Abstract
The fungal genus Stemphylium (Ascomycota) contains selfing species that evolved from outcrossing ancestors. To find out how selfing originated, we analyzed the Stemphylium MAT loci that regulate sexual reproduction in ascomycetes and compared MAT structures and phylogeny with a multigene Stemphylium species phylogeny. We found that some Stemphylium species' MAT loci contained a single gene, either MAT1-1 or MAT1-2, whereas others contained a unique fusion of the MAT1-1 and MAT1-2 regions. In all fused MAT regions, MAT1-1 was inverted and joined to a forward-oriented MAT1-2 region. As in the closely related Cochliobolus, Stemphylium species with fused MAT regions were able to self. Structural and phylogenetic analyses of the MAT loci showed that the selfing-conferring fused MAT regions were monophyletic with strong support. However, in an organismal phylogeny of Stemphylium species based on 106 isolates and four loci unrelated to mating, selfing arose in two clades, each time with strong support. Isolates with identical fused MAT regions were present in both clades. We showed that a one-time origin of the fused MAT loci, followed by a horizontal transfer across lineages, was compatible with the data. Another group of selfers in Stemphylium only had forward-oriented MAT1-1 at their MAT loci, constituting an additional and third origin of selfing in Stemphylium.
Keywords: filamentous ascomycetes, nonvertical inheritance, evolution, Pleospora, horizontal transfer
Among bacteria, lateral gene flow has redistributed characters of evolutionary importance (1). In eukaryotes including fungi, evolution through lateral gene transfer is often proposed, but alternative explanations for character distributions have been difficult to rule out (2). The mating type locus in the ascomycetous fungal genus Stemphylium has provided an opportunity to detect an ancient lateral transfer that changed a fungal breeding system. We used phylogenetic analyses based on five loci in conjunction with gene rearrangement data to show that the distribution of mating strategies in Stemphylium is best explained by a lateral transfer of the genes controlling mating.
In haploid filamentous ascomycetes, self-sterility and self-fertility depend on the configuration of genes at MAT, the mating type locus (3). Each self-sterile individual has only one of the two alternative single-copy genes, MAT1-1 or MAT1-2, at its MAT locus. Like large portions of the human X and Y chromosomes, the DNA and amino acid sequences of MAT1-1 and MAT1-2 are no more similar than expected by chance. Outside of the ≈1,100- to 4,200-bp MAT region, sequence similarity between homologous chromosomes reappears and haploid individuals of opposite mating types have syntenous flanking genes (4, 5).
In contrast to self-sterile species, haploid self-fertile species in Stemphylium (this study), as in the closely related Cochliobolus (6), contain both MAT1-1 and MAT1-2 genes in the same genome. Demonstrating that the presence of a MAT1-1/MAT1-2 fusion was enough to confer self-fertility, transformation of a mating-type deletion strain of the self-sterile species Cochliobolus heterostrophus with the fused MAT genes produced self-fertile individuals (7). Suggesting that self-fertility originated convergently, each of the four selfing species of Cochliobolus had unique splice sites adjacent to its MAT1-1 and/or MAT1-2 genes, and the direction of transcription of the two genes along with the length of the intergenic intervening sequences differed across species. In some cases, the physical linkage of the MAT alleles could be accounted for by a crossover event, for other arrangements the mechanism remained unknown (7). Phylogenies from housekeeping loci were congruent with one another, and they supported the MAT gene arrangements in showing that each self-fertile Cochliobolus species evolved independently from a self-sterile ancestor.
To investigate the origin of self-fertility in Stemphylium, we compared the position of self-fertile species in an organismal phylogeny and in a phylogeny of the mating-type genes. We analyzed the mating-type loci to assess homology of the gene arrangements in selfing and outcrossing species. We had expected that, as in Cochliobolus, self-fertility would have originated in different species by independent mating-type gene fusions. Instead, we found evidence for a single fusion that was transferred across lineages.
Materials and Methods
Fungal Strains Used. The 106 isolates of Stemphylium used were from the collection of P.I. or from other culture collections. Isolates were chosen to represent the genetic diversity of known Stemphylium strains, with an increased sampling around the type species S. herbarum. See Table 1, which is published as supporting information on the PNAS web site, for details.
Assessment of Self-Fertility. Self-fertility of the fungal strains was evaluated in the laboratory. Single ascospores or conidia were germinated on water agar, transferred to V8 agar plates upon germination (8), stored at room temperature or at 4°C, and periodically examined for the formation of sexual spores (ascospores) (9).
