Abstract
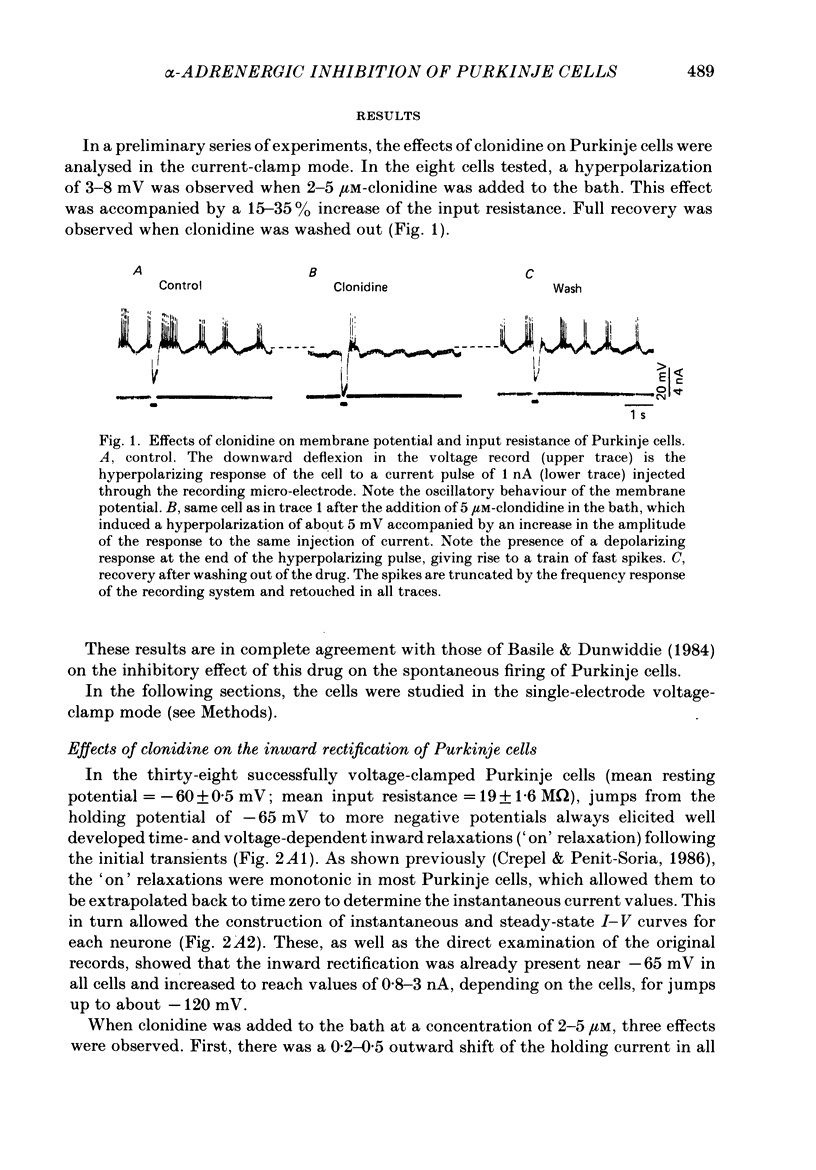
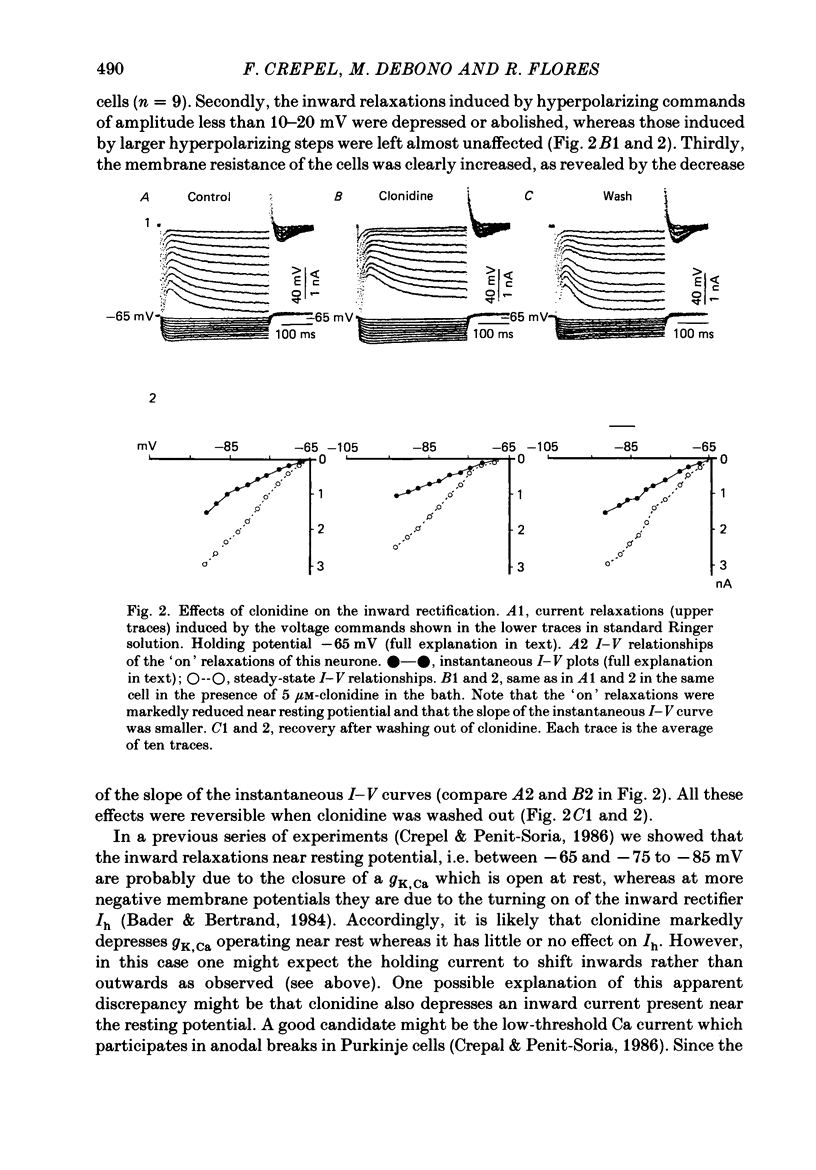
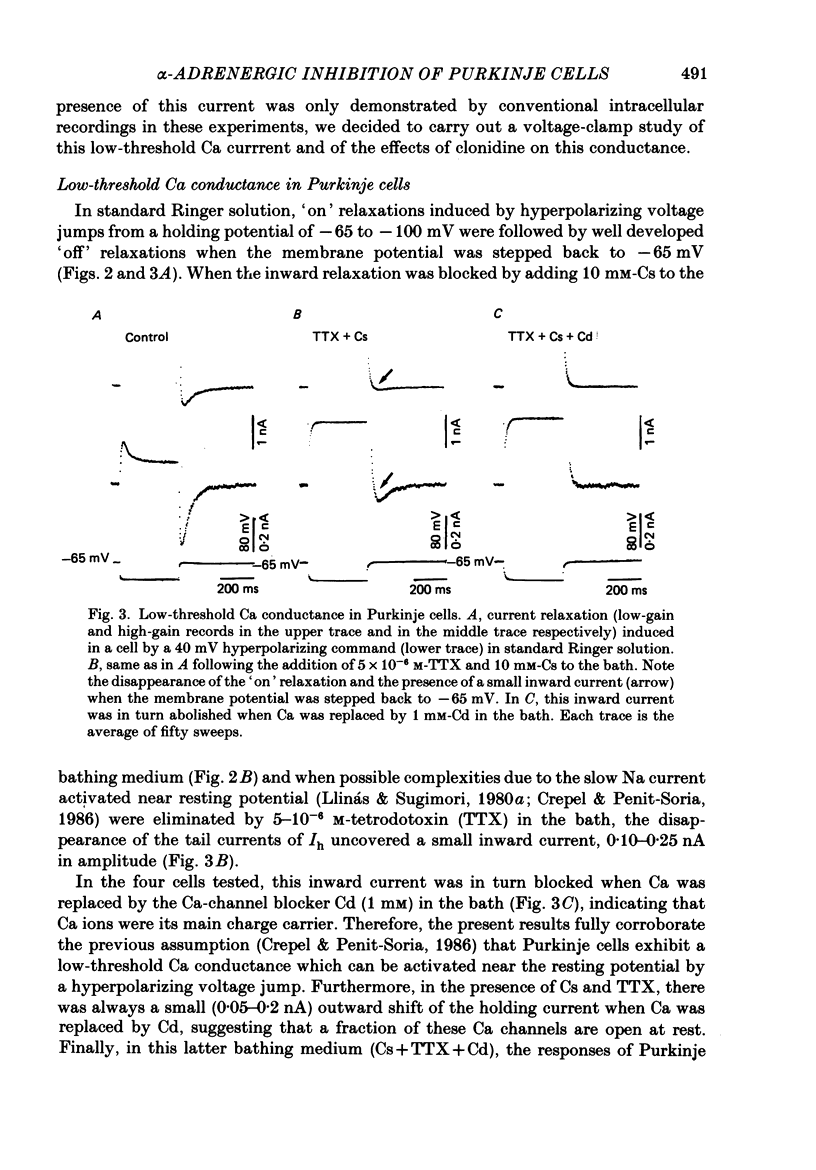
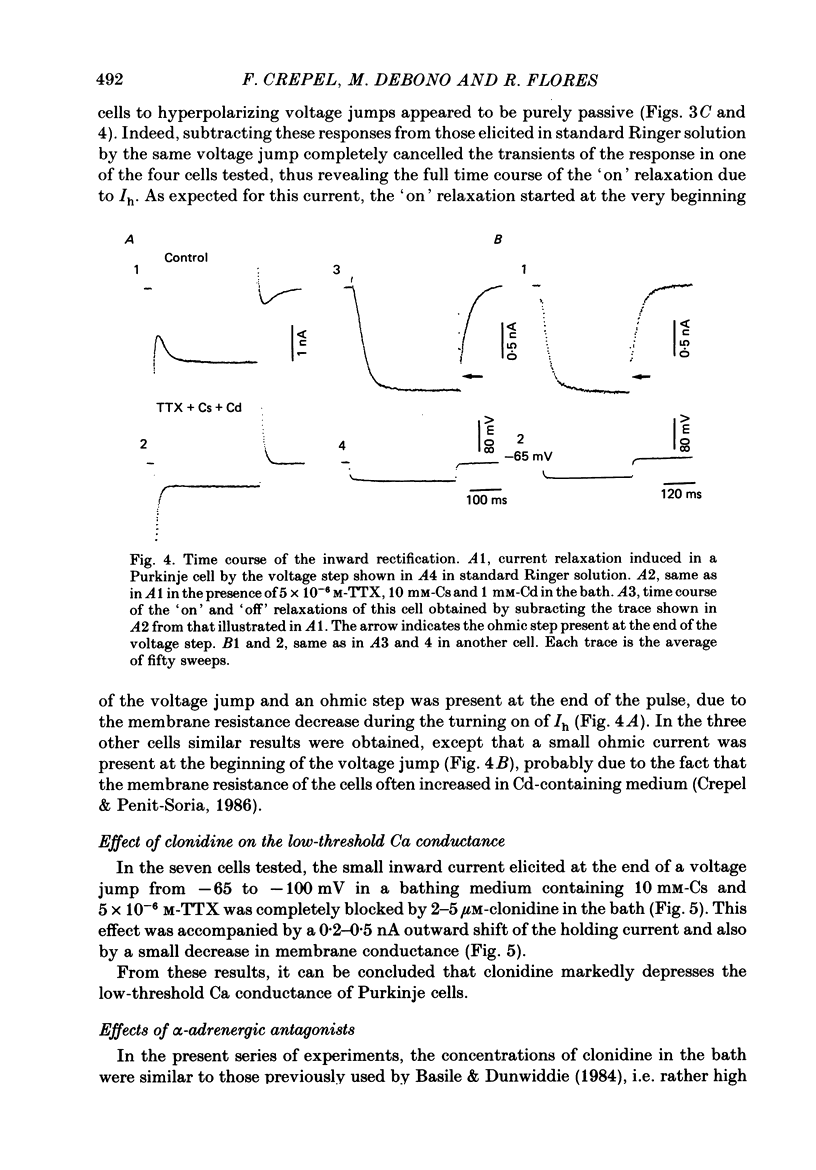
1. The effects of the alpha 2-adrenergic agonist clonidine on the membrane properties of Purkinje cells were analysed in sagittal slices of adult rat cerebellum by the use of intracellular recordings performed at a somatic level in the single-electrode voltage-clamp mode. 2. In preliminary current-clamp experiments, clonidine elicited in all cells a hyperpolarization 3-8 mV in amplitude, accompanied by a 15-35% increase of the input resistance when it was added to the bath at a concentration of 2-5 microM. 3. In voltage-clamped cells at a potential of -65 mV. the same concentration of clonidine always induced an outward shift of the holding current (0.2-0.5 nA in amplitude), thus corresponding to the hyperpolarization