Abstract
A plasmid-based gene reporter system has been developed to construct lacZ gene fusions for monitoring intrinsic promoter expression in Methanosarcina acetivorans. Constructs transform with high efficiency that can be readily screened by color selection on plates and exhibit a consistent copy number on different substrates negating the need for gene copy normalization. Expression of the CO dehydrogenase-acetyl coenzyme A synthase promoter fusion to lacZ revealed 18- to 54-fold down-regulation in cells grown on methylotrophic substrates compared with acetate-grown cells, which is up to an order of magnitude greater than the range of regulation previously reported by enzyme activity assays. This system complements and expands the current techniques for studying genetics of the methanosarcinal Archaea by providing a rapid method for monitoring and quantifying gene expression.
Methanosarcina is the most metabolically diverse genus among the methanogens (12). Whereas most methanogenic Archaea grow by CO2 reduction with H2, these species grow by the dismutation of acetate, by methylotrophic catabolism of methanol, methylated amines, and dimethylsulfide, and in some cases also by CO2 reduction with H2. The aceticlastic methanogens exhibit a hierarchy of substrate preference coinciding with the free-energy yields of the substrates. Previous reports indicate that acetate catabolism is regulated at the transcriptional level in Methanosarcina spp. in response to alternative substrates, but the mechanism of catabolic gene regulation by these Archaea is currently unknown (3, 11, 15). With the complete sequencing of genomes from two methanosarcinal species (4, 5) and partial completion of a third species (http://genome.jgi-psf.org), this genus is well poised for genetic studies on archaeal gene expression and physiological pathways.
One of the difficulties encountered when studying the Archaea has been the dearth of genetic techniques for transferring DNA into cells and selecting mutants. Although gene transfer and recombination techniques are straightforward in many bacterial and eukaryal systems, application of these techniques in the methanogenic Archaea has been problematic and is only in the developing stages. A genetic system developed for the Methanosarcina spp. is among the most advanced available for the methanogenic Archaea. Clonal colonies of Methanosarcina spp. can be grown on solidified medium by streaking or replica plating (1, 13). Development of an efficient transformation system for transfer of both plasmid and linear DNA into Methanosarcina spp. (9), combined with transposon-mediated random mutagenesis (19) and directed gene disruption (20), provides approaches for constructing and isolating mutants. The recent report of a markerless genetic exchange system for Methanosarcina spp. overcomes the current problem of limited selectable markers by allowing the marker to be removed and reused after gene disruption (10).
A genetic system is required for functional analysis of the available methanosarcinal genomic data to confirm and quantify gene expression. Herein, we describe a plasmid-mediated gene reporter system that utilizes fusions of archaeal promoters to the lacZ gene from Escherichia coli for in vivo expression in Methanosarcina acetivorans. The advantage of this system is that protein fusions can be made in a single cloning step, allowing constructs to be prepared rapidly for transformation and in vivo analysis in M. acetivorans. Differential expression of the gene encoding the catabolic CO dehydrogenase from Methanosarcina thermophila fused to lacZ is described during growth of M. acetivorans with different substrates.
Development of a plasmid-mediated cdh promoter-lacZ gene fusion for monitoring cdh expression.
