Abstract
In silico analysis of the Listeria monocytogenes genome revealed lmo0292, a gene predicted to encode a HtrA-like serine protease. A stable insertion mutant was constructed, revealing a requirement for htrA in the listerial response to heat, acid, and penicillin stress. Transcriptional analysis revealed that htrA is not induced in response to heat shock but is induced in response to low pH and penicillin G stress. Furthermore, htrA expression was shown to be dependent upon the LisRK two-component sensor-kinase, a system known to respond to changes in integrity of the cell envelope. In addition, we demonstrated that a second in-frame start codon, upstream of that previously annotated for L. monocytogenes htrA, incorporating a putative signal sequence appears to influence virulence potential. Finally, a significant virulence defect was observed for the htrA mutant, indicating that this gene is required for full virulence in mice. Our findings suggest that L. monocytogenes lmo0292 encodes an HtrA-like serine protease that is not part of the classical heat shock response but is involved in stress responses and virulence.
L. monocytogenes is a food-borne pathogen with an ability to sense and appropriately respond to hostile changes in its environment, an adaptive response, which is pivotal to mounting a successful infection. To overcome stresses encountered in food and during infection, L. monocytogenes has evolved elaborate systems for sensing and responding to a variety of adverse environments (2, 9, 15, 28).
The publication of the complete chromosomal sequence of L. monocytogenes EGDe (11) facilitated significant advancements in the identification and characterization of loci which potentially play important roles in listerial growth and survival in foods and during infection, one such locus is lmo0292 (encoding an HtrA-like homologue). Initially characterized in Escherichia coli, HtrA is one of several proteins, collectively known as heat shock proteins, whose expression is essential for survival of bacteria at high temperatures (17). In addition, htrA has been shown to be essential for the pathogenicity of several gram-negative and gram-positive bacteria, namely, Salmonella enterica serovar Typhimurium (13), Klebsiella pneumoniae (3), Streptococcus pyogenes (14), and Streptococcus pneumoniae (12), as well as the antibiotic stress response in Lactococcus lactis (7) and Staphylococcus aureus (30).
Three HtrA homologues—HtrA, YvtA, and YycK—are encoded in Bacillus subtilis (22, 23). In silico analysis revealed that the immediate genomic organization of the region encoding the HtrA-like serine protease in L. monocytogenes corresponds to the B. subtilis six-gene operon (yycF-yycK). The B. subtilis yycF gene and its ortholog in S. aureus encode a response regulator that is essential for cell growth (6, 21). Fukuchi et al. (8) showed that the two-component system encoded by yycF and yycG is essential and has the potential to modulate expression of the cell division operon ftsAZ in B. subtilis. Attempts to disrupt the corresponding response regulator in L. monocytogenes (lmo0287) proved unsuccessful, suggesting a crucial role for this gene in cell growth (15). The essential role of the upstream response regulator in B. subtilis, S. aureus, and L. monocytogenes, the involvement of htrA in the virulence potential of several gram-positive and gram-negative pathogens, and its role in the antibiotic stress response in certain genera prompted further investigation into the HtrA-like serine protease in L. monocytogenes.
Prior to completion of the present study, Wonderling et al. (31), using a Tn917-based approach, isolated an NaCl-sensitive strain of L. monocytogenes 10403s, which they showed to be disrupted in an htrA-like locus (equivalent to lmo0292 in L. monocytogenes EGDe). In the present study we extend the work of Wonderling et al. by demonstrating a significant role for HtrA in the virulence potential of L. monocytogenes. Furthermore, transcriptional analysis of the locus under a variety of stress conditions has revealed that, unlike classical htrA genes, the L. monocytogenes htrA homologue (lmo0292) is not induced by heat shock. This analysis has also led to the identification of the LisRK two-component signal transduction system as a positive regulator of the gene. In addition, using an in silico-based approach we reveal that htrA has an alternative in-frame upstream start codon, which we analyzed for its possible role in listerial stress responses.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids are listed in Table 1. L. monocytogenes strains were grown in brain heart infusion (BHI) broth (Oxoid). Escherichia coli was grown in Luria-Bertani (LB) medium. In each case, strains were incubated at 37°C with shaking. Concentrated stocks of erythromycin (EM; 20 mg/ml), chloramphenicol (CM; 33 mg/ml), and ampicillin (25 mg/ml) were prepared and added to media at the required levels. Where necessary, the medium pH was adjusted by using concentrated HCl.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| EC101 | Derivative of E. coli with pWV01 RepA integrated into the chromosome | 16 |
| DH5α | supE44 Δlac U169 (φ80lacZΔM15) R17 recA1 endA1 gyrA96 thi-1 relA1 | Gibco-BRL |
| X10Gold | Ultracompetent cells | QuikChange XL site-directed mutagenesis kit |
| L. monocytogenes | ||
| EGDe | Wild-type strain, serotype 1/2a | W. Goebel |
| LO28 | Wild-type strain, serotype 1/2c | P. Cossart |
| EGDe-pHS1::htrA | EGDe derivative with pHS1 inserted in htrA | This study |
| EGDe-htrA-rev | EGD-pHS1::htrA with pHS1 cured from the chromosome | This study |
| HtrASS* | EGDe derivative with a frameshift mutation in the −73 to −74-bp region of the putative signal sequence of htrA | This study |
| LO28Δlisk | LO28 with a 498-bp deletion in lisK | 4 |
| Plasmids | ||
| pORI19 | EMr, Ori+, RepA− derivative of pOR128 | 16 |
| pVE6007 | CMr, temperature sensitive, RepA+ derivative of pWV01 | 18 |
| pKSV7 | CMr, temperature sensitive | 29 |
| pHS1 | pORI19 containing 721-bp region central to htrA | This study |
| pHS2 | pKSV7 containing 797-bp region incorporating both potential start codons of htrA | This study |
| pHS3 | pKSV7 containing the desired frameshift mutation in the 797-bp region of htrA | This study |
Nucleic acid manipulations.