Molecular Work. Mycelium was scraped off the surface of a Petri plate, and DNA was extracted by using a standard phenol-chloroform extraction method (10). PCR reactions were performed by using a variety of conditions (see Supporting Methods in Supporting Text, which is published as supporting information on the PNAS web site).
Loci sequenced for species phylogenies were the ribosomal internal transcribed spacer (ITS), glyceraldehyde-3-phosphate dehydrogenase (GPD), elongation factor-1 α (EF-1 α) and the noncoding region between the vacuolar membrane ATPase catalytic subunit A gene (vmaA), and vpsA, a gene involved in vacuolar biogenesis (11). Primers were from refs. 12–14 or designed for this study based on DNA sequences available from GenBank (see Table 2, which is published as supporting information on the PNAS web site). Primers for the intergenic spacer between vmaA and vpsA were based on Cochliobolus heterostrophus sequences that G. Saenz and B. G. Turgeon obtained from the former Torrey Mesa Research Institute.
Primers used for amplification of the MAT regions were designed based on DNA sequences (15, 16), and later, on sequences from this study (see Tables 3–8, which are published as supporting information on the PNAS web site).
To determine DNA sequences of the entire mating-type genes and flanking regions, the chromosome walking kit Vectorette was used (Sigma-Genosys, The Woodlands, TX). Additional primers were designed from previously sequenced conserved DNA binding regions of the MAT genes and used together with the kit primers for PCR amplifications of MAT flanks (see Supporting Methods and Tables 9–11, which are published as supporting information on the PNAS web site).
PCR products were completely sequenced in both directions by using a Big Dye Terminator Cycle Sequencing Kit v2.0 or 3.0 (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions with a variety of sequencing primers (see Supporting Methods and Tables 9–11).
Generation of Species Phylogenies. DNA sequences were assembled by using autoassembler 1.4.0 (Applied Biosystems PerkinElmer) and DNA sequence alignments were generated by clustalx 1.8 (17) by using default settings and manually optimized in se-al 1.d1 (18). For some of the isolates used in this study, DNA sequences were retrieved from GenBank (accession nos. AF442783, AF442788, AF442789, AF443882, AF443887, and AF443888). All other sequences were generated in this study. Phylogenies were inferred with four algorithms by using paup* 4.0b10 for parsimony, likelihood, and neighbor-joining (19), and mrbayes 3.0b4 for Bayesian analyses (20).
The most parsimonious trees were inferred by using 30 heuristic searches with random addition of taxa but default settings otherwise, coding gaps as missing characters. Bootstrap support was from 500 replicates by using random taxon addition but, otherwise, default settings.
For likelihood analyses, base frequencies, transition-transversion ratio, proportion of invariable sites, and gamma shape parameter were estimated from a most parsimonious tree. The gamma distribution was approximated by four rate categories. Most likely trees were estimated by using 30 heuristic searches with random taxon addition and, otherwise, default settings. Bootstrap support values were based on 185 replicates.
Neighbor-joining analyses were done by using likelihood modeled distances with parameters estimated on a most parsimonious tree. Branch support was evaluated with 500 bootstrap replicates.
For Bayesian analyses, a general time reversible model of evolution was used. Rate heterogeneity across sites was modeled with a gamma distribution. Four chains starting with a random tree were run for 1 million generations, retaining each 100th tree. The first 1,000 trees of the 10,000 collected trees were discarded, and the posterior probabilities were based on the remaining 9,000 trees. The 50% majority rule consensus tree was calculated in paup*.
Combinability of data sets was evaluated by using the partition homogeneity test as implemented in paup* 4.0b10 (19).
Mating Type Screening. All 106 Stemphylium strains were screened for the presence of MAT genes and MAT gene arrangement. At first, specific PCR primers were designed based on ref. 15. For MAT1-1, primer pair Jen1f/Jen1r was designed on the conserved DNA binding motif alpha box (see Supporting Methods). The MAT1-2 primer pair was Jen2f/Jen2r, situated on the conserved DNA binding high mobility group box motif. Later, new primers were designed to fit all Stemphylium strains. For MAT1-1, these primers were Alpha181f, used together with Jen1r, and HMG85f, used with Jen2r for MAT1-2 screening (see Supporting Methods).
Generation of Mating-Type DNA Phylogenies. For MAT1-1 analyses, the inverted parts of the fused MAT region from 11 selfing species were combined in an alignment with homologous regions from 11 species with separate MAT regions. Similarly, the MAT1-2 parts from species with fused MAT regions were used for analyses together with homologous regions from five species with separate MAT regions. Settings for phylogenetic analyses were as for the four loci species data set except that likelihood bootstrap support percentages were based on 500 replicates.