To develop a gene reporter assay for Methanosarcina spp., the DNA sequence upstream of the M. thermophila TM-1 cdhABCDE operon (GenBank accession no. U66032), which had been shown previously to express the catabolic CO dehydrogenase-acetyl coenzyme A synthase (8, 15), was cloned upstream of the reporter gene β-galactosidase (lacZ). A 1,153-bp fragment containing the cdhA promoter from M. thermophila TM-1 was PCR amplified from pCDH1 (15) forward primer 5′-CACGGTACCTTCTGCAGCACG-3′ and reverse primer 5′-AGTTTGCCCATGGGAGCTTTAC-3′, which introduced flanking NsiI/NcoI restriction sites and directly ligated into pCR2.1 (Invitrogen). Conditions for PCR amplification were as follows: initial denaturation at 94°C for 5 min followed by 30 amplification cycles of 94°C for 30 s, 48°C for 30 s, and 72°C for 1.5 min. A 1,170-bp NsiI/NcoI restriction fragment was cloned into NsiI and NcoI sites in pBAD-TOPOlacZ (Invitrogen) upstream of the lacZ gene with the ATG of the cdh operon fused in-frame with the ATG of lacZ. This translational fusion, which was designated pcdhP-lacZ, conserved the 371 untranslated leader sequence of the wild-type cdh operon with only the substitution of a cytosine for adenine two bases upstream of the translational start site. The 4,306-bp cdh promoter-lacZ fusion was digested with KpnI and PmeI and cloned into the KpnI and EcoRV sites of the E. coli/M. acetivorans shuttle plasmid pWM315 (9). This construct, designated pKJ104, was transformed into E. coli strain DH5α/λpir. To create a cdh-lacZ fusion in the opposite orientation pKJ104 was digested with StuI/NaeI. These two restriction ends of the 4,343-bp fragment containing the cdh promoter-lacZ fusion were filled-in with Vent Polymerase (NEB). The DNA fragment was ligated into the AscI site of pWM307 to create pEA103, which was transformed into E. coli strain DH5α/λpir (Fig. 1). The sequences of all constructs were confirmed by dye terminator cycle sequencing on an ABI 377 automated sequencer (PE Applied Biosystems). The reporter plasmids were identical except pEA103 lacked a partial lac promoter, lac repressor binding site, and the CAP (catabolite gene activator protein) binding site that were present in pKJ104 from the parent pWM315 vector and the second B repeat of the putative origin of replication in the pC2A cassette was inadvertently deleted. Promoterless lacZ vectors were constructed by PCR using pEA64 as template, which contained the cdh promoter fused to cat (chloramphenicol acetyltransferase) (unpublished data). Reverse primer (5′-GCCATTGGGATATATCAACGG-3′) was combined with forward primer (5′-TTATATCGATTTGGTACATTT-3′) to generate a PCR product with a ClaI restriction site (underlined) 29 bp upstream of the transcriptional start site. The fragment was cloned into pCR2.1 (Invitrogen), then digested with NsiI/SalI and ligated into pcdhP-lacZ, in place of the wild-type cdh promoter sequence to create a cdh::lacZ deletion fusion. An XhoI/BamHI-digested fragment, which included the cdh deletion and 5′ flanking end of lacZ, was cloned into pEA103 and pKJ104 in place of the wild-type cdh::lacZ fusion sequence, creating the promoterless vectors pEA110 and pCDHΔ64, respectively.
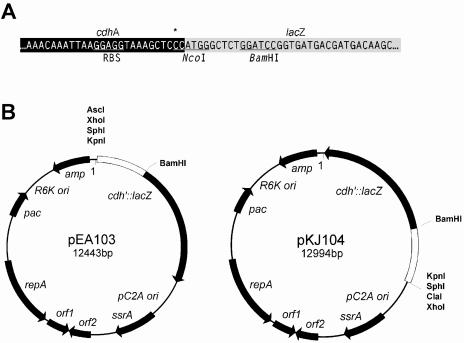
FIG. 1.
Archaeal reporter plasmids pEA103 and pKJ104. (A) Detailed map showing the sequence of the Methanosarcina thermophila TM-1 cdh promoter-lacZ translational fusion reporter. The cdhA promoter, which included the putative archaeal ribosomal binding site (RBS), was amplified with a 3′ NcoI restriction site by substituting a C for an A (shown with an asterisk) and ligated to the 5′ NcoI site of lacZ. The BamHI restriction site 9 bp downstream of the translational start site provides an alternative restriction site for constructing translational lacZ fusions. (B) The complete maps of the reporter plasmids pEA103 and pKJ104, constructed from the autonomously replicating shuttle vector pWM315 (9), which contain the native Methanosarcina acetivorans strain C2A plasmid with an archaeal origin of replication (pC2A ori), open reading frames with sequence similarity to a putative replication initiation protein (repA), site-specific recombinase (ssrA), and two open reading frames without significant sequence similarity to known proteins. The constructs also include a puromycin N-acetyltransferase gene (pac) under the control of an archaeal methyl coenzyme M reductase promoter and terminator for puromycin selection in Methanosarcina spp.; an origin of replication (R6K ori) and β-lactamase gene (bla) for replication and ampicillin selection, respectively, in E. coli.