DNA extraction was performed by using the QIAGEN gel extraction kit. Plasmid DNA isolation was performed by using the QIAGEN QIAprep spin miniprep kit. T4 DNA ligase, PCR reagents, and restriction enzymes were purchased from Roche Diagnostics GmbH (Mannheim, Germany) and used according to the manufacturer's instructions. E. coli was transformed by standard methods (19), while electrotransformation of L. monocytogenes was achieved by using the protocol outlined by Park and Stewart (24). PCR was performed by using a Hybaid (Middlesex, United Kingdom) PCR express system. Oligonucleotide primers for PCR were synthesized by Sigma-Genosys Biotechnologies, and Taq DNA polymerase (Biotaq; Bioline) was used for all reactions. Colony PCR was carried out after lysis of cells with IGEPAL CA-630 (Sigma). For transcriptional analysis, nucleic acid was extracted by using either QIAGEN RNA preparation kit or the Macaloid method described by Raya et al. (25).
pORI19 insertional mutagenesis.
pORI19 insertional mutagenesis is based on gene disruption, followed by a single crossover event as described by Law et al. (16). Primers HtrAF and HtrAR (Table 2) were used to amplify a 721-bp fragment central to htrA by PCR. The resulting PCR product was digested and ligated to similarly digested pORI19 creating pHS1. The resulting pHS1 plasmid construct was transformed into E. coli EC101. L. monocytogenes EGDe containing pVE6007 was then transformed with pHS1 isolated from E. coli EC101. Transformants were selected and grown overnight in 10 ml of broth prewarmed to 42°C (the nonpermissive temperature for pVE6007 replication in Listeria) before they were plated onto BHI agar containing 5 μg of EM/ml to select for chromosomal integration and disruption of htrA. Integration was confirmed by PCR.
TABLE 2.
Oligonucleotide primers
| Primer | Sequence (5′-3′) |
|---|---|
| HtrAF | GGTCTAGACGCGCTGGTACAACTGGa |
| HtrAR | TCGGATCCGTCGCCATTTGTATCAACa,c |
| HtrA-out | ACCGGAGAGCGCGCAACCAGc |
| HtrASSF | AACGGATCCTGGTTATGTAAGa |
| HtrASSR | ACGGTACCCGTTACCACTTCa |
| HtrASSQ1F | CACCGTTTTTTTAAAGCTTTATTCATC GTTTTTTCATATTTGGGGb |
| HtrASSQ1R | CCCCAAATATGAAAAAACGATGAATA AAGCTTTAAAAAAACGGTb |
| DnaKF | GCTGGTCTTGAAGTAGAACc |
| DnaKR | GTTCATCAAATTTAGCACGAGTc |
| GroELF | GTAGTAGCCGTGAAAGCc |
| GroELR | GTAGAGCGGAACGTGTTACc |
| 0291F | GATTGTGCTTTGAGTGGAAAGc |
| 0291R | CCCCCAACATCAATACCTTCc |
| 0293F | GTTGAAGTTCCGGATGAGAAAc |
| 0293R | CAACCTCATCAACTGGTGCc |
Restriction sites incorporated into primer sequences are underlined.
Frameshift mutation incorporated into primer sequence are underlined.
Primers used for transcriptional analysis.
Reversion by precise excision.
The mutant was transformed with pVE6007 to induce chromosomal excision of the integrated pHS1. One transformant was selected on BHI agar containing 10 μg of CM/ml and grown overnight at 30°C. Plasmid excision and curing was facilitated by continual passaging at 30°C. Plasmid excision and loss was confirmed phenotypically by sensitivity to EM and CM and at the molecular level by PCR.
Frameshift mutagenesis of the putative htrA signal sequence.