Generation of Mating-Type Protein Phylogenies. The MAT gene DNA sequences were translated into amino acids. To the MAT1-1 protein alignment, five outgroup taxa from GenBank were added (Alternaria alternata, AB009451; Cochliobolus cymbopogonis, AF129744; C. heterostrophus, AF029913; C. ellisii, AF129746; C. sativus AF275373). The MAT1-2 alignment contained the same outgroup taxa as MAT1-1 (A. alternata, AB009452; C. cymbopogonis, AF129745; C. heterostrophus, AF027687; C. ellisii, AF129747; C. sativus, AF275374). The alignments were generated by clustalx 1.8 (17). For parsimony analyses, the phylip 3.572 package was used (21). Most parsimonious trees were inferred by using protpars. Default options were used, except that input order was randomized and 30 random sequence additions were used (jumble set to 30). Strict consensus trees were calculated and drawn by using paup* (19). For bootstrap analyses, 500 random data sets were created by using seqboot with default settings and analyzed in protpars with one random taxon addition per data set (jumble set to 1). For likelihood trees, the quartet puzzling algorithm in tree-puzzle 5.0 (22) was used with default settings, except that heterogeneous rates were approximated with four gamma rate categories. For distance trees, the distance matrix obtained with tree-puzzle 5.0 was imported into the fitch component of phylip 3.572 (21) to construct and optimize the tree topology by using a Fitch–Margoliash method with default settings, except that the global optimization option was in effect and the input order was randomized with the jumble option set to 30. The resulting tree was manipulated in macclade 4.03 (23), and printed in paup* (19).
Testing of Alternative Phylogenetic Hypotheses. Alternative phylogenetic hypotheses were evaluated with the Shimodaira-Hasegawa test as implemented in paup* 4.0b10 (19) by using default settings, except that the test was one-sided and full optimization was chosen. For each round of testing, the most likely tree was evaluated against the most parsimonious trees obtained given a topological constraint. Topological constraints generated in macclade 4.03 (23) were monophyly of the taxa with fused MAT regions in the species phylogeny and nonmonophyly of the taxa with fused MAT regions for the MAT1-1 and MAT1-2 DNA phylogenies. Constrained most parsimonious trees were inferred as described for the species phylogenies.
Results
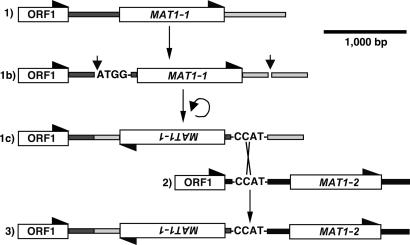
MAT Genes from Self-Sterile Isolates. In 23 self-sterile isolates, one of the two opposite mating type genes, either MAT1-1 or MAT1-2, was detected (Fig. 1). Presence of a single gene at the MAT locus was confirmed by sequencing of the MAT locus in 17 representatives. Sequencing coverage was 2203–4269 bp for the 11 MAT1-1 regions (from 34–280 bp inside ORF1, a conserved ORF of unknown function, to 322–2,373 bp downstream of MAT1-1) and 2,571–7,248 bp for the six MAT1-2 regions [from 21–321 bp inside ORF1 to 397–4,804 bp downstream of MAT1-2, including 291 bp of β glucosidase gene (BGL1) in strain P232]. MAT1-1 and MAT1-2 were respectively 1,193 and 1,093 bp in length.
Fig. 1.
Alternative arrangements of the opposite mating-type genes MAT1-1 and MAT1-2 at the mating-type locus in Stemphylium. Obligate outcrossers, in their dominant haploid phase, have the ancestral condition, either a forward-oriented MAT1-1 (1) or a forward-oriented MAT1-2 (2). Fused genes of self-fertile species carry a reversed MAT1-1 (3) with its flank fused to a forward MAT1-2. White boxes are genes, and half arrowheads indicate direction of transcription. ORF1 is a flanking gene of unknown function (7). The fused mating-type region may have evolved through 1b, an inversion of a MAT1-1 region that had a short “ATGG” sequence producing 1c with a CCAT motif. The juxtaposition of the CCAT from MAT1-1 may have permitted crossover with a forward-oriented MAT1-2 region (2), leading to the fused MAT regions (3) found in Stemphylium. The CCAT motif is always present at fusion sites where MAT1-1 is joined to MAT1-2 (Fig. 2). A CCAT motif also appears in the 5′ flank of unfused MAT1-2 regions, homologous to the putative crossover site (Fig. 2).
