Abstract
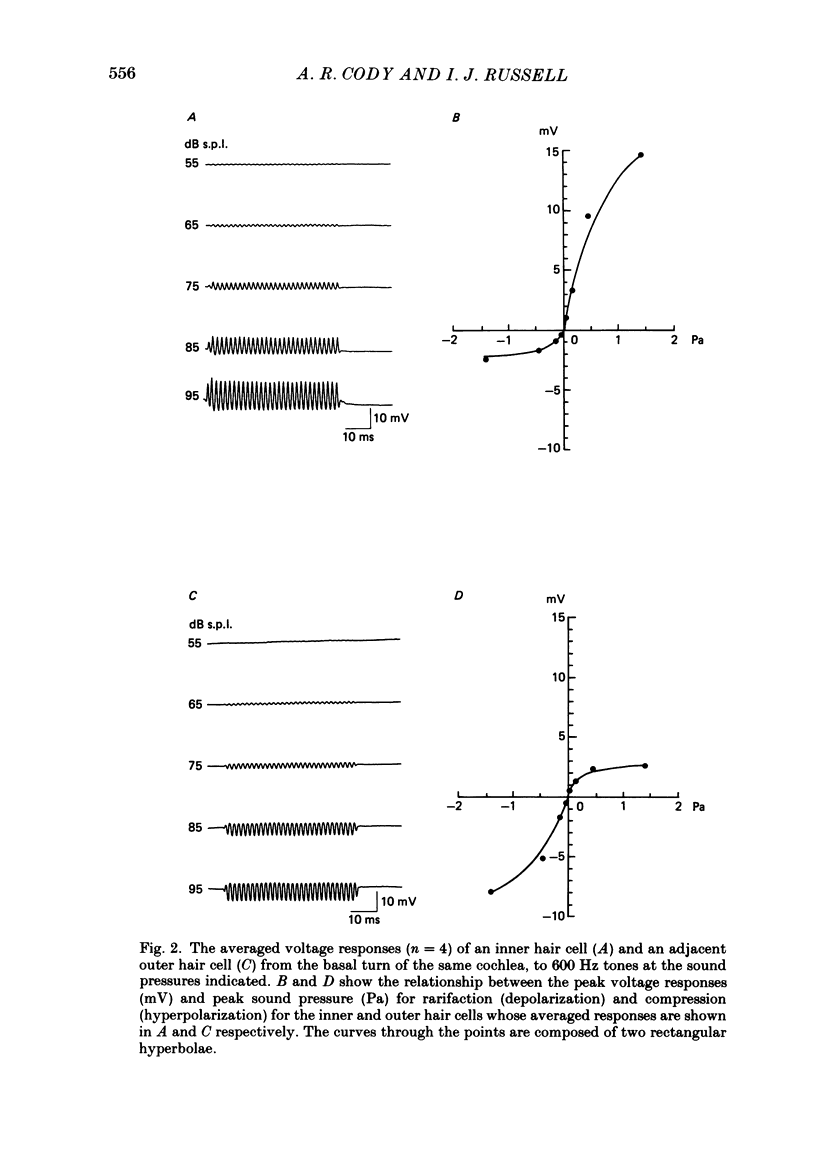
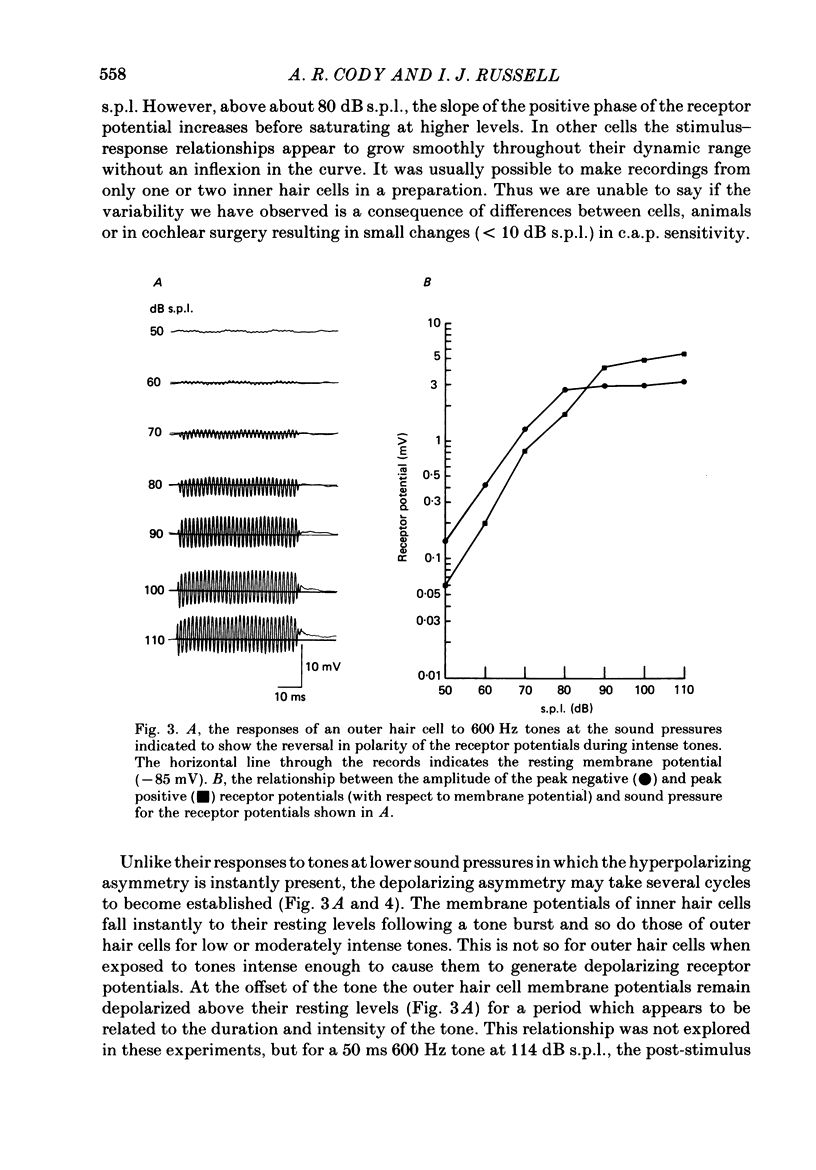
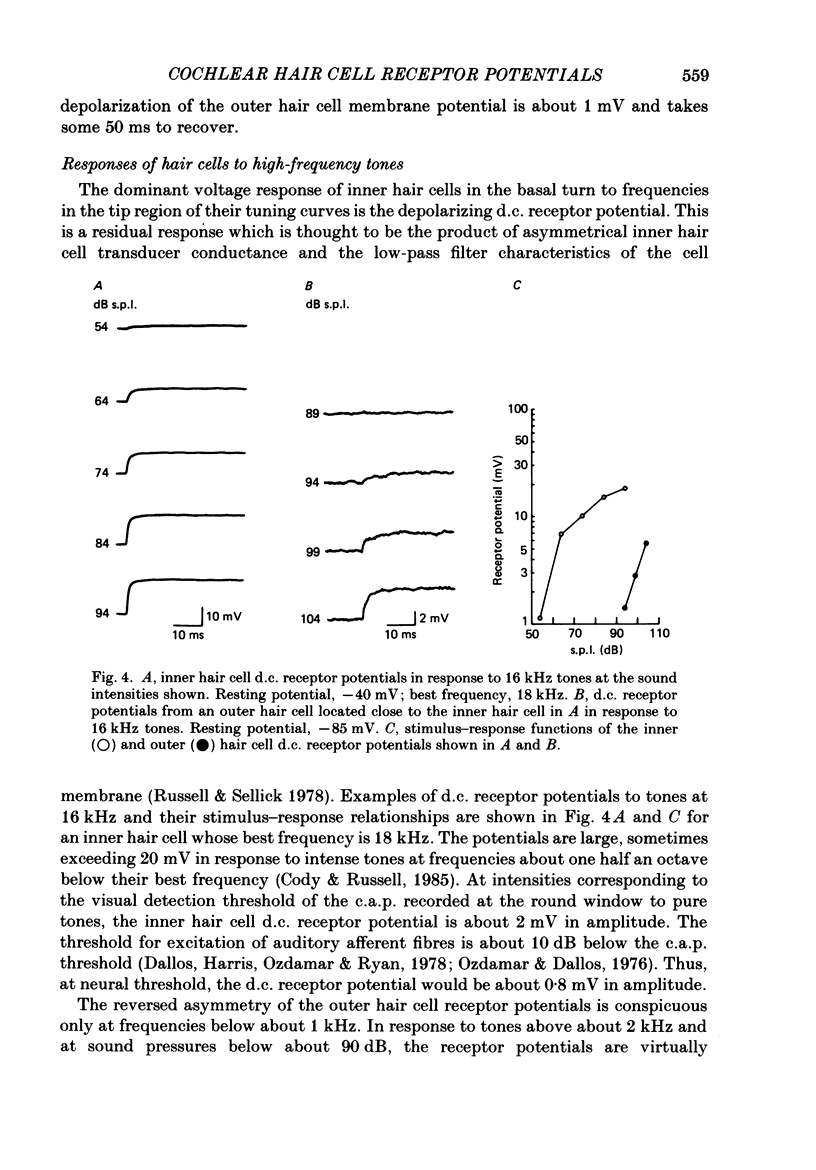
1. Intracellular recordings were made from inner and outer hair cells in the basal turn of the guinea-pig cochlea. The resting membrane potentials of the inner hair cells are more positive than -50 mV while those of outer hair cells are usually more negative than -70 mV. 2. At low frequencies the receptor potentials of inner hair cells are predominantly depolarizing while those from outer hair cells are hyperpolarizing at low and moderate sound pressure (e.g. less than 90 dB re 2 X 10(-5) Pa at 600 Hz). The potentials then become predominantly depolarizing at high sound pressure. 3. The asymmetry of the inner and outer hair cell receptor potentials are manifested instantaneously except at high stimulus levels when the depolarizing responses of outer hair cells take several cycles to develop. 4. At the offset of intense tones outer hair cell membrane potentials remain depolarized by 1-2 mV above their resting value and return to normal over a period depending on the level and duration of the tone. 5. In response to tones above about 2 kHz and at levels below about 90 dB the wave forms of outer hair cell receptor potentials are virtually symmetrical without measurable d.c. components. In response to tones close to their best frequencies (16-21 kHz), inner hair cells in the basal turn generate large depolarizing (d.c.) receptor potentials while outer hair cells from this region of the cochlea do not generate significant voltage responses. 6. Frequency tuning curves were derived for inner and outer hair cells from the amplitude-intensity relationships of their d.c. and phasic (a.c.) receptor potentials respectively. When the latter were compensated for the low-pass characteristics of the recording system and the hair cell time constant, the frequency selectivity of inner and outer hair cells are similar. 7. The response properties of inner and outer hair cells in the basal turn of the guinea-pig cochlea are discussed in relation to their proposed roles in mechano-electric transduction.