The frameshift mutation was created in the region encoding a putative signal sequence by incorporating an adenine between positions −73 and −74 by using a QuikChangeXL site-directed mutagenesis kit. Briefly, a 797-bp region incorporating both potential start codons was amplified by PCR (by using primers HtrASSF and HtrASSR; Table 2). The resulting product was digested and ligated to similarly digested pKSV7, creating pHS2. The resulting plasmid construct was transformed into E. coli DH5α. After sequencing, primers (HtrAQ1F and HtrAQ1R; Table 2) containing the desired frameshift mutation were used to amplify across the multiple cloning site of pHS2. The resulting PCR product was then treated with DpnI, creating a new double-stranded mutated DNA construct, pHS3. XL10-Gold supercompetent E. coli cells were subsequently transformed with pHS3. Transformants were selected and grown overnight in broth containing 50 μg of ampicillin/ml. Once the desired point mutation was confirmed by sequencing, pHS3 was electroporated into L. monocytogenes EGDe, and transformants were selected on BHI agar containing 10 μg of CM ml (BHI/CM). Chromosomal integration of pHS3 at 42°C was selected for by serial passage of a transformant in prewarmed BHI/CM broth, followed by streaking onto prewarmed BHI/CM agar plates. Plasmid excision and curing was facilitated by continuous passage in BHI at 30°C. Replica plating onto BHI and BHI/CM allowed selection of the frameshift mutation, which was confirmed by PCR.
Growth curves.
Overnight cultures were centrifuged (12,000 × g for 6 min), washed, resuspended in an equal volume of one-quarter-strength Ringer’s solution (Merck), inoculated (4 × 108 CFU/ml) into BHI broth supplemented with sublethal levels of various stressors (heat at 30, 37, 42, and 44°C; HCl [pH 5]; and penicillin G at 87.5 ng/ml), and incubated at 37°C with shaking. Cell growth was determined spectrophotometrically by measuring the optical density at 595 nm.
Disk assays for hydrogen peroxide analysis.
Log- and stationary-phase cultures (10% inoculum) of the wild-type, htrA mutant (EGDe-pHS1::htrA), HtrASS* (frameshift mutant), and revertant (EGDe-htrA-rev) strains were incorporated into 4 ml of BHI molten agar (0.7% agar) and overlaid onto 20 ml of BHI agar (1.5% agar). Hydrogen peroxide (20 μl at log phase and 50 μl at stationary phase of 30% [wt/wt] H2O2; Sigma) was subsequently added to blank filter disks (Oxoid) placed on these agar plates. All disk assay plates were incubated at 37°C, overnight and the zones of inhibition were measured by using Vernier calipers.
Virulence assays.
Groups of 8- to 12-week-old BALB/c mice were inoculated intraperitoneally with overnight cultures of the parent, htrA mutant, HtrASS*, and revertant strains suspended in 0.2 ml of phosphate-buffered saline to a final concentration of 2 × 106 CFU/ml. Mice were sacrificed 3 days postinfection, and the numbers of viable organisms in the spleens of infected animals were determined by plating serial 10-fold dilutions of organ homogenates on BHI agar.
RESULTS
Sequence analysis.
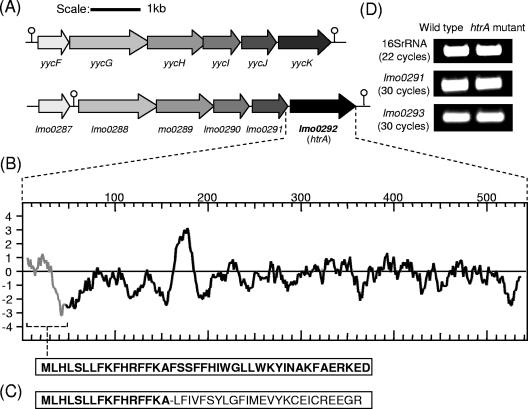
htrA of L. monocytogenes EGDe exhibits significant sequence homologies (52% identity over 342 amino acid [aa] residues) to YycK, an HtrA-like serine protease in B. subtilis (Fig. 1A). Indeed, the overall chromosomal organization of this region in the listerial genome exhibits considerable synteny with that of B. subtilis (Fig. 1A). One significant difference between the two operons is the existence of a stem-loop structure (ΔG = −6.9 kcal/mol) directly upstream of lmo0288 separating the gene from the putative two-component regulator lmo0287. In B. subtilis the two-component system (yycF-yycG) forms part of the operon and is flanked by proximal and distal stem-loop structures. Downstream of the listerial htrA a stem-loop structure (ΔG = −17.5 kcal/mol) potentially functions as a rho-independent transcription termination signal, preventing readthrough into downstream sequences. Indeed, reverse transcription-PCR (RT-PCR) analysis proved that this is the case, since there is no coordinate transcription observed between htrA and its downstream gene lmo0293 (data not shown). lmo0292 was disrupted by pHS1 integration as described above (see Materials and Methods). As expected, RT-PCR analysis revealed that the integrated plasmid had no effect on transcription of the upstream (lmo0291) or downstream (lmo0293) genes (Fig. 1D), confirming that pHS1 insertion has no polar effects in the htrA mutant (26). Furthermore, a strategy of reversion by precise excision was performed by allowing the plasmid to excise and restore the original preinsertion genotype. This confirmed that mutant phenotypes can be attributed to site-specific plasmid insertion rather than simultaneous random mutation(s) in regions outside of the htrA locus. This strategy permits complete reversion of the original disruption, since the original gene order, location, and copy number are precisely restored. A potential drawback to plasmid insertion mutants is possible spontaneous resolution of the integrated plasmid, but earlier studies by Rea et al. (26) illustrated 100% stability of the insertion mutant after four consecutive days of passaging. In the present study, 100 colonies were replica plated on day 5, giving a frequency of 1% reversion. Thus, the mutation is both nonpolar and sufficiently stable to allow genotype-phenotype comparisons.