MAT Genes from Self-Fertile Isolates with Fused MAT Regions. PCR screens showed that 76 self-fertile isolates contained an inverted MAT1-1 gene less than ≈1.5 kb from a MAT1-2 gene (Fig. 1). A representative selection of 17 fused MAT regions were sequenced. Sequencing coverage was 3,851–6,102 bp [from 255 bp inside GTPase activating protein gene (GAP1) in strain P1, for remaining isolates beginning 44–167 bp within ORF1, to 418-1300 bp downstream of MAT1-2]. MAT genes from fused regions were equal in length to the ones from separate MAT regions.
MAT Genes from Self-Fertile Isolates with Only MAT1-1 Detected. The remaining seven isolates included in this study were also self-fertile and all were confined to group SP (Fig. 3; group SP is represented by strains P56 and P107). However, based on PCR screening of all isolates in SP, only MAT1-1 was detected, in forward orientation as in outcrossers. A total of 15 isolates from meiotic spores (ascospores) in group SP were screened for the presence of MAT genes by using PCR primer sets Alpha181f/Jen1ri and Jen2f/Jen2r (Tables 3–6). These seven isolates were in Fig. 5, which is published as supporting information on the PNAS web site, and eight additional isolates of Stemphylium sp. strain P56 were not listed in Table 1. Assuming that opposite mating-type genes MAT1-1 and MAT1-2 segregate in equal proportions as expected after meiosis, the odds that all 15 ascospores selected at random are MAT1-1 mating type would be one in 32,768, a rare event (P < 0.0001). Thus, MAT1-2 might be absent in this group, or we were unable to detect it. In the three isolates sequenced, MAT1-2 was not present 2,450 bp upstream or 4,813 bp downstream of the MAT1-1 gene (isolates Stemphylium sp. strains P56, P107, and P342; GenBank accession nos. AY339851, AY339852, and AY339862, respectively).
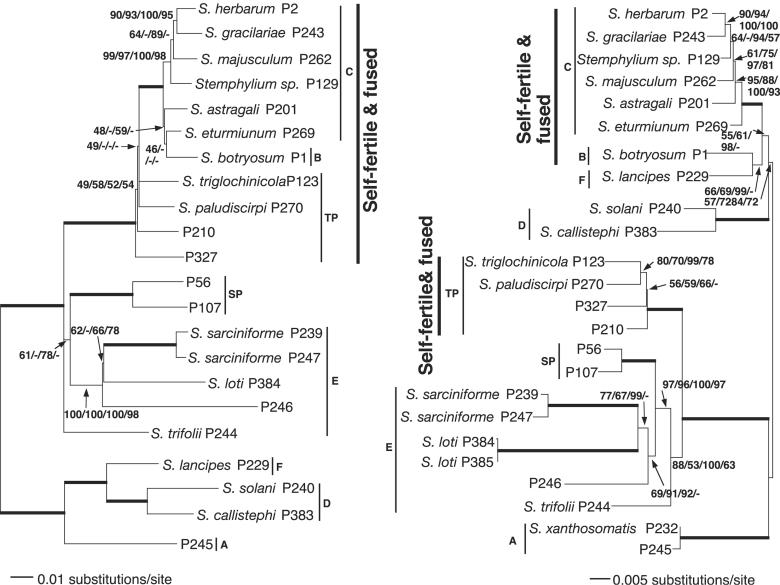
Fig. 3.
The self-fertile species with fused mating-type regions are monophyletic in a mating-type region phylogeny but polyphyletic in a phylogeny based on four other loci. The most likely phylogenetic tree from the Stemphylium mating-type MAT1-1 region (Left) is thus incongruent with the most likely, four-locus species tree (Right). Trees are rooted based on mating-type amino acid phylogenies by using outgroups (see Figs. 8 and 9). Lengths of horizontal branches are proportional to substitutions per site. Support values by the branches are from likelihood/parsimony/Bayesian/neighbor-joining. Branches in bold were supported by 100% in all analyses. Phylogentic groups from ref. 24 and this study are indicated by the narrow vertical lines. Self-fertile species with fused mating-type regions are indicated by bold vertical lines. For unnamed taxa, only strain numbers are given.