M. acetivorans C2A (DSM 2834) was transformed with pKJ104, pEA103, pCDHΔ64, and pEA110 as described previously (9), and transformants were selected for puromycin resistance. All clones were reisolated by streaking on agar-solidified medium. Transformants were further screened for β-galactosidase activity by transferring colonies to a nitrocellulose filter (NitroPure, catalog no. WP4H5; GE Osmonics, Inc., Minnetonka, MN) in an anaerobic glove box and then treating the filter with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) outside of the glove box to expose it to air.
To measure the effects of substrates on cdh promoter expression, M. acetivorans transformed with pKJ104, pEA103, pCDHΔ64, and pEA110 was inoculated into marine disaggregating medium prepared anaerobically under a N2-CO2 (4:1) atmosphere as described previously (14). M. acetivorans transformants containing the reporter plasmids were inoculated (5% vol/vol) into 10 ml of liquid medium containing 2 μg ml−1 puromycin and 0.1 M of either sodium acetate, trimethylamine, or methanol. Cultures were sampled (1.0 ml) with a syringe at mid-exponential growth, and aliquots were held on ice until assayed. The optical densities at 550 nm for mid-exponential growth on each substrate were as follows: acetate, 0.135 to 0.145; methanol, 0.18 to 0.25; trimethylamine, 0.35 to 0.40. Cells were pelleted by centrifugation at 14,900 × g for 5 min at 4°C and the supernatant was decanted. Cell pellets were resuspended in 1 ml chilled Z buffer (60 mM Na2HPO4 · 7H2O, 40 mM NaH2PO4 · 2H2O, 10 mM KCl, 1 mM MgSO4 · 7H2O, pH 7.0) and lysed completely by sonication for 20 s with 2-s on/off pulses at an output setting of 3 (Fisher 550 Sonic Dismembrator). Lysed cells were centrifuged at 14,900 × g for 15 min at 4°C. The supernatant was transferred to new microcentrifuge tubes and maintained on ice. Triplicate samples of 50 to 100 μl were dispensed into 750 to 700 μl Z buffer with 50 mM β-mercaptoethanol and prewarmed 5 min at 30°C. ONPG solution (160 μl Z buffer with 4 mg/ml o-nitrophenyl-β-d-galactopyranoside) was added to start the reaction, and 400 μl 1 M Na2CO3 was added to stop the reaction. Absorbance was measured at 420 nm and 550 nm, and activity was calculated by using an extinction coefficient of 0.0035 M−1 cm−1. Protein concentration was measured using 100 μl cell extract in a Branford assay with bovine serum albumin as the standard (2). One unit of β-galactosidase activity is given as 1 nmol of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per min per mg of protein (Table 1).
TABLE 1.
E. coli and M. acetivorans plasmid constructs
| Plasmid | Parent | Characteristicsa | Source or reference |
|---|---|---|---|
| pCR2.1 | Cloning vector, Ampr, lacZα | Invitrogen | |
| pBAD-TOPO | Cloning vector, Ampr, lacZ | Invitrogen | |
| pWM307 | Ori R6K, Ori pC2A, Ampr, Purr | 9 | |
| pWM315 | Ori R6K, Ori pC2A, Ampr, Purr, lacZα | 9 | |
| pCDH1 | pUC18 | cdhA,′ Ampr | 15 |
| pcdhP-lacZ | pBAD-TOPO | cdhA′::lacZ; Ampr, Ori pBR322 | This study |
| pKJ104 | pWM315 | cdhA′::lacZ, Ori pC2A, Ampr, Purr, Ori R6K | This study |
| pEA103 | pWM307 | cdhA′::lacZ, Ori pC2A, Ampr, Purr, Ori R6K | This study |
| pCDHΔ64 | pKJ104 | cdhA′Δpromoter::lacZ, Ori pC2A, Ampr, Purr, Ori R6K | This study |
| pEA110 | pEA103 | cdhA′Δpromoter::lacZ, Ori pC2A, Ampr, Purr, Ori R6K | This study |
cdhA′, cdh promoter; cdhA′ Δpromoter::lacZ, promoterless vector.
