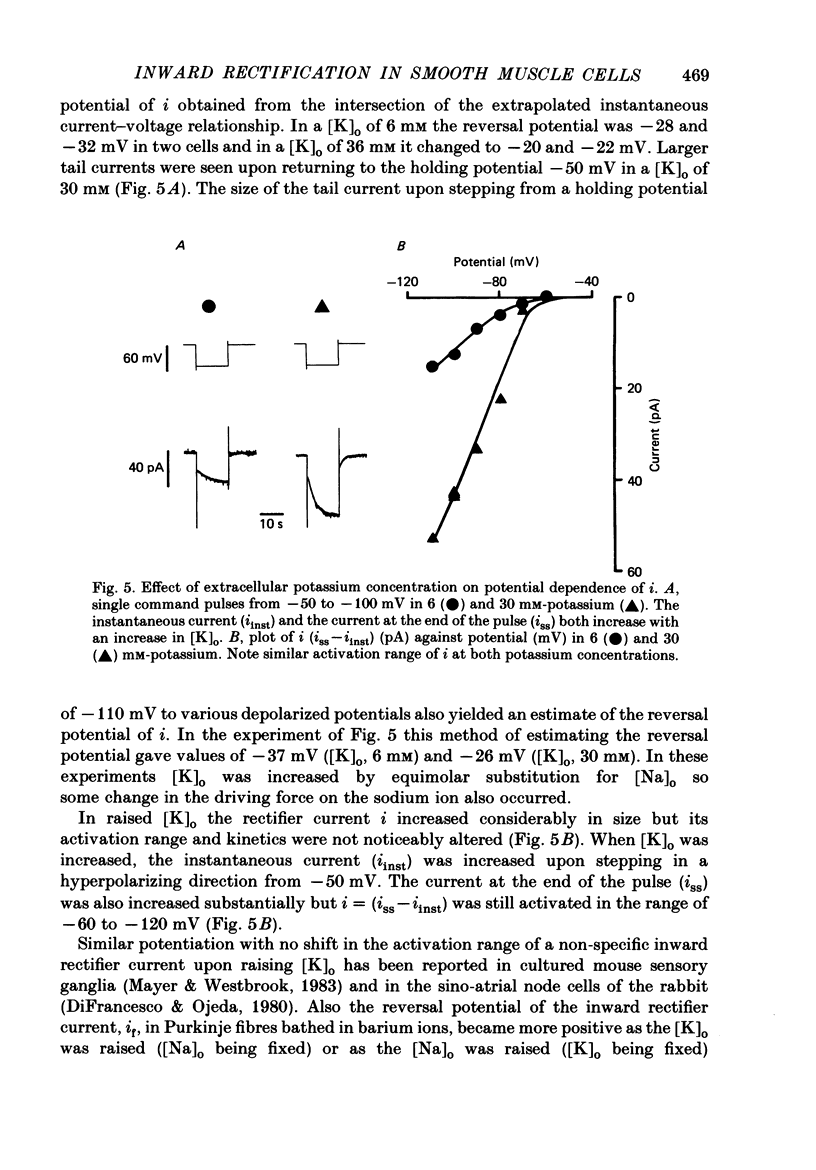
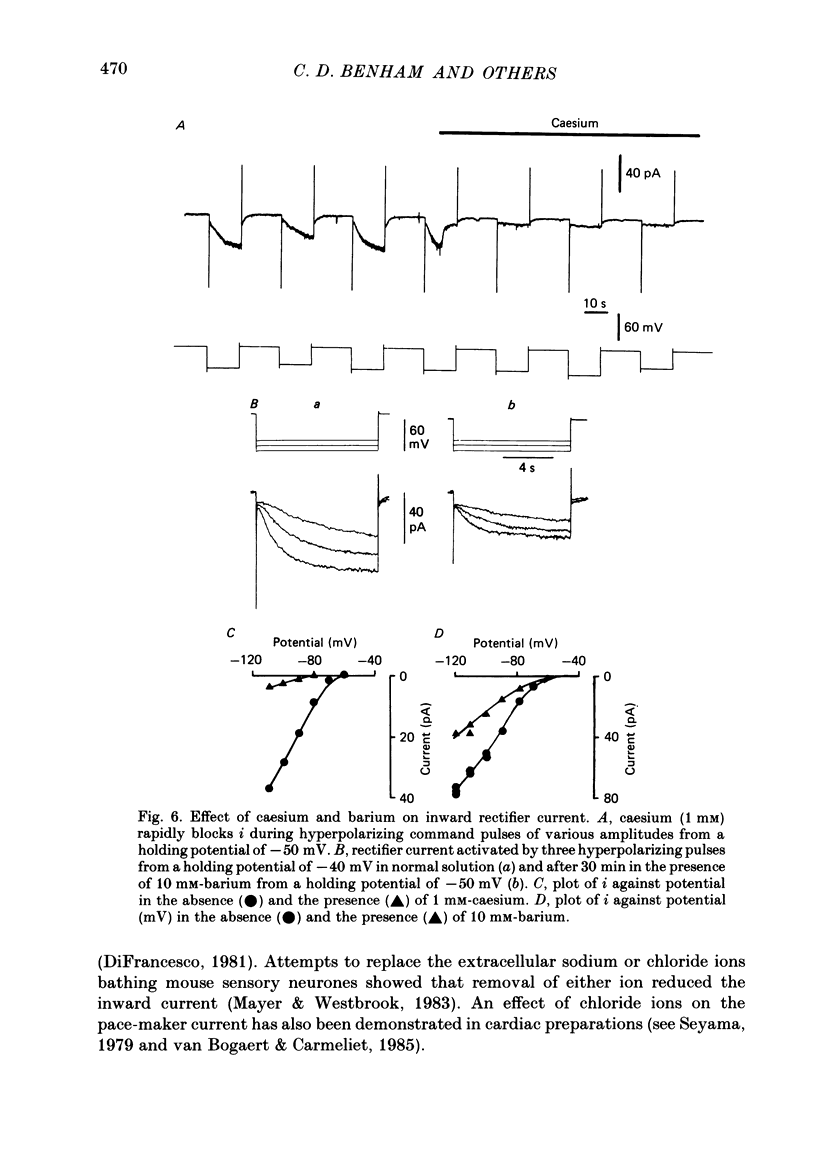
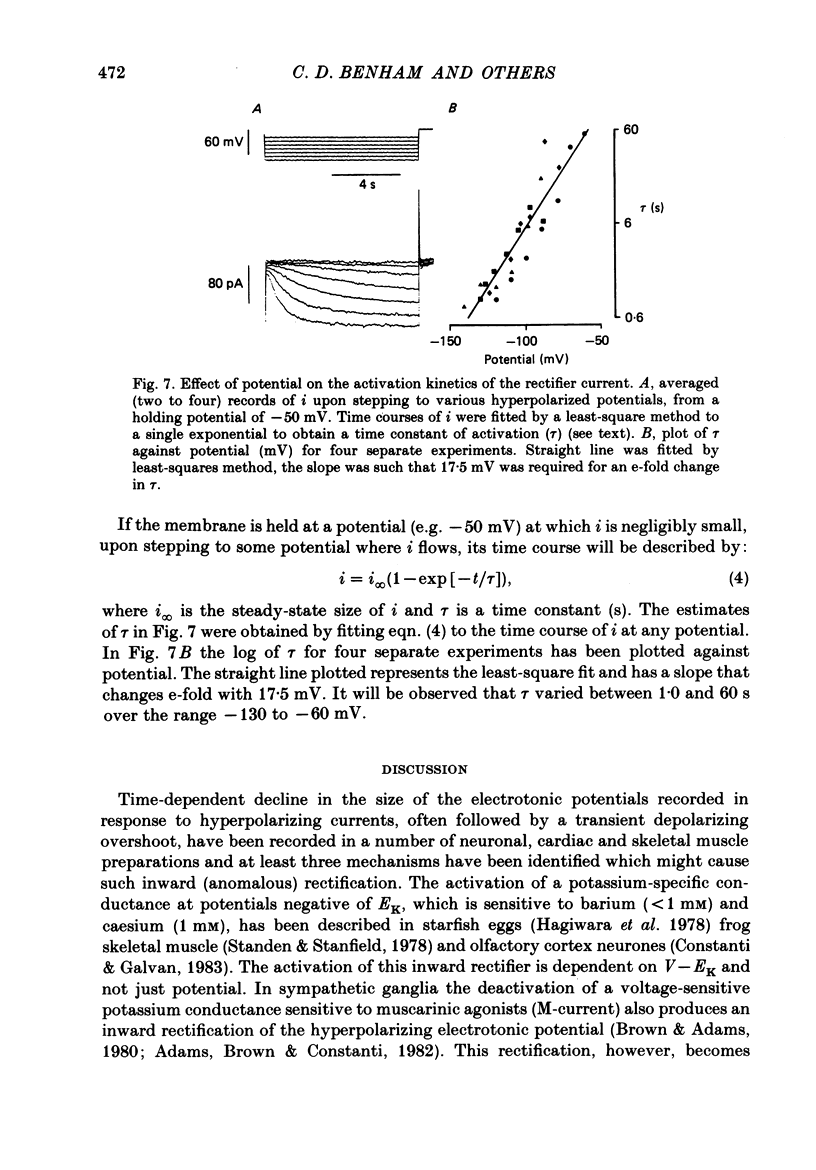
1. Single smooth muscle cells were obtained by collagenase digestion of longitudinal muscle of rabbit jejunum. Membrane potential or current under voltage clamp was recorded by patch-clamp technique in the whole-cell recording mode at 22-25 degrees C. 2. At a membrane potential of -50 mV small hyperpolarizing current pulses produced electrotonic potentials which asymptotically approached a steady-state size and did not 'sag'. Stronger hyperpolarization resulted in a 'sag' of the electrotonic potential. Under voltage clamp an inward current, i, was activated in the range -60 to -110 mV. 3. This current had an equilibrium potential of -24.5 +/- 3.5 mV which was shifted negatively by reducing the sodium concentration, and positively by raising the potassium concentration of the bathing solution. 4. This current was blocked by caesium (1 mM) but less affected by barium ions up to 10 mM. 5. The time course of i upon stepping into its activation range was accelerated by increasing negativity and following an initial short delay could be described by a single exponential with a time constant in the range 60 s(-60 mV) to 1 s(-130 mV). 6. It is concluded that these jejunal cells possess a current to which sodium and potassium ions make a contribution which is responsible for the inward rectification they show upon hyperpolarization. This current is activated in a range which would allow it to contribute to the slow potential changes shown by longitudinal jejunal muscle.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Bennett M. R. Rebound excitation of the smooth muscle cells of the guinea-pig taenia coli after stimulation of intramural inhibitory nerves. J Physiol. 1966 Jul;185(1):124–131. doi: 10.1113/jphysiol.1966.sp007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T. Nerve-mediated excitation and inhibition of the smooth muscle cells of the avian gizzard. J Physiol. 