Full text
PDF






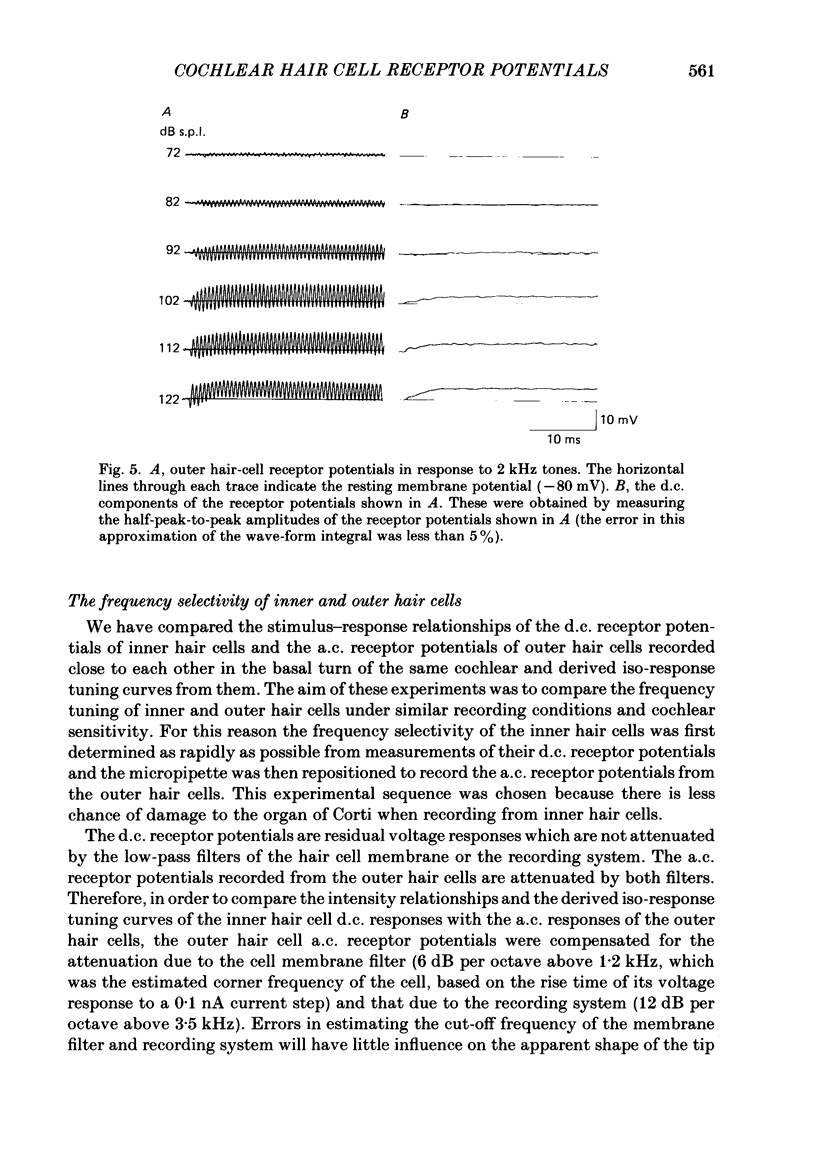
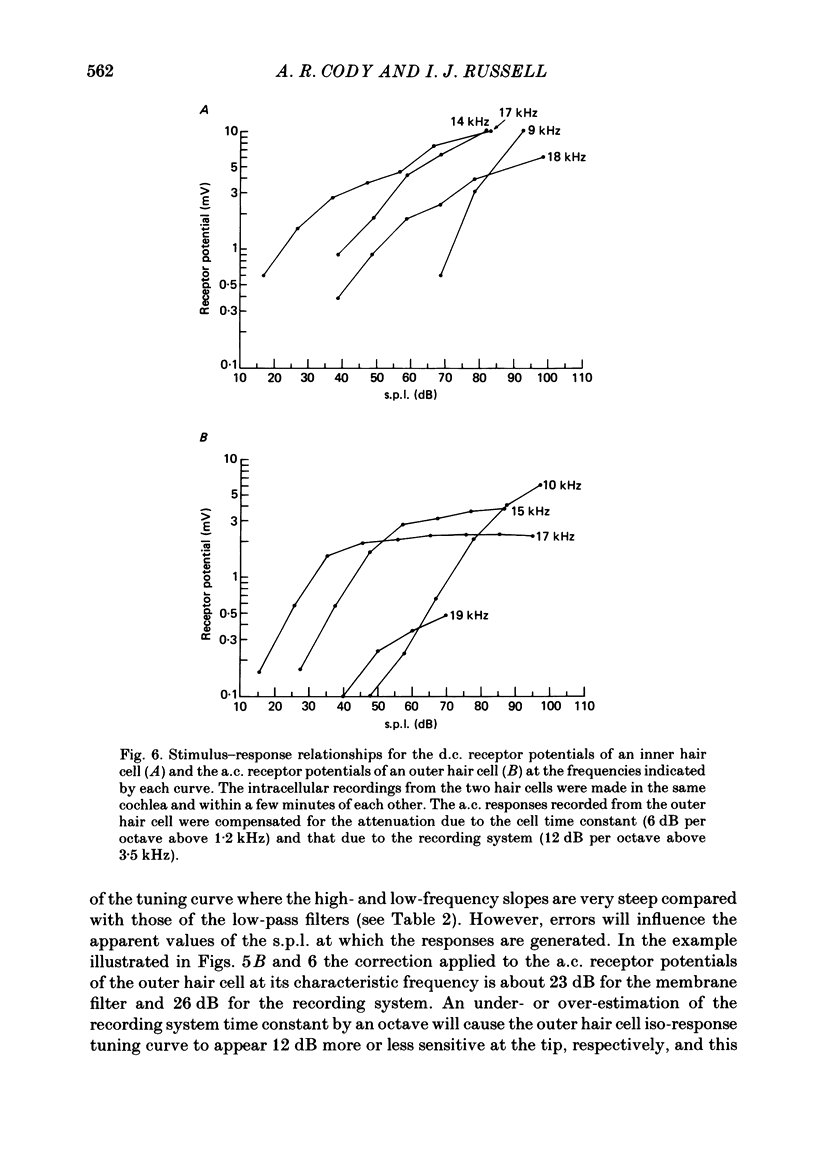
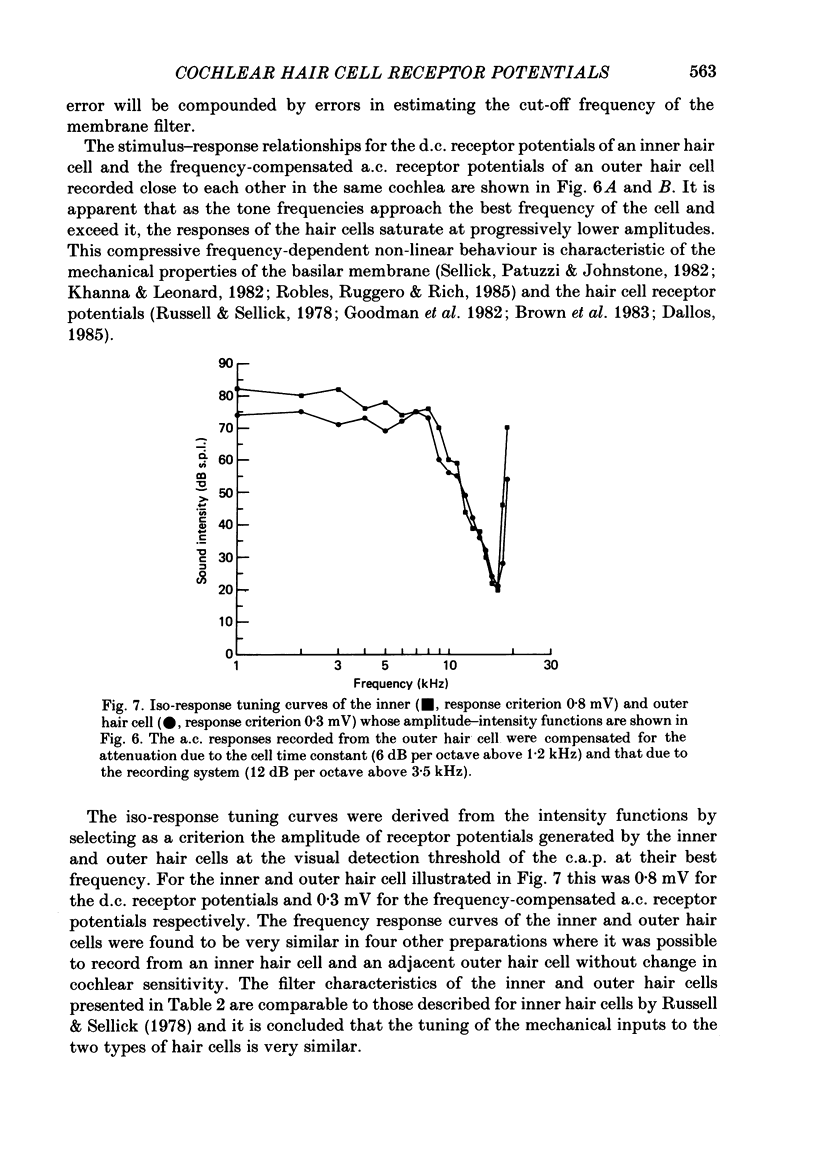






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baden-Kristensen K., Weiss T. F. Receptor potentials of lizard hair cells with free-standing stereocilia: responses to acoustic clicks. J Physiol. 1983 Feb;335:699–721. doi: 10.1113/jphysiol.1983.sp014559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boston J. R. A model of lateral line microphonic response to high-level stimuli. J Acoust Soc Am. 1980 Mar;67(3):875–881. doi: 10.1121/1.383967. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Nuttall A. L. Efferent control of cochlear inner hair cell responses in the guinea-pig. J Physiol. 1984 Sep;354:625–646. doi: 10.1113/jphysiol.1984.sp015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Nuttall A. L., Masta R. I. Intracellular recordings from cochlear inner hair cells: effects of stimulation of the crossed olivocochlear efferents. Science. 1983 Oct 7;222(4619):69–72. doi: 10.1126/science.6623058. [DOI] [PubMed] [Google Scholar]
- Brownell W. E., Bader C. R., Bertrand D., de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985 Jan 11;227(4683):194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Cody A. R., Russell I. J. Outer hair cells in the mammalian cochlea and noise-induced hearing loss. Nature. 1985 Jun 20;315(6021):662–665. doi: 10.1038/315662a0. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983 May;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS H., FERNANDEZ C., McAULIFFE D. R. The excitatory process in the cochlea. Proc Natl Acad Sci U S A. 1950 Oct;36(10):580–587. doi: 10.1073/pnas.36.10.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS H. [A mechano-electrical theory of cochlear action]. Ann Otol Rhinol Laryngol. 1958 Sep;67(3):789–801. doi: 10.1177/000348945806700315. [DOI] [PubMed] [Google Scholar]