seen in current-clamp experiments, and this effect was accompanied by a clear increase of membrane resistance. Furthermore, clonidine markedly depressed the inward relaxations induced by hyperpolarizing commands of amplitude less than 10-20 mV whereas those induced by larger steps were much less affected. All these effects of clonidine were reversible when the drug was washed out. 4. When the slices were bathed in a medium containing 10 mM-Cs and 5 X 10(-6) M-tetrodotoxin, the inward relaxations induced by hyperpolarizing steps were abolished. However, a small inward current was still present when the membrane potential was stepped back to -65 mV, which was in turn blocked by the Ca-channel blocker Cd. This inward Ca current was also blocked by 2-5 microM-clonidine in the bath. 5. All these effects of clonidine were abolished by the alpha 1-adrenergic antagonists prazosin and phentolamine at concentrations of 0.5 and 40 microM respectively in the bath. In contrast, they were only weakly antagonized or unaffected by 2 microM of the alpha 2-adrenergic antagonist yohimbine. 6. On the basis of these results and of a previous work on the ionic basis of the inward rectification of Purkinje cells (Crepel & Penit-Soria, 1986), it appears that these neurones exhibit a well developed alpha (possibly alpha 1)-adrenergic inhibition of a low-threshold Ca conductance and a Ca-dependent K conductance operating near resting potential.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K. Modulation of a transient outward current in serotonergic neurones by alpha 1-adrenoceptors. Nature. 1985 Jun 6;315(6019):501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- Akasu T., Gallagher J. P., Nakamura T., Shinnick-Gallagher P., Yoshimura M. Noradrenaline hyperpolarization and depolarization in cat vesical parasympathetic neurones. J Physiol. 