Phylogenetic Analyses and Taxon Selection for Figs. 2, 3, 4. Two rounds of phylogenetic analyses attributed the 106 Stemphylium isolates to 22 phylogenetic species represented by 28 strains in Figs. 2, 3, 4. Preliminary parsimony analyses of the initial 106 taxa in the combined ITS, GPD, and EF-1 α data set was used to select 53 isolates for sequencing of the fourth locus, vmaA-vpsA (see Fig. 5). After a partition homogeneity test indicated that the 53 taxa, ITS, GPD, EF-1 α, and vmaA-vpsA data sets were not significantly different (P = 0.179), they were pooled into a 2,371 character data set and analyzed by using likelihood, parsimony, Bayesian, and neighbor-joining methods (see Supporting Results in Supporting Text and Fig. 6, which is published as supporting information on the PNAS web site, for additional details). A representative taxon sampling for the species tree in Figs. 3 and 4 was achieved by choosing isolates from the phylogenetic species as follows. If the species had fused MAT regions, one MAT region was used for final analyses. If the species had MAT1-1 and MAT1-2 in separate individuals, then one of each was chosen, if available. For ease of interpretation and to facilitate analyses, the MAT1-2 strains P306, P310, and P252 were excluded from species analyses and Figs. 3 and 4. In preliminary phylogenetic analyses, these isolates had identical four locus genotypes to the respective cospecific MAT1-1 strains P247, P239, and P240 (see Fig. 6).
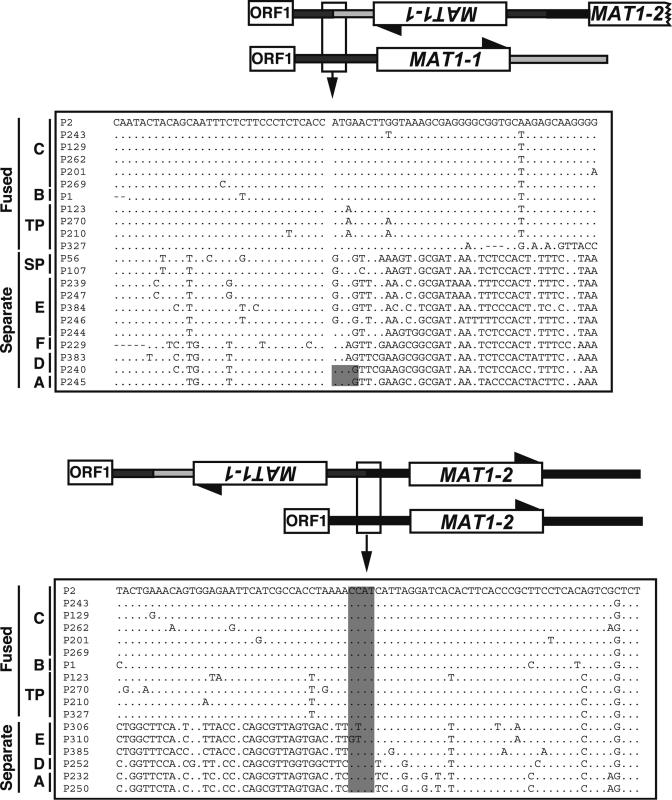
Fig. 2.
DNA sequence alignments (within boxes) showing the splice sites in the fused region containing mating-type genes MAT1-1 and MAT1-2 in self-fertile species of Stemphylium. The fused regions are aligned with their unfused homologs from other species. ORF1 is a flanking gene of unknown function (7). The top part of the diagram is an ORF1 proximal splice junction; bottom part is an ORF1 distal junction. The sequences are arranged by phylogenetic groups as in Fig. 3. Dots indicate nucleotide identity to P2. In the upper sequence box, the column of spaces indicates the junction between the forward flank and the inverted MAT1-1 region, where sequence homology between fused and unfused regions is lost. The shaded nucleotide positions indicate the ATGG that would, through inversion, produce a CCAT motif. In the lower sequence box, the shaded CCAT is the postulated crossover site with MAT1-1 to the left and MAT1-2 to the right.