- Dallos P. Cochlear physiology. Annu Rev Psychol. 1981;32:153–190. doi: 10.1146/annurev.ps.32.020181.001101. [DOI] [PubMed] [Google Scholar]
- Dallos P., Harris D., Ozdamar O., Ryan A. Behavioral, compound action potential, and single unit thresholds: relationship in normal and abnormal ears. J Acoust Soc Am. 1978 Jul;64(1):151–157. doi: 10.1121/1.381980. [DOI] [PubMed] [Google Scholar]
- Dallos P. Response characteristics of mammalian cochlear hair cells. J Neurosci. 1985 Jun;5(6):1591–1608. doi: 10.1523/JNEUROSCI.05-06-01591.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P., Santos-Sacchi J., Flock A. Intracellular recordings from cochlear outer hair cells. Science. 1982 Nov 5;218(4572):582–584. doi: 10.1126/science.7123260. [DOI] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983 Jan;9(1):79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Evans E. F. Neuroleptanesthesia for the guinea pig. An ideal anesthetic procedure for long-term physiological studies of the cochlea. Arch Otolaryngol. 1979 Apr;105(4):185–186. doi: 10.1001/archotol.1979.00790160019004. [DOI] [PubMed] [Google Scholar]
- Evans E. F. The frequency response and other properties of single fibres in the guinea-pig cochlear nerve. J Physiol. 1972 Oct;226(1):263–287. doi: 10.1113/jphysiol.1972.sp009984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A., Russell I. Inhibition by efferent nerve fibres: action on hair cells and afferent synaptic transmission in the lateral line canal organ of the burbot Lota lota. J Physiol. 1976 May;257(1):45–62. doi: 10.1113/jphysiol.1976.sp011355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. A., Smith R. L., Chamberlain S. C. Intracellular and extracellular responses in the organ of Corti of the gerbil. Hear Res. 1982 Jul;7(2):161–169. doi: 10.1016/0378-5955(82)90012-0. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J., Corey D. P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B. M., Sellick P. M. The peripheral auditory apparatus. Q Rev Biophys. 1972 Feb;5(1):1–57. doi: 10.1017/s0033583500000032. [DOI] [PubMed] [Google Scholar]