Plasmids pKJ104 and pEA103 (cdh promoter-lacZ) were transformed into M. acetivorans, as this species exhibits greater than 100-fold greater transformation efficiency than M. thermophila (9). Both species have nearly identical physiological responses to the substrate and the upstream regulatory sequences of the catabolic CO dehydrogenase-acetyl coenzyme A synthase homologs from M. thermophila and M. acetivorans (MA3860) are nearly identical (>99% similarity). Colonies of pKJ104 or pEA103 transformants transferred to nitrocellulose filters developed a blue color when the filters were treated with X-Gal and exposed to air. Quantitative assays with o-nitrophenyl-β-d-galactosidase showed at least 50-fold greater levels of activity in acetate-grown transformants than with methanol-grown transformants (Table 2). Negligible activity was detected in wild-type cells and in transformants that contained either reporter vector with a deleted archaeal promoter sequence (pEA110 and pCDHΔ64). Although gene fusions in both plasmid orientations yielded similar results, pEA103 exhibited up to 100-fold greater transformation efficiencies in E. coli than pKJ104, possibly due to absence of sequence containing the residual E. coli lac operator and/or the pC2A direct repeat present in the latter construct.
TABLE 2.
Effect of substrates on cdh promoter-lacZ expression
| Vector(s) | Substrate | Specific activity (mean ± SD)a |
|---|---|---|
| pEA103 | Sodium acetate | 23.77 ± 1.62 |
| pEA103 | Methanol | 0.44 ± 0.09 |
| pEA103 | Trimethylamine | 0.77 ± 0.51 |
| pEA103 | Monomethylamine | 0.66 ± 0.06 |
| pKJ104 | Sodium acetate | 24.22 ± 1.25 |
| pKJ104 | Methanol | 0.95 ± 0.20 |
| pKJ104 | Trimethylamine | <0.1 |
| pEA110, pCDHΔ64 | Sodium acetate | <0.1 |
| pEA110, pCDHΔ64 | Methanol | <0.1 |
| pEA110, pCDHΔ64 | Trimethylamine | <0.1 |
| pWM315 | Sodium acetate | <0.1 |
β-Galactosidase specific activity is defined as 1 nmol of ONPG-hydrolyzed min−1 μg−1 of protein. The lower detection limit is 0.1 unit. Averages are from three or more replicate cultures.
Effect of plasmid copy number on cdh promoter-lacZ gene fusion expression.
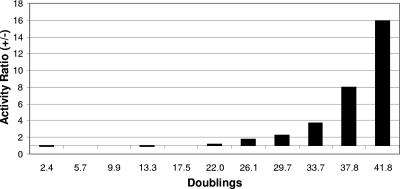
Reporter plasmids transformed into M. acetivorans were quantified throughout growth in media containing puromycin to normalize for the effect of gene copy numbers on β-galactosidase activity. Since growth rates with methylotrophic substrates are up to 4.5 times greater than with acetate, plasmid copies per cell were also determined in cells grown on both acetate and methanol. M. acetivorans transformed with pKJ104 or pEA103 was inoculated into five tubes of liquid medium containing 2 μg/ml puromycin and 0.1 M sodium acetate or methanol. To determine plasmid copy numbers per cell, total DNA was extracted from pooled cultures by using a modified hexadecyltrimethylammonium bromide DNA extraction protocol (18). RNase-treated total DNA was resuspended in 50 μl Tris-EDTA and stored at −20°C. Total DNA in amounts of 5 μg, 1 μg, 0.5 μg, and 0.1 μg was digested with HindIII/XbaI (NEB). Plasmid DNA standards (pEA103) of 100 pg, 500 pg, 1 ng, 2.5 ng, and 12.5 ng were digested with HindIII (NEB). Duplicate restriction digests of the DNA samples and standards were separated by electrophoresis in a 1.2% Tris-borate-EDTA agarose gel and transferred to two Biotrans nylon membranes (ICN) by capillary blotting using standard protocols. A 1,022-bp fragment of a deoxyhypusine synthetase-orotidine 5′-monophosphate decarboxylase gene was generated by PCR from M. acetivorans genomic DNA as a hybridization probe for genome copies with forward primer 5′-GAG ACT GAA AGT GCA GGC GC-3′ and reverse primer 5′-GTG CCT CAG AAC TCA TAG GGC TC-3′. A 1,136-bp fragment of lacZ was isolated from a BamHI/EcoRV digest of pEA103 as a hybridization probe for plasmid copies. Both the genomic and plasmid probes were digoxigenin (DIG) labeled by using DIG High-Prime (Roche Molecular Biochemicals) following the manufacturer’s directions and hybridized to the membranes at 68°C overnight. DIG-labeled probes, hybridized to target DNA, were detected by Nitro Blue Tetrazolium/BCIP (5-bromo-4-chloro-3-indolylphosphate) colorimetric reaction using a DIG nucleic acid detection kit (Roche Molecular Biochemicals) and quantified with a Gel-Doc 2000 digital imaging system (Bio-Rad). Both pKJ104 and pEA103 copy numbers (mean ± standard deviation) remained constant at 15 ± 3 copies per genomic pyrF gene copy during exponential growth with either acetate or methanol. The results indicate that the level of gene expression using pKJ104 or pEA103 does not have to be normalized for variation in the gene copy number when comparing gene expression in cells grown with different substrates. The long-term stability of pKJ104 in M. acetivorans was determined by monitoring β-galactosidase activity for over 42 generations in the presence and absence of puromycin. A decrease in activity was detected after 22 generations in the absence of antibiotic selection, but no significant changes in activity were detected after 42 generations if the cells were maintained with puromycin (Fig. 2). These results indicate that the recombinant vector is stable in M. acetivorans maintained with antibiotic. In addition, the observed stability of the reporter vector in the absence of antibiotic for at least 20 generations negates the requirement for expensive antibiotic selection during high-volume mass culturing, which requires only 5 to 10 generations (16).