1969 Oct;204(3):669–686. doi: 10.1113/jphysiol.1969.sp008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Clark J. P., Kitamura K., Lang R. J. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981 Nov;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T., Benham C. D. Patch and whole-cell voltage clamp of single mammalian visceral and vascular smooth muscle cells. Experientia. 1985 Jul 15;41(7):887–894. doi: 10.1007/BF01970006. [DOI] [PubMed] [Google Scholar]
- Bolton T. B. The depolarizing action of acetylcholine or carbachol in intestinal smooth muscle. J Physiol. 1972 Feb;220(3):647–671. doi: 10.1113/jphysiol.1972.sp009728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Properties of the inhibitory potential of smooth muscle as observed in the response to field stimulation of the guinea-pig taenia coli. J Physiol. 1967 Apr;189(2):299–315. doi: 10.1113/jphysiol.1967.sp008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Carmeliet E., Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. J Physiol. 1984 Apr;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. Nerve-mediated excitation of the taenia of the guinea-pig caecum. J Physiol. 1966 Jul;185(1):148–159. doi: 10.1113/jphysiol.1966.sp007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Galvan M. Fast inward-rectifying current accounts for anomalous rectification in olfactory cortex neurones. J Physiol. 1983 Feb;335:153–178. doi: 10.1113/jphysiol.1983.sp014526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E., Helmy-Elkholy A., Jager L. P., Kannan M. S. Neither a purine nor VIP is the mediator of inhibitory nerves of opossum oesophageal smooth muscle. J Physiol. 1983 Mar;336:243–260. doi: 10.1113/jphysiol.1983.sp014579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ojeda C. Properties of the current if in the sino-atrial node of the rabbit compared with those of the current iK, in Purkinje fibres. J Physiol. 1980 Nov;308:353–367. doi: 10.1113/jphysiol.1980.sp013475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy, Daniel E. E. The electrophysiological basis of the motor inhibitory effect of adrenaline on rabbit small intestinal smooth muscle. Can J Physiol Pharmacol. 