FIG. 1.
(A) Genomic organization of the yyc operon in B. subtilis and the corresponding region of the L. monocytogenes EGDe genome. (B) Kyte and Doolittle hydrophobicity plots of HtrA in L. monocytogenes EGDe (gray line indicates putative leader peptide). (C) The frameshift mutation in the potential signal sequence of HtrA is represented by a dash. (D) Transcriptional analysis of lmo0291 and lmo0293. Total RNA was isolated from exponential-phase cultures of EGDe and the htrA mutant grown in BHI at 37°C. RNA was converted to cDNA, and PCRs were performed with lmo0291 and lmo0293 specific primers.
Computer-aided analysis of the htrA gene revealed two potential initiation codons. The first ATG encodes a methionine (Met 1) and is preceded by a weak ribosomal binding site (AGA 8 bp upstream of the start codon), as well as a consensus σB-dependent promoter binding site (GGGAAT-13 bp-GTTT) 358 bp upstream of Met 1. However, the annotation of the sequenced EGDe genome predicts that the translational start site is encoded by an ATG 120 bp downstream of Met 1, most likely due to the existence of a strong ribosomal binding site (AGGA) 4 bp upstream of Met 2 and also to the gene context, in that the second ATG is downstream of the stop codon of the previous gene, lmo0291, whereas Met 1 is within the lmo0291 coding region. Hydrophobicity analysis of the HtrA protein (from the Met 1 site) reveals two potential transmembrane domains (Fig. 1B). Significantly, the first transmembrane segment corresponding to the region between Met 1 and Met 2 is predicted (by Sigcleave and Signal P analysis) to function as a signal peptide, with a predicted cleavage site between positions 35 and 36. Thus, under certain conditions it is possible that HtrA is secreted into the extracellular environment. We created a frameshift mutation (HtrASS*) in the region encoding this potential signal sequence (Met 1-Met 2) (Fig. 1C) and analyzed its role in a variety of different stress conditions. Interestingly, the nonpathogenic strain L. innocua also possesses a potential leader peptide, which at 47 aa is even longer than that in the pathogenic species L. monocytogenes strains EGDe and 10403s (41 aa). Lastly, both L. monocytogenes EGDe and L. innocua lack a threonine at amino acid position 140 relative to L. monocytogenes 10403s. In addition, L. innocua also lacks a glycine at amino acid position 153.
Analysis of the heat stress response.
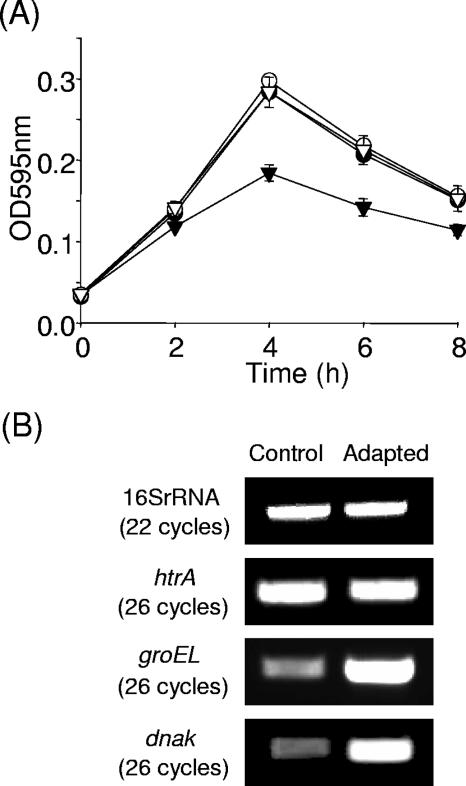
The wild-type, htrA mutant, HtrASS*, and revertant strains were analyzed for their responses to different temperatures (30, 37, and 42°C), but no difference in growth rate was observed (data not shown). Increasing the temperature to 44°C did, however, result in a significant reduction (P ≤ 0.001) in the growth rate of the htrA mutant but not of HtrASS* relative to the wild-type and revertant (Fig. 2A). Reflecting the observations of Wonderling et al. (31), the htrA mutant exhibited a bacteriostatic effect at the same time but at a lower level than the wild-type/revertant and HtrASS* strains. The absence of any growth defect in the frameshift mutant suggests that transcription of htrA under heat stress initiates at the annotated start codon, Met 2. Interestingly, despite HtrA playing a significant role in growth of Listeria at elevated temperatures, transcriptional analysis of htrA revealed no significant upregulation after heat shock (Fig. 2B). Both groEL and dnaK were used as controls for this experiment, since they are known to be transcriptionally upregulated under the conditions used (10).