FIG. 2.
Stability of pKJ104 in M. acetivorans grown in the absence of puromycin selection. The activity ratio is based on the specific activity ratio of β-galactosidase in cultures grown with and without puromycin for 10 sequential 5% vol/vol transfers. Each datum point is the mean value from three replicate cultures.
Stability of recombinant β-galactosidase in M. acetivorans.
In order to determine the intrinsic promoter strength from a lacZ fusion under different growth conditions, β-galactosidase is assayed during steady-state exponential growth. During this phase of growth, the synthesis rate and dilution rate resulting from cell doubling have achieved equilibrium (6). However, when comparing cultures that exhibit different growth rates, such as M. acetivorans strains grown aceticlastically versus strains grown methylotrophically, rapid breakdown of β-galactosidase may not yield accurate relative levels of gene expression if the growth rate in the more rapidly growing culture exceeds the rate of β-galactosidase degradation. The stability of the recombinant lacZ in M. acetivorans was therefore determined in the presence of sparsomycin (Sigma, St. Louis, MO), which inhibits protein synthesis (6). Triplicate cultures of M. acetivorans transformed with pEA103 and pKJ104 fusions were grown in 0.1 M sodium acetate. At mid-exponential growth, filter-sterilized sparsomycin was added to a final concentration of 25 μg ml−1 in each culture. β-Galactosidase activity was assayed immediately after sparsomycin addition and 1 h, 1 day, 2 days, 4 days, and 8 days after sparsomycin addition as described above. Levels of β-galactosidase remained constant over 2 days and decreased by 11% on day 4 and 22% on day 8, which indicates that the protein has a low turnover rate in M. acetivorans. Results indicate that β-galactosidase stability is not a factor when comparing the intrinsic promoter strength of lacZ fusions in M. acetivorans strains grown aceticlastically or methylotrophically.
The effects of substrates on cdh expression.
The effects of substrates on cdh expression were determined for pKJ104, pEA103, pCDHΔ64, and pEA110 transformants grown with acetate and methylotrophic substrates (Table 2). Down-regulated expression of β-galactosidase ranged from an 18-fold decrease in activity for monomethylamine to a 31-fold-decreased activity for trimethylamine and 54-fold-decreased activity for methanol. No β-galactosidase was detected in cells transformed with pEA110 that lacked cdh promoter sequence. The results are consistent with earlier reports on enzyme activity assays and Northern analyses showing that CO dehydrogenase-acetyl coenzyme A synthase is up-regulated in the cells grown with acetate and down-regulated in the presence of alternative substrates (3, 11, 15, 17). Although the range of promoter expression is similar to that reported for other genes encoding catabolic enzymes, this range is significantly greater than the five- to sixfold difference in CO dehydrogenase activity detected in acetate-grown M. thermophila and Methanosarcina barkeri compared to methanol-grown M. thermophila and M. barkeri (7, 17). Similarly, Western analysis cell extracts with antibody to methanosarcinal CO dehydrogenases (13) also revealed a difference in expression (mean ± standard deviation) of only (10.6 ± 0.6)-fold between methanol- and acetate-grown cells (data not shown). The results suggest that the full range of gene expression by the regulated catabolic cdh in this study may have been masked in the previous CO dehydrogenase activity assays by the coexpression of multiple regulated or constitutively expressed cdh genes. Likewise, cross-reactivity of cdh antiserum with multiple CO dehydrogenases would have also masked the full range of cdh regulation by Western analysis. Annotated genome sequences of M. acetivorans, Methanosarcina mazei, and M. barkeri reveal the existence of two CO dehydrogenase genes associated with acetyl coenzyme A decarbonylase-synthase operons and at least two other putative CO dehydrogenases genes (4, 5) (http://genome.jgi-psf.org). The role of these other CO dehydrogenases has not yet been determined.