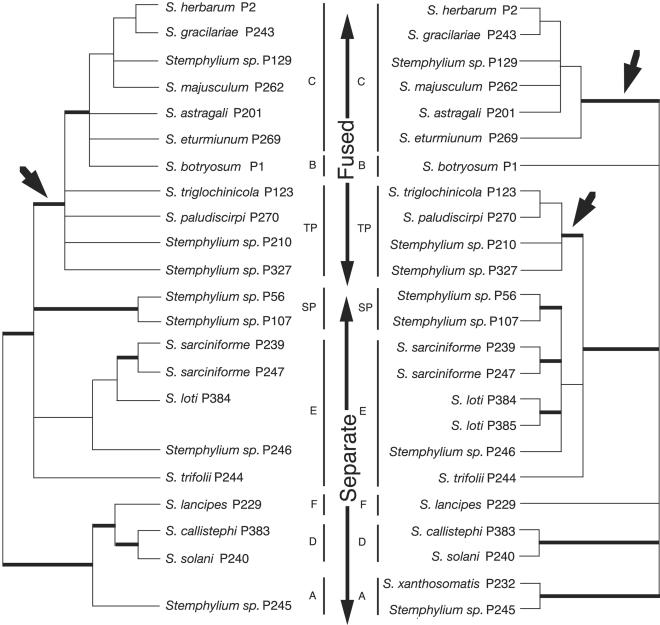
Fig. 4.
The Stemphylium MAT1-1 mating-type region phylogeny contradicts the four-locus species phylogeny. Fused mating-type region sequences were monophyletic in the consensus tree (Left), but the species with the fusions were polyphyletic in the four-locus species consensus phylogeny (Right). Branches with <70% support in bootstrap and Bayesian analyses were collapsed. Branches in bold had 100% support in all analyses. Distribution of fused and separate mating-type regions is given by long vertical arrows. Short arrows represent conflicting inferred evolutionary origins of fused mating-type regions between the MAT1-1 region phylogeny and four-locus species phylogeny. Phylogenetic groups from ref. 24 and this study are given by the narrow vertical lines. The MAT1-1 tree has fewer taxa than the species phylogeny because of the absence of isolates of the opposite mating type.
The representative most likely species tree (-ln likelihood = 8,197) containing 24 strains is illustrated in Fig. 3 as a phylogram and in Fig. 4 as a simplified diagram to highlight well supported branches relevant to the conclusions of this study. Branches supporting the polyphyly of isolates with fused MAT regions received maximal support in all four methods of analysis (Figs. 3 and 4).
In the MAT DNA analyses, the MAT1-1 data set consisted of 22 strains representing 22 species and the MAT1-2 data set contained 16 strains from 16 species. Eleven strains from 11 species were present in both data sets. The MAT1-1 data set of 1,688 characters was analyzed by using likelihood, parsimony, Bayesian, and neighbor-joining methods. The most likely tree is shown in Figs. 3 and 4 (-ln likelihood = 8,389). Parsimony, Bayesian, and neighbor-joining analyses were congruent for branches relevant in this study, with 100% support from all analyses for the monophyly of MAT1-1 from fused regions (Figs. 3 and 4). MAT1-2 also strongly supported the monophyly of the fused MAT regions. The MAT1-2 data set consisted of 1,632 characters. It was analyzed as MAT1-1, and the most likely tree is illustrated in Fig. 7, which is published as supporting information on the PNAS web site (-ln likelihood = 6,215).
Phylogenetic Analyses of MAT1-1 Proteins. The MAT1-1 data set contained 26 representatives reflecting the total diversity of the 31 Stemphylium strains for which MAT1-1 was sequenced and five outgroup species. The amino acid alignment of 429 characters was analyzed by using parsimony, likelihood, and distance algorithms yielding congruent results (see Supporting Results in Supporting Text). The Fitch–Margoliash distance tree in Fig. 8, which is published as supporting information on the PNAS web site, shows that Stemphylium was monophyletic with 100% parsimony and 92% quartet puzzling support. A monophyletic clade (groups B, C, and TP) of MAT1-1 proteins from fused MAT regions was the sister group, with 88% parsimony bootstrap support and 81% quartet puzzling support, to a clade of proteins from unfused MAT regions (groups SP plus E). A second, basal clade also consisted of proteins from unfused mating type regions (groups F, A, and D). This phylogenetic configuration indicated that the fused MAT region was derived from ancestral separate MAT regions (Fig. 8).
Phylogenetic Analyses of MAT1-2 Proteins. The MAT1-2 data set contained 19 representatives reflecting the total diversity of the 23 Stemphylium isolates for which MAT1-2 was sequenced and five outgroups. The amino acid alignment of 365 characters was analyzed with the same three algorithms as MAT1-1. MAT1-2 protein analyses were congruent with MAT1-1 analyses in showing that Stemphylium was monophyletic and that the fused MAT regions of the self-fertile species were monophyletic within Stemphylium and were derived from separate MAT regions (see Supporting Results in Supporting Text; see also Fig. 9, which is published as supporting information on the PNAS web site).