FIG. 2.
(A) Growth of wild-type EGDe (•), revertant (○), htrA mutant (▾), and HtrASS* (▿) under sublethal heat conditions (44°C). Error bars represent standard deviations of triplicate experiments. (B) Transcriptional analysis of htrA by RT-PCR. Total RNA was isolated from exponential-phase cultures of EGDe grown in BHI at 37°C (Control) and exposed to 45°C for 30 min (Adapted). RNA was converted to cDNA and PCRs were performed with htrA, groEL, and dnaK specific primers.
Analysis of the general stress responses.
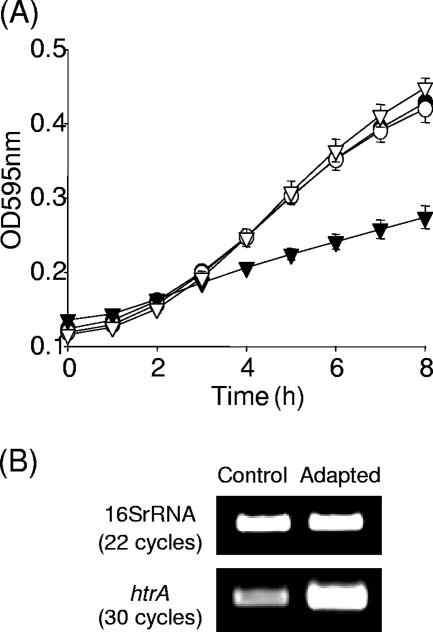
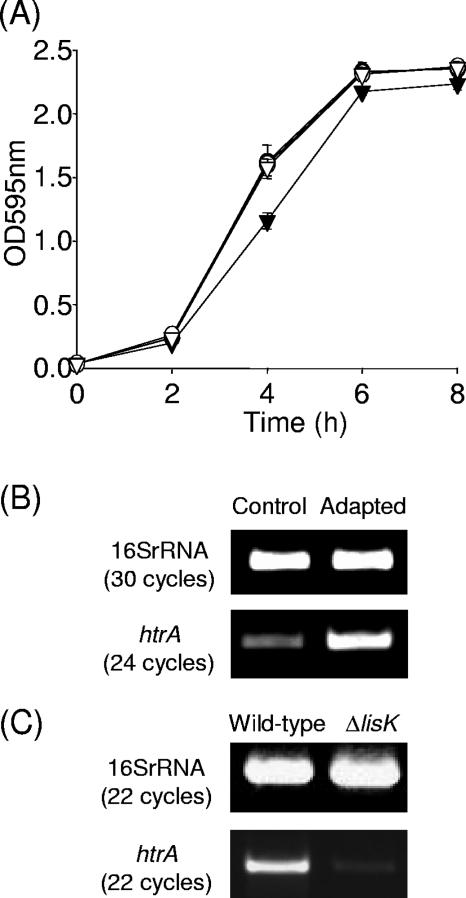
The sensitivity of the strains to acid, penicillin G, and H2O2 were examined. When the strains were cultured in media adjusted to pH 5 (mildly acidic condition representing a sublethal stress), a significant reduction (P ≤ 0.001) in growth rate was observed for the htrA mutant, but not HtrASS*, relative to the wild-type and revertant strains (Fig. 3A). This suggests an important role for HtrA in listerial growth under acidic stress and also implies that htrA is transcribed from Met 2 under acidic conditions. In support of this phenotypic analysis, transcriptional studies reveal a significant increase in levels of htrA transcript after exposure to pH 5 for 30 min (Fig. 3B). Since penicillin is widely used as the antibiotic of choice for the treatment of listerial infections (27), this antibiotic was chosen for further study. The penicillin phenotype was analyzed in broth supplemented with penicillin G (87.5 ng/ml, i.e., the level at which the greatest difference in survival of the htrA mutant relative to the wild type was observed [data not shown]). Penicillin G stress significantly (P ≤ 0.01) reduces the growth rate of the htrA mutant relative to the HtrASS* and wild-type or revertant strains (Fig. 4A), indicating a role for HtrA in listerial growth under penicillin stress. These data also suggest that transcription of htrA under penicillin G conditions initiates at Met 2. In addition, transcriptional analysis revealed that htrA was upregulated in response to this stress (Fig. 4B), suggesting a role for HtrA in the physiological response to this antibiotic. Disk assays were used to analyze the contribution of the htrA and HtrASS* mutants to H2O2 resistance. Interestingly, although Wonderling et al. (31) observed that the HtrA serine protease in L. monocytogenes 10403s was involved in H2O2 stress response at 37°C, we observed no difference in sensitivity to H2O2 (data not shown). This difference may be due to strain variation, a disparity in experimental protocol, or a combination of both.
FIG. 3.