Summary.
This report describes a plasmid-mediated gene reporter system for assaying in vivo gene expression in Methanosarcina spp. The cdhA promoter was fused to lacZ at the NcoI restriction site, and the translational fusion cassette was ligated into the archaeal shuttle vector pWM315 to construct pEA103 and pJK104. Subsequent translational fusions can be directly constructed in pEA103 and pJK104 by substituting alternative archaeal promoters for the cdhA promoter by using multiple upstream restriction sites and the BamHI site within the N terminus of β-galactosidase. The autonomously replicating vector does not require integration into the genome enabling greater recovery efficiency of recombinants that can be screened by color on colony blots. Recombinant plasmids are stable in M. acetivorans, and numbers are consistent on different substrates throughout exponential growth negating the need for normalization due to fluctuations in gene copy numbers. Finally, there is negligible β-galactosidase background activity in M. acetivorans and the assay is simple and sensitive. This system complements and expands the current genetic techniques for studying genetics of the methanosarcinal Archaea by providing a rapid method for monitoring and quantifying gene expression.
Acknowledgments
The first two authors contributed equally to the manuscript and are listed in alphabetical order.
We thank S. MacAuley for assisting with β-galactosidase assays and W. Metcalf for the gift of pWM307 and pWM315.
This work was supported by a grant to K.S. from the Department of Energy, Energy Biosciences Program (DE-FG02-93ER20106), and by an NSF Postdoctoral Fellowship in Microbial Biology to K.J.
REFERENCES
- 1.Apolinario, E. A., and K. R. Sowers. 1996. Plate colonization of Methanococcus maripaludis and Methanosarcina thermophila in a modified canning jar. FEMS Microbiol. Lett. 145:131-137. [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Clements, A. P., and J. G. Ferry. 1992. Cloning, nucleotide sequence, and transcriptional analyses of the gene encoding a ferredoxin from Methanosarcina thermophila. J. Bacteriol. 174:5244-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deppenmeier, U., A. Johann, T. Hartsch, R. Merkl, R. A. Schmitz, R. Martinez-Arias, A. Henne, A. Wiezer, S. Bäumer, C. Jacobi, H. Brüggemann, T. Lienard, A. Christmann, M. Bömeke, S. Steckel, A. Bhattacharyya, A. Lykidis, R. Overbeek, H.-P. Klenk, R. P. Gunsalus, H. J. Fritz, and G. Gottschalk. 2002. The genome of Methanosarcina mazei: evidence for lateral gene transfer between Bacteria and Archaea. J. Mol. Microbiol. Biotechnol. 4:453-461. [PubMed] [Google Scholar]
- 5.Galagan, J. E., C. Nusbaum, A. Roy, M. G. Endrizzi, P. Macdonald, W. FitzHugh, S. Calvo, R. Engels, S. Smirnov, D. Atnoor, A. Brown, N. Allen, J. Naylor, N. Stange-Thomann, K. DeArellano, R. Johnson, L. Linton, P. McEwan, K. McKernan, J. Talamas, A. Tirrell, W. J. Ye, A. Zimmer, R. D. Barber, I. Cann, D. E. Graham, D. A. Grahame, A. M. Guss, R. Hedderich, C. Ingram-Smith, H. C. Kuettner, J. A. Krzycki, J. A. Leigh, W. X. Li, J. F. Liu, B. Mukhopadhyay, J. N. Reeve, K. Smith, T. A. Springer, L. A. Umayam, O. White, R. H. White, E. C. de Macario, J. G. Ferry, K. F. Jarrell, H. Jing, A. J. L. Macario, I. Paulsen, M. Pritchett, K. R. Sowers, R. V. Swanson, S. H. Zinder, E. Lander, W. W. Metcalf, and B. Birren. 2002. The genome of Methanosarcina acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12:532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guss, A. M., B. Mukhopadbyay, J. K. Zhang, and W. W. Metcalf. 2005. Genetic analysis of mch mutants in two Methanosarcina species demonstrates multiple roles for the methanopterin-dependent C-1 oxidation/reduction pathway and differences in H2 metabolism between closely related species. Mol. Microbiol. 55:1671-1680. [DOI] [PubMed] [Google Scholar]