1985 Apr;361:165–184. doi: 10.1113/jphysiol.1985.sp015639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Bertrand D. Effect of changes in intra- and extracellular sodium on the inward (anomalous) rectification in salamander photoreceptors. J Physiol. 1984 Feb;347:611–631. doi: 10.1113/jphysiol.1984.sp015086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile A. S., Dunwiddie T. V. Norepinephrine elicits both excitatory and inhibitory responses from Purkinje cells in the in vitro rat cerebellar slice. Brain Res. 1984 Mar 26;296(1):15–25. doi: 10.1016/0006-8993(84)90507-9. [DOI] [PubMed] [Google Scholar]
- Crepel F., Delhaye-Bouchaud N. Distribution of climbing fibres on cerebellar Purkinje cells in X-irradiated rats. An electrophysiological study. J Physiol. 1979 May;290(2):97–112. doi: 10.1113/jphysiol.1979.sp012762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Penit-Soria J. Inward rectification and low threshold calcium conductance in rat cerebellar Purkinje cells. An in vitro study. J Physiol. 1986 Mar;372:1–23. doi: 10.1113/jphysiol.1986.sp015993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978 Dec 21;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
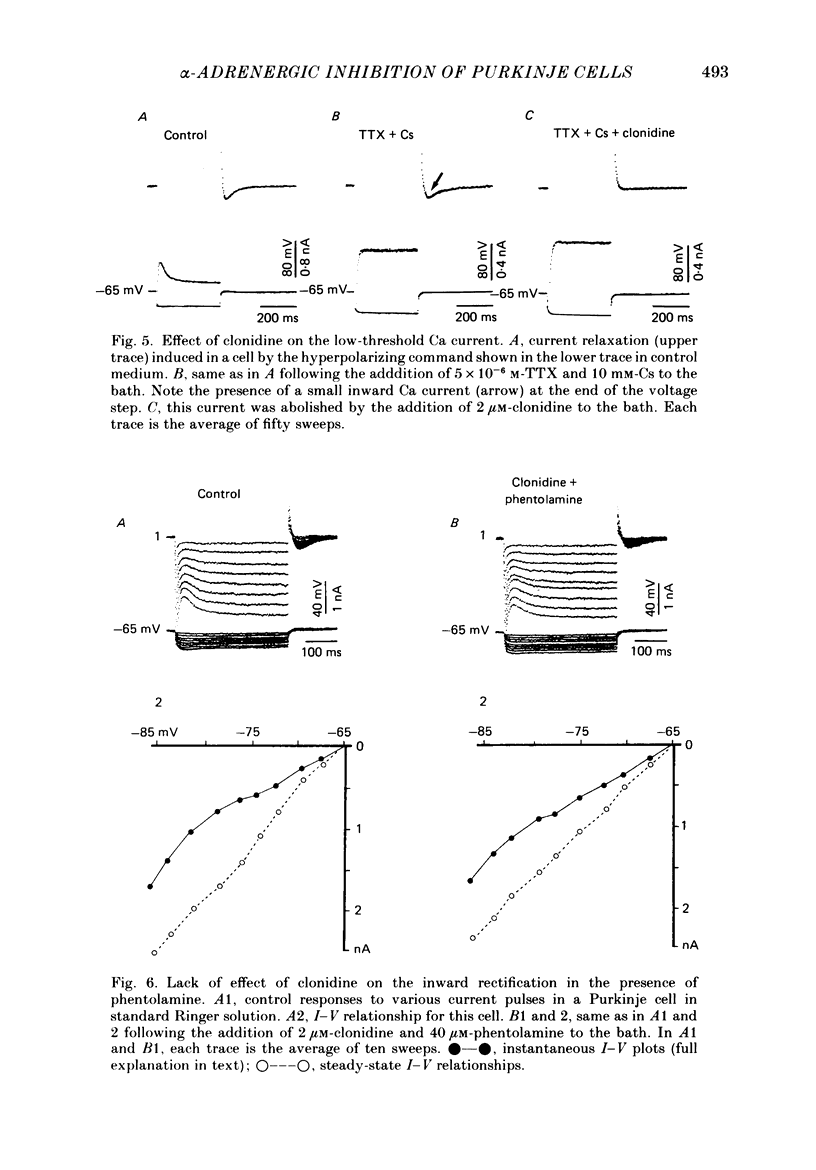
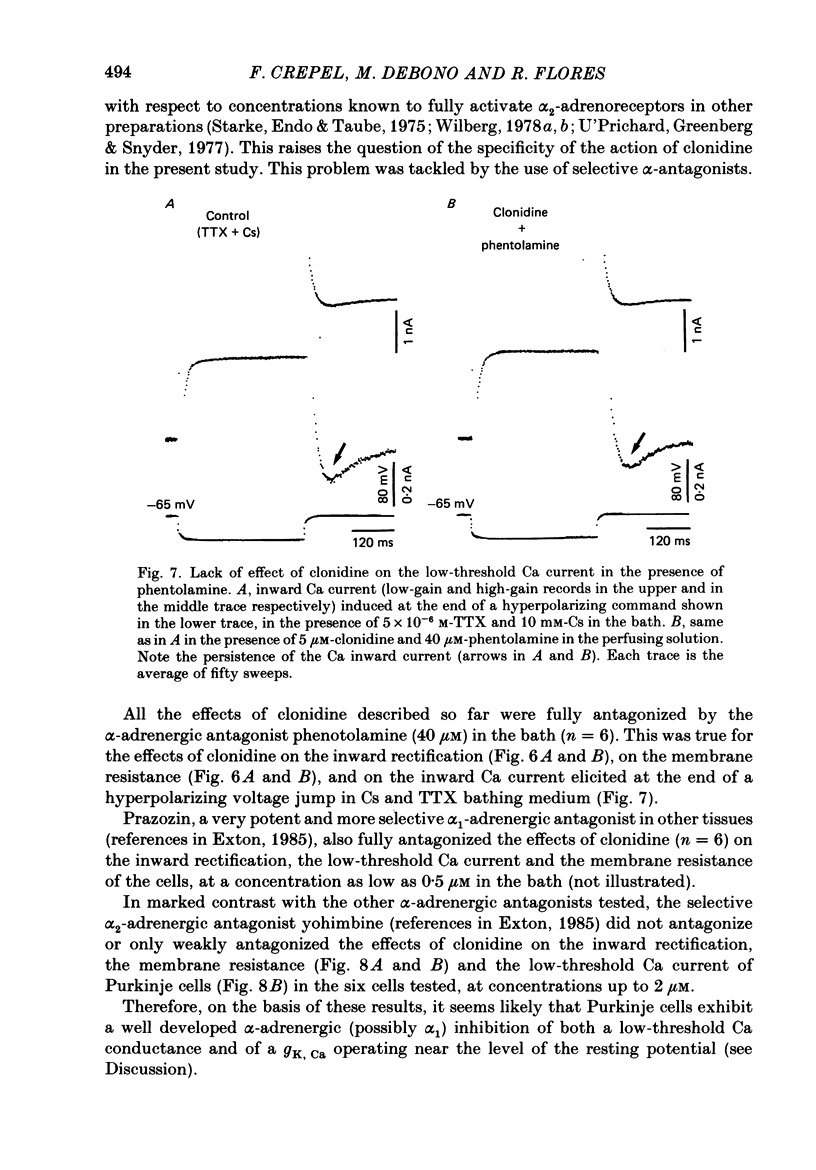
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- Galvan M., Adams P. R. Control of calcium current in rat sympathetic neurons by norepinephrine. Brain Res. 1982 Jul 22;244(1):135–144. doi: 10.1016/0006-8993(82)90911-8. [DOI] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
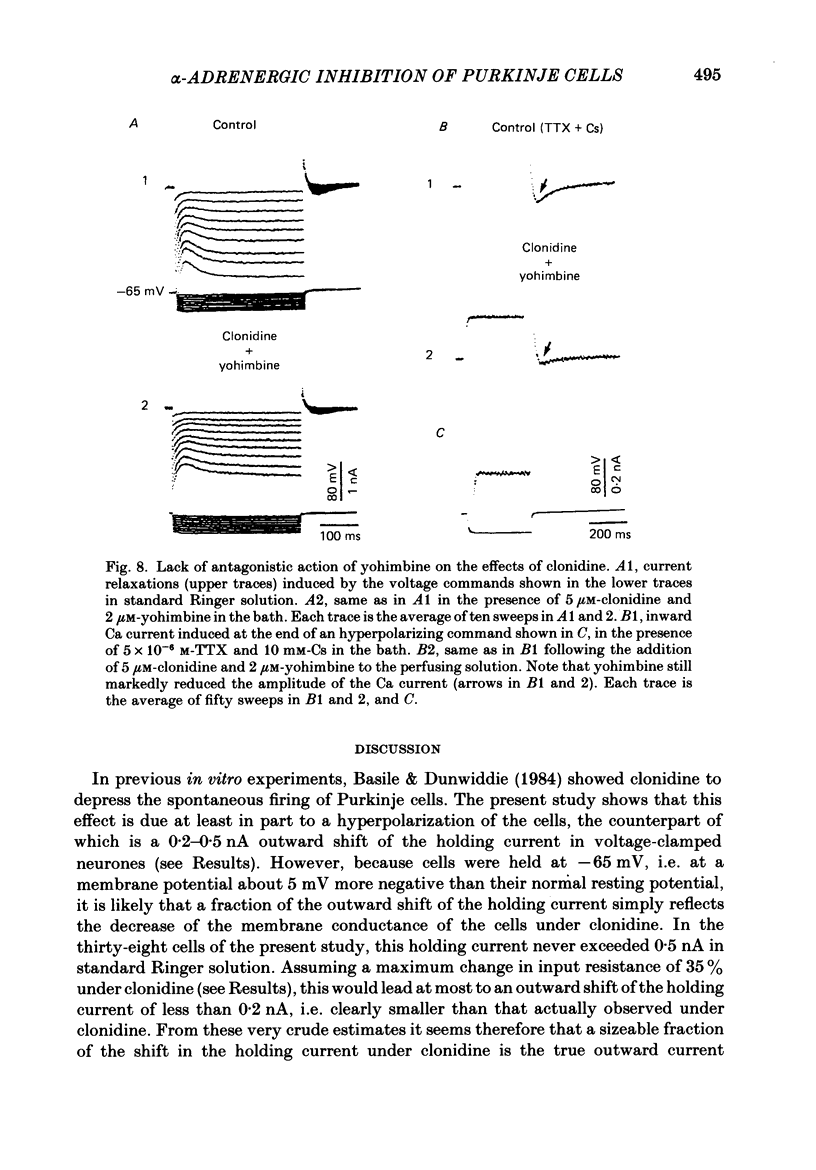