(A) Growth of wild-type EGDe (•), revertant (○), htrA mutant (▾), and HtrASS* (▿) under sublethal acid conditions (pH 5, HCl). Error bars represent standard deviations of triplicate experiments. (B) Transcriptional analysis of htrA by RT-PCR. Total RNA was isolated from exponential-phase cultures of EGDe grown in pH 7 BHI (Control) and exposed to pH 5 for 30 min (Adapted). RNA was converted to cDNA, and PCRs were performed with htrA specific primers.
FIG. 4.
(A) Growth of wild-type EGDe (•), revertant (○), htrA mutant (▾), and HtrASS* (▿) under sublethal penicillin G conditions (87.5 ng/ml). Error bars represent standard deviations of triplicate experiments. (B and C) Transcriptional analysis of htrA by RT-PCR. (B) Total RNA was isolated from exponential-phase cultures of EGDe growing in BHI (Control) and exposed to penicillin G at 87.5 ng/ml for 30 min (Adapted). (C) Total RNA was extracted from stationary-phase cultures of LO28 wild-type and ΔlisK strains. RNA was converted to cDNA, and PCRs were performed with htrA specific primers.
Transcriptional regulation of htrA.
Given the altered acid and antibiotic resistance profile of the LisRK two-component regulatory system in L. monocytogenes LO28 (4, 5), as well as an observed role in heat tolerance (R. Sleator, unpublished data), we investigated the possible role of this two-component system in regulating htrA transcription in L. monocytogenes LO28. The level of htrA transcript observed against the ΔlisK background was greatly reduced relative to the wild type, suggesting a role for LisRK as a positive regulator of htrA (Fig. 4C).
Virulence.
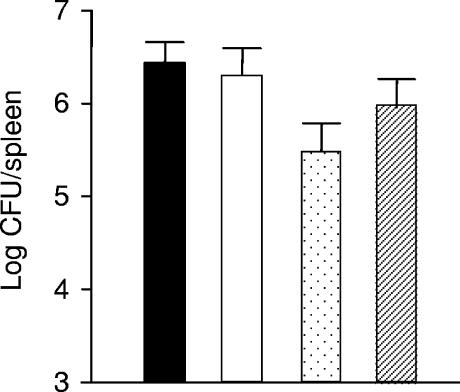
Given the significant role for HtrA in the virulence of gram-positive and gram-negative organisms (3, 12-14), we examined the influence of this locus on pathogenesis of L. monocytogenes in the murine model of infection. The htrA mutants isolated from mice were analyzed for the presence of the integrated plasmid (100 colonies analyzed), and no revertants were detected, indicating the inherent stability of the gene disruptions throughout the infection studies. Disrupting the htrA gene and creating a frameshift mutation in its putative signal sequence resulted in ∼1-log (P ≤ 0.01) and ∼0.5-log (P ≤ 0.1) reductions, respectively, in the numbers of the mutants relative to wild type or revertant strains in the spleens of infected mice 3 days after intraperitoneal infection (Fig. 5). This illustrates an important role for HtrA in contributing to listerial growth and survival during infection. Although this phenotype probably results from the increased sensitivity of the htrA mutant to the various stresses encountered during infection, the exact role of HtrA in the overall pathogenesis of L. monocytogenes remains to be determined. These data also demonstrate that a frameshift in the region upstream of Met 2 influences virulence potential.
FIG. 5.
Levels of EGDe (black bar), revertant (white bar), htrA mutant (dotted bar), and HtrASS* (hatched bar) in the spleens of BALB/c mice 3 days after intraperitoneal infection. Error bars represent the standard deviations of four experiments.
DISCUSSION
In silico analysis revealed that the immediate genomic organization of the HtrA-like serine protease in L. monocytogenes corresponds to the B. subtilis six-gene operon (yycF-yycK). Interestingly, the three HtrA homologues in B. subtilis—HtrA, YvtA, and YycK (22, 23)—have different chromosomal locations, and analysis revealed that the operon encoding yycK (yycF-yycK) exhibits highest homology to the putative operon in which the listerial htrA homologue exists (lmo0287-lmo0288) (SubtiList, ListiList). Noone et al. (23) showed that YycK is not heat shock inducible, unlike its HtrA homologues YkdA and YvtA. Given the conserved nature of the listerial and Bacillus operons and similar expression profiles for yycK and htrA (lmo0292), it is likely that the listerial HtrA is more similar to YycK than the classical heat shock-inducible HtrA serine proteases.
We have determined that both the EGDe and L. innocua HtrA proteins are missing a threonine at amino acid position 140 relative to that of L. monocytogenes strain 10403s. Interestingly, L. innocua, as well as lacking the threonine at amino acid position 140, also lacks a glycine at amino acid position 153. The close proximity of these two frameshift mutations suggests that this region may represent an important regulatory domain that is possibly linked to the virulence phenotype associated with the protein. In addition, by using an in silico-based approach we showed that htrA possesses a 123-bp extension upstream of the predicted start codon and in frame with the coding region. This 5′ extension is itself preceded by a consensus σB-dependent promoter-binding site (GGGAAT-13 bp-GTTTTA). Computer-aided analysis predicts that this 41-aa N-terminal extension may function as a leader peptide, suggesting that HtrA may, under certain conditions (possibly under the transcriptional control of σB), be secreted into the external environment. However, a frameshift mutation in the predicted signal region, followed by in vitro analysis under a variety of different stresses, revealed that htrA is transcribed from Met 2, the annotated start codon. Conversely, in vivo analysis suggests that the frameshift mutation in the region upstream of Met 2 influences virulence potential. The reduced virulence phenotype observed for the frameshift mutation suggests that stresses associated with the in vivo environment may signal HtrA secretion. Interestingly, HtrA homologues in B. subtilis are thought to have dual localization (anchored and secreted [1]), a situation which may hold true for Listeria.