- 7.Krzycki, J. A., R. H. Wolkin, and J. G. Zeikus. 1982. Comparison of unitrophic and mixotrophic substrate metabolism by an acetate-adapted strain of Methanosarcina barkeri. J. Bacteriol. 149:247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maupin-Furlow, J. A., and J. G. Ferry. 1996. Analysis of the CO dehydrogenase/acetyl-coenzyme A synthase operon of Methanosarcina thermophila. J. Bacteriol. 178:6849-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf, W. W., J. K. Zhang, E. Apolinario, K. R. Sowers, and R. S. Wolfe. 1997. A genetic system for Archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proc. Natl. Acad. Sci. USA 94:2626-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pritchett, M. A., J. K. Zhang, and W. W. Metcalf. 2004. Development of a markerless genetic exchange method for Methanosarcina acetivorans C2A and its use in construction of new genetic tools for methanogenic archaea. Appl. Environ. Microbiol. 70:1425-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhwissmann, K., and J. G. Ferry. 1995. Transcriptional regulation of the phosphotransacetylase-encoding and acetate kinase-encoding genes (pta and ack) from methanosarcina thermophila. J. Bacteriol. 177:1699-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowers, K. R. 2000. Methanogenesis, p. 204-226. In J. Lederberg, M. Alexander, B. Bloom, D. Hopwood, R. Hull, B. Iglewski, A. Laskin, S. Oliver, M. Schaechter, and W. Summers (ed.), Encyclopedia of microbiology, 2nd ed. Academic Press, Inc., New York, N.Y.
- 13.Sowers, K. R., J. E. Boone, and R. P. Gunsalus. 1993. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl. Environ. Microbiol. 59:3832-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowers, K. R., and R. P. Gunsalus. 1988. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J. Bacteriol. 170:998-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowers, K. R., and R. P. Gunsalus. 1993. Transcriptional regulation of the carbon monoxide dehydrogenase gene (cdhA) in. Methanosarcina thermophila. J. Biol. Chem. 268:23172-23178. [PubMed] [Google Scholar]
- 16.Sowers, K. R., M. J. K. Nelson, and J. G. Ferry. 1984. Growth of acetotrophic, methane-producing bacteria in a pH auxostat. Curr. Microbiol. 11:227-230. [Google Scholar]
- 17.Terlesky, K. C., M. J. K. Nelson, and J. G. Ferry. 1986. Isolation of an enzyme complex with carbon monoxide dehydrogenase activity containing a corrinoid and nickel from acetate-grown Methanosarcina thermophila. J. Bacteriol. 168:1053-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson, K. 1994. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4.5. In F. M. Ausubel, R. E. Brent, E. Kingston, D. D. Moore, J. G. Seidman, J. G. Smith, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 1. John Wiley & Sons, New York, N.Y. [Google Scholar]
- 19.Zhang, J. K., M. A. Pritchett, D. J. Lampe, H. M. Robertson, and W. W. Metcalf. 2000. In vivo transposon mutagenesis of the methanogenic archaeon Methanosarcina acetivorans C2A using a modified version of the insect mariner-family transposable element Himar1. Proc. Natl. Acad. Sci. USA 97:9665-9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, J. K., A. K. White, H. C. Kuettner, P. Boccazzi, and W. W. Metcalf. 2002. Directed mutagenesis and plasmid-based complementation in the methanogenic archaeon Methanosarcina acetivorans C2A demonstrated by genetic analysis of proline biosynthesis. J. Bacteriol. 184:1449-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]