Testing of Alternative Phylogenetic Hypotheses. The Shimodaira–Hasegawa test showed that constrained monophyly of the taxa with fused MAT regions in the species phylogeny yielded significantly worse trees. Of the six constrained most parsimonious trees of 902 steps each, all were significantly worse than the most likely tree (P < 0.05). In MAT DNA analyses, constraining taxa with fused MAT regions to be polyphyletic yielded 24 and 2 most parsimonious trees of 1,176 and 784 steps each for MAT1-1 and MAT1-2, respectively, all worse than the corresponding most likely tree (P < 0.05) (data not shown).
Discussion
The genus Stemphylium is closely related to Cochliobolus, suggesting that the mating systems of the two genera might have evolved in similar ways. Our results showed that, as in self-sterile isolates of Cochliobolus, each of the 23 self-sterile isolates of Stemphylium that was tested had only one mating-type gene, either MAT1-1 or MAT1-2 (Fig. 1). Unexpectedly and unlike Cochliobolus, in Stemphylium, only one type of fused MAT region was found and it was present in 76 self-fertile isolates, all lacking separate MAT regions (Fig. 1). In all selfers with fused MAT regions, an inverted MAT1-1 gene was inserted between a MAT1-2 and its flanks (Fig. 1). Also in all fused MAT regions, the 5′ and 3′ splices were at homologous positions in the DNA sequence alignment (Fig. 2). Further, in gene genealogies, all MAT1-1 genes from fused MAT regions were monophyletic with 100% bootstrap support in all analyses (Fig. 3). Similarly, in genealogies of MAT1-2, the genes from fused MAT regions were monophyletic with 100% bootstrap support (see Fig. 7). Phylogenetic testing supported monophyly of the fused MAT regions, because constraining the fused MAT regions to be polyphyletic resulted in significantly worse trees. In both cases, direction of evolution from separate to fused MAT regions was suggested by phylogenetic analyses. Using amino acid translations of the MAT sequences and including outgroup sequences in the analyses, separate MAT regions were basal to fused MAT regions for both MAT1-1 and MAT1-2, suggesting ancestry of the separate MAT regions (see Figs. 8 and 9). Evolution of fused MAT regions from separate progenitors was supported by DNA sequence comparison showing that selfers with fused MAT regions could have originated from a self-sterile ancestor by the inversion of an ancestral MAT1-1 gene plus flanking regions, creating a crossover site allowing the fusion of the inverted MAT1-1 region to MAT1-2 (Fig. 1). However it arose, in Stemphylium, unlike Cochliobolus, the fused mating-type genes characterizing the self-fertile isolates had a single origin. The Stemphylium species phylogeny, on the other hand, suggested otherwise.
In contrast to the single origin of the fused mating-type genes, the four-locus Stemphylium species phylogeny showed two independent origins of the self-fertile isolates with fused MAT regions (Fig. 3). The self-fertile species with the mating type gene fusion in the TP cluster (Fig. 4) form a strongly supported monophyletic group with the species with separate mating-type genes in cluster E rather than with the self-fertile species with the fusion in cluster C. Constraining clusters C and TP to be monophyletic resulted in significantly worse trees, lending additional support to the polyphyly of the fused MAT regions. The contradiction between MAT gene trees and the species phylogenies can be resolved by invoking a lateral transfer of the MAT1-1 and MAT1-2 gene fusion across lineages in the evolution of Stemphylium.
The mechanism and direction of transfer of the mating-type region between lineages is unknown. A rare sexual or asexual hybridization event, followed by lineage sorting, might have led to the gene distribution found in selfers. In our studies, we have used 106 strains of Stemphylium and found no evidence for any other hybridization across lineages. Kondo et al. (25) showed that a fragment of at least 11 kb was transferred between a bacterial endosymbiont and an insect host. The fused MAT region in Stemphylium is <10 kb and, thus, not unreasonably long to be transferred asexually.
No other evolutionary event could have caused the discontinuous distribution of the fused MAT regions in the Stemphylium species phylogeny (Fig. 3). We found no evidence for sources of phylogenetic error such as extreme substitution rate heterogeneity or paralogy (26). Convergent evolution of the fusion is ruled out by the monophyly of the individual MAT1-1 (Figs. 3 and 4) and MAT1-2, right of gray box components of the fusions and by the identical arrangement and splice sites of all of the fused MAT genes (Fig. 2). The high similarity of the MAT1-1 (see sequences in Fig. 2 Upper, right of gray box, and Lower, left of gray box) sequences of self-fertile species in clusters B, C, and TP indicates that the fusion originated recently relative to the divergence of B and C from TP in the species tree (Figs. 3 Right and 4 Right). Its recent age suggests that the fusion had not persisted as an ancient polymorphism during the evolution of the shared ancestors of B, C, E, and TP.