Differentiating the HtrA-like serine protease of L. monocytogenes EGDe from the classical HtrA family of serine proteases prompted an analysis of the role of HtrA in the listerial general stress response. Consistent with the findings of Wonderling et al. (31), in vitro analysis reveals an important role for HtrA in listerial growth at elevated temperatures (>44°C). Overcoming the hurdle imposed by acidic conditions is essential for L. monocytogenes to initiate successful infection in the host even during systemic infection (20). We have shown that HtrA is necessary for listerial growth under acidic conditions and is transcriptionally upregulated after acid shock. Furthermore, HtrA appears to play a role in the vulnerability of susceptible bacteria to antibiotics (7, 30), specifically the cell wall acting antibiotic, penicillin G, and is transcriptionally upregulated in response to this stress.
Bacterial proteases have the potential to destroy the structural and functional proteins of host defense mechanisms. The HtrA serine protease of S. enterica serovar Typhimurium has been associated with virulence of this intracellular pathogen (13). In the present study we confirm a role for HtrA in listerial pathogenesis revealing an ∼1-log reduction in the level of the htrA mutant relative to the wild-type 3 days after intraperitoneal infection. Although this phenotype probably results from the increased sensitivity of the htrA mutant to the various stresses encountered during infection, the exact role of HtrA in the overall pathogenesis of L. monocytogenes remains to be determined. The current study adds L. monocytogenes to a growing list of gram-positive pathogens (12, 14) that require HtrA for full virulence potential. It is evident from our study that although HtrA is required for full virulence potential in L. monocytogenes, mutants in this locus demonstrate diminished rather than abrogated pathogenicity. This may reflect the fact that, after invasion of the host cell, L. monocytogenes escapes from the stressful environment of the phagosome into the relatively benign environment of the cell cytoplasm, where a requirement for HtrA may be diminished.
The listerial two-component regulatory system LisRK is involved in modulating a number of stress responses, namely, acid, antibiotic (4, 5), and heat stress (R. Sleator, unpublished data), as well as contributing to listerial pathogenesis (4). These phenotypes have all been linked to HtrA in the present study, thereby suggesting a possible role for LisRK in regulating htrA expression. Indeed, RT-PCR analysis revealed that htrA transcription is significantly reduced against the ΔlisK background, suggesting a possible role for LisRK as a positive regulator of htrA. We suggest that LisRK may sense perturbations in the external environment and in turn transcriptionally activate htrA either directly or through regulation of another system.
In conclusion, the HtrA-like serine protease appears to play an important role in listerial growth and survival under adverse conditions that may be encountered in foods and subsequently during host colonization. The role for HtrA in the virulence of a number of gram-positive and gram-negative pathogens suggests that this protein may represent an attractive target for the development of novel broad-spectrum antibiotic therapies.
Acknowledgments
We acknowledge the financial assistance of the Irish Government under National Development Plan 2000-2006 and funding of the Alimentary Pharmabiotic Centre by Science Foundation Ireland. R.D.S. is funded by an Embark postdoctoral fellowship and a joint ESCMID-FEMS research fellowship.