- Kemp D. T. Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea. Arch Otorhinolaryngol. 1979;224(1-2):37–45. doi: 10.1007/BF00455222. [DOI] [PubMed] [Google Scholar]
- Khanna S. M., Leonard D. G. Basilar membrane tuning in the cat cochlea. Science. 1982 Jan 15;215(4530):305–306. doi: 10.1126/science.7053580. [DOI] [PubMed] [Google Scholar]
- McMullen T. A., Mountain D. C. Model of d.c. potentials in the cochlea: effects of voltage-dependent cilia stiffness. Hear Res. 1985 Feb;17(2):127–141. doi: 10.1016/0378-5955(85)90016-4. [DOI] [PubMed] [Google Scholar]
- Morrison D., Schindler R. A., Wersäll J. A quantitative analysis of the afferent innervation of the organ of corti in guinea pig. Acta Otolaryngol. 1975 Jan-Feb;79(1-2):11–23. doi: 10.3109/00016487509124649. [DOI] [PubMed] [Google Scholar]
- Mountain D. C. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980 Oct 3;210(4465):71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Neely S. T., Kim D. O. An active cochlear model showing sharp tuning and high sensitivity. Hear Res. 1983 Feb;9(2):123–130. doi: 10.1016/0378-5955(83)90022-9. [DOI] [PubMed] [Google Scholar]
- Ozdamar O., Dallos P. Input-output functions of cochlear whole-nerve action potentials: interpretation in terms of one population of neurons. J Acoust Soc Am. 1976 Jan;59(1):143–147. doi: 10.1121/1.380818. [DOI] [PubMed] [Google Scholar]
- Pickles J. O. Recent advances in cochlear physiology. Prog Neurobiol. 1985;24(1):1–42. doi: 10.1016/0301-0082(85)90014-0. [DOI] [PubMed] [Google Scholar]
- Robertson D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res. 1984 Aug;15(2):113–121. doi: 10.1016/0378-5955(84)90042-x. [DOI] [PubMed] [Google Scholar]