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Brown T. H. Interpretation of voltage-clamp measurements in hippocampal neurons. J Neurophysiol. 1983 Aug;50(2):464–486. doi: 10.1152/jn.1983.50.2.464. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Phillis J. W. Stimulation of cerebral cortical synaptosomal Na-K-ATPase by biogenic amines. Can J Physiol Pharmacol. 1977 Aug;55(4):961–964. doi: 10.1139/y77-130. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984 Apr;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry B. S., Phillis J. W. Antagonism of biogenic amine-induced depression of cerebral cortical neurones by Na+, K+-ATPase in inhibitors. Can J Physiol Pharmacol. 1977 Apr;55(2):170–179. doi: 10.1139/y77-025. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of norepinephrine in the rat hippocampus: intracellular studies in the slice preparation. Brain Res. 1981 Feb 9;206(1):107–128. doi: 10.1016/0006-8993(81)90104-9. [DOI] [PubMed] [Google Scholar]
- Starke K., Endo T., Taube H. D. Relative pre- and postsynaptic potencies of alpha-adrenoceptor agonists in the rabbit pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(1):55–78. doi: 10.1007/BF00510821. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Greenberg D. A., Snyder S. H. Binding characteristics of a radiolabeled agonist and antagonist at central nervous system alpha noradrenergic receptors. Mol Pharmacol. 1977 May;13(3):454–473. [PubMed] [Google Scholar]
- Wikberg J. E. Pharmacological classification of adrenergic alpha receptors in the guinea pig. Nature. 1978 May 11;273(5658):164–166. doi: 10.1038/273164a0. [DOI] [PubMed] [Google Scholar]
- Williams J. T., Henderson G., North R. A. Characterization of alpha 2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. Neuroscience. 1985 Jan;14(1):95–101. doi: 10.1016/0306-4522(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]