REFERENCES
- 1.Antelmann, H., E. Darmon, D. Noone, J.-W. Veening, H. Westers, S. Bron, O. P. Kuipers, K. M. Devine, M. Hecker, and J. M. van Diji. 2003. The extracellular proteome of Bacillus subtilis under secretion conditions. Mol. Micrbiol. 49:143-156. [DOI] [PubMed] [Google Scholar]
- 2.Begley, M., C. Hill, and C. G. M. Gahan. 2003. Identification and disruption of btlA, a locus involved in bile tolerance and general stress resistance in Listeria monocytogenes. FEMS Microbiol. Lett. 218:31-38. [DOI] [PubMed] [Google Scholar]
- 3.Cortes, G., B. de Astorza, V. J. Benedi, and S. Alberti. 2002. Role of the htrA gene in Klebsiella pneumoniae virulence. Infect. Immun. 70:4772-4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter, P. D., C. G. M. Gahan, and C. Hill. 1999. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 181:6840-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter, P. D., C. M. Guinane, and C. Hill. 2002. The LisRK signal transduction system determines the sensitivity of Listeria monocytogenes to nisin and cephalosporins. Antimicrob. Agents Chemother. 46:2784-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabret, C., and J. A. Hoch. 1998. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J. Bacteriol. 180:6375-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foucaud-Scheunemann, C., and L. Poquet. 2003. HtrA is a key factor in the response to specific stress conditions in Lactococcus lactis. FEMS Microbiol. Lett. 224:53-59. [DOI] [PubMed] [Google Scholar]
- 8.Fukuchi, K., Y. Kasahara, K. Asai, K. Kobayashi, S. Moriya, and N. Ogasawara. 2000. The essential two-component regulatory system encoded by yycF and yycG modulates expression of the ftsAZ operon in Bacillus subtilis. Microbiology 146:1573-1583. [DOI] [PubMed] [Google Scholar]
- 9.Gahan, C. G. M., and C. Hill. 1999. The relationship between acid stress responses in virulence in Salmonella typhimurium and Listeria monocytogenes. Int. J. Food Microbiol. 50:93-100. [DOI] [PubMed] [Google Scholar]
- 10.Gahan, C. G. M., J. O'Mahony, and C. Hill. 2001. Characterization of the groESL operon in Listeria monocytogenes: utilization of two reporter systems (gfp and hly) for evaluating in vivo expression. Infect. Immun. 69:3924-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-Del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. Mata Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueterm, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cosart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim, Y. M., A. R. McCluskey, and T. J. Mitchell. 2004. Role of HtrA in the virulence and competence of Streptococcus pneumoniae. Infect. Immun. 72:3584-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, K., L. Charles, G. Dougan, D. Pickard, P. O'Gaora, G. Costa, T. Ali, I. Miller, and C. Hormaeche. 1991. The role of a stress-response protein in Salmonella typhimurium virulence. J. Mol. Microbiol. 5:401-407. [DOI] [PubMed] [Google Scholar]
- 14.Jones, C. H., T. C. Bolken, K. F. Jones, G. O. Zeller, and D. E. Hruby. 2001. Conserved DegP protease in gram-positive bacteria is essential for thermal and oxidative tolerance and full virulence in streptococcus pyogenes. Infect. Immun. 69:5538-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kallipolitis, B. H., and H. Ingmer. 2001. Listeria monocytogenes response regulators important for stress tolerance and pathogenesis. FEMS Microbiol. Lett. 204:111-115. [DOI] [PubMed] [Google Scholar]
- 16.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipinska, B., O. Fayet, L. Baird, and C. Georgopoulos. 1989. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose gene product is essential for bacterial growth only at elevated temperatures. J. Bacteriol. 171:1574-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maguin, E., P. Duwat, T. Hege, D. Ehrlich, and A. Gruss. 1992. New thermosensitive plasmid for gram-positive bacteria. J. Bacteriol. 174:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Marron, L., N. Emerson, C. G. M. Gahan, and C. Hill. 1997. A mutant of Listeria monocytogenes LO28 unable to induce an acid tolerance response displays diminished virulence in a murine model. Appl. Environ. Microbiol. 63:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin, P. K., T. Li, D. Sun, D. P. Biek, and M. B. Schmid. 1999. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J. Bacteriol. 181:3666-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noone, D., A. Howell, and K. M. Devine. 2000. Expression of ykdA, encoding a Bacillus subtilis homologue of HtrA, is heat shock inducible and negatively autoregulated. J. Bacteriol. 182:1592-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noone, D., A. Howell, R. Collery, and K. M. Devine. 2001. YkdA and YvtA, HtrA-like serine protease in Bacillus subtilis, engage in negative auroregulation and reciprocal cross-regulation of ykdA and yvtA gene expression. J. Bacteriol. 183:654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park, S. F., and G. S. Stewart. 1990. High efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene 94:129-132. [DOI] [PubMed] [Google Scholar]
- 25.Raya, R., J. Bardowski, P. S. Andersen, S. D. Ehrlich, and A. Chopin. 1998. Multiple transcriptional control of the Lactococcus lactis trp operon. J. Bacteriol. 180:3174-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rea, R. B., C. G. M. Gahan, and C. Hill. 2004. Disruption of putative regulatory loci in Listeria monocytogenes demonstrates a significant role for Fur and PerR in virulence. Infect. Immun. 72:717-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlech, W. F., III. 2000. Food-borne listeriosis. Clin. Infect. Dis. 31:770-775. [DOI] [PubMed] [Google Scholar]
- 28.Sleator, R. D., C. G. M. Gahan, and C. Hill. 2003. A postgenomic appraisal of osmotolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 69:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, K., and P. Youngman. 1992. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochemie 74:705-711. [DOI] [PubMed] [Google Scholar]
- 30.Utaida, S., P. M. Dunman, D. Macapagal, E. Murphy, J. S. Projan, V. K. Singh, R. K. Jayaswal, and B. J. Wilkinson. 2003. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiol. 149:2719-2732. [DOI] [PubMed] [Google Scholar]
- 31.Wonderling, L. D., B. J. Wilkinson, and D. O. Bayles. 2004. The htrA (degP) gene of Listeria monocytogenes 10403S is essential for optimal growth under stress conditions. Appl. Environ. Microbiol. 70:1935-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]