- Russell I. J. Origin of the receptor potential in inner hair cells of the mammalian cochlea--evidence for Davis' theory. Nature. 1983 Jan 27;301(5898):334–336. doi: 10.1038/301334a0. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Richardson G. P., Cody A. R. Mechanosensitivity of mammalian auditory hair cells in vitro. 1986 May 29-Jun 4Nature. 321(6069):517–519. doi: 10.1038/321517a0. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol. 1983 May;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J. A re-evaluation of cell coupling in the organ of Corti. Hear Res. 1984 May;14(2):203–204. doi: 10.1016/0378-5955(84)90019-4. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J., Dallos P. Intercellular communication in the supporting cells of the organ of Corti. Hear Res. 1983 Mar;9(3):317–326. doi: 10.1016/0378-5955(83)90034-5. [DOI] [PubMed] [Google Scholar]
- Sellick P. M., Patuzzi R., Johnstone B. M. Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J Acoust Soc Am. 1982 Jul;72(1):131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Asanuma A., Yanagisawa K. Potentials of outer hair cells and their membrane properties in cationic environments. Hear Res. 1980 Jun;2(3-4):431–438. doi: 10.1016/0378-5955(80)90079-9. [DOI] [PubMed] [Google Scholar]
- Weiss T. F. Bidirectional transduction in vertebrate hair cells: a mechanism for coupling mechanical and electrical processes. Hear Res. 1982 Aug;7(3):353–360. doi: 10.1016/0378-5955(82)90045-4. [DOI] [PubMed] [Google Scholar]
- Weiss T. F., Peake W. T., Sohmer H. S. Intracochlear potential recorded with micropipets. 3. Relation of cochlear microphonic potential to stapes velocity. J Acoust Soc Am. 1971 Aug;50(2):602–615. doi: 10.1121/1.1912676. [DOI] [PubMed] [Google Scholar]
- Wever E. G., Bray C. W. ACTION CURRENTS IN THE AUDITORY NERVE IN RESPONSE TO ACOUSTICAL STIMULATION. Proc Natl Acad Sci U S A. 1930 May 15;16(5):344–350. doi: 10.1073/pnas.16.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenner H. P., Zimmermann U., Schmitt U. Reversible contraction of isolated mammalian cochlear hair cells. Hear Res. 1985 May;18(2):127–133. doi: 10.1016/0378-5955(85)90004-8. [DOI] [PubMed] [Google Scholar]