1976 Aug;54(4):446–456. doi: 10.1139/y76-064. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Weinrich J. P. The effects of calcium and magnesium on inhibitory junctional transmission in smooth muscle of guinea pig small intestine. Pflugers Arch. 1975 Oct 28;360(2):109–119. doi: 10.1007/BF00580534. [DOI] [PubMed] [Google Scholar]
- Kitamura K. Comparative aspects of membrane properties and innervation of longitudinal and circular muscle layers of rabbit jejunum. Jpn J Physiol. 1978;28(5):583–601. doi: 10.2170/jjphysiol.28.583. [DOI] [PubMed] [Google Scholar]
- Kurachi Y. Voltage-dependent activation of the inward-rectifier potassium channel in the ventricular cell membrane of guinea-pig heart. J Physiol. 1985 Sep;366:365–385. doi: 10.1113/jphysiol.1985.sp015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niel J. P., Bywater R. A., Taylor G. S. Effect of substance P on non-cholinergic fast and slow post-stimulus depolarization in the guinea-pig ileum. J Auton Nerv Syst. 1983 Dec;9(4):573–584. doi: 10.1016/0165-1838(83)90114-5. [DOI] [PubMed] [Google Scholar]
- Publicover N. G., Sanders K. M. A technique to locate the pacemaker in smooth muscles. J Appl Physiol Respir Environ Exerc Physiol. 1984 Nov;57(5):1586–1590. doi: 10.1152/jappl.1984.57.5.1586. [DOI] [PubMed] [Google Scholar]
- Seyama I. Characteristics of the anion channel in the sino-atrial node cell of the rabbit. J Physiol. 1979 Sep;294:447–460. doi: 10.1113/jphysiol.1979.sp012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims S. M., Singer J. J., Walsh J. V., Jr Cholinergic agonists suppress a potassium current in freshly dissociated smooth muscle cells of the toad. J Physiol. 1985 Oct;367:503–529. doi: 10.1113/jphysiol.1985.sp015837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. V., Jr, Singer J. J. Voltage clamp of single freshly dissociated smooth muscle cells: current-voltage relationships for three currents. Pflugers Arch. 1981 May;390(2):207–210. doi: 10.1007/BF00590209. [DOI] [PubMed] [Google Scholar]