In addition to selfers with fused MAT regions, Stemphylium contained another, not closely related, clade of selfers. This clade was group SP, where only MAT1-1 was detected, present in forward orientation as in self-sterile isolates. Group SP may represent a third lineage that evolved selfing independently, with unknown genetic changes responsible for its evolution. Self-fertile isolates with only one detectable MAT allele have also been reported in Neurospora africana (27).
Our data thus show convincingly that selfing in Stemphylium evolved three times from self-sterile ancestors. One time was by fusion of MAT1-1 and MAT1-2, a second time by horizontal transfer of the fused MAT locus across lineages, and a third time by unknown means in the presence of only MAT1-1.
Supplementary Material
Acknowledgments
We thank the following institutions and individuals: the former Torrey Mesa Research Institute/Syngenta for access to the vmaA-vpsA genes in the unpublished C. heterostrophus genome sequence; G. Saenz for assistance with annotation of the vmaA-vpsA genes; A. and R. Bandoni, S. Landvik, and G. Zhang (University of British Columbia, Vancouver), M. E. Barr (Sidney, BC, Canada), N. O'Neill (U.S. Department of Agriculture Research Service, Beltsville, MD), E. Simmons (Crawfordsville, IN), and the Culture Collection of Fungi, Technical University of Denmark, Department of Biotechnology, Lyngby, Denmark, for generous contributions of fungi and/or help with field work; Seara Lim for laboratory assistance; L. Sigler of the University of Alberta Microfungus Collection and Herbarium and T. Merkx of the Centraalbureau voor Schimmelcultures for incorporating our strains into their collections. This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) operating grants (to M.L.B.), National Science Foundation Grant DEB-9806935 (to B.G.T.) (subcontract M.L.B.), and a University of British Columbia Graduate Fellowship and an NSERC postgraduate fellowship (to P.I.).
Author contributions: P.I., B.G.T., and M.L.B. designed research; P.I. and J.H. performed research; P.I. analyzed data; and P.I. and M.L.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: ITS, internal transcribed spacers.
Data deposition: The DNA sequences reported in this paper have been deposited in the GenBank database (accession nos. AY316968–AY316973, AY316975–AY316978, AY316980, AY316982–AY316989, AY316991–AY317074, AY324671–AY324776, AY329168–AY329270, AY329271–AY329323, AY335164–AY335180, AY339853–AY339864, and AY340940–AY340945). Alignments have been submitted to TreeBASE (study accession no. S1313; Matrix accession nos. M2301–M2305).
References
- 1.Ochman, H., Lawrence, J. G. & Groisman, E. A. (2000) Nature 405, 299-304. [DOI] [PubMed] [Google Scholar]
- 2.Rosewich, U. L. & Kistler, H. C. (2000) Annu. Rev. Phytopathol. 38, 325-363. [DOI] [PubMed] [Google Scholar]
- 3.Coppin, E., Debuchy, R., Arnaise, S. & Picard, M. (1997) Microbiol. Mol. Biol. Rev. 61, 411-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turgeon, B. G., Bohlmann, H., Ciuffetti, L. M., Christiansen, S. K., Yang, G., Schafer, W. & Yoder, O. C. (1993) Mol. Gen. Genet. 238, 270-284. [DOI] [PubMed] [Google Scholar]
- 5.Cozijnsen, A. J. & Howlett, B. J. (2003) Curr. Genet. 43, 351-357. [DOI] [PubMed] [Google Scholar]
- 6.Turgeon, B. G. (1998) Annu. Rev. Phytopathol. 36, 115-137. [DOI] [PubMed] [Google Scholar]
- 7.Yun, S.-H., Berbee, M. L., Yoder, O. C. & Turgeon, B. G. (1999) Proc. Natl. Acad. Sci. USA 96, 5592-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawksworth, D. L., Kirk, P. M., Sutton, B. C. & Pegler, D. N. (1995) Dictionary of the Fungi (CAB International, Wallingford, Oxon, U.K.).
- 9.Simmons, E. G. (1969) Mycologia 61, 1-26. [PubMed] [Google Scholar]
- 10.Lee, S. B. & Taylor, J. W. (1990) in PCR Protocols, eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J. (Academic, San Diego), pp. 282-287.
- 11.Tarutani, Y., Ohsumi, K., Arioka, M., Nakajima, H. & Kitamoto, K. (2001) Gene 268, 23-30. [DOI] [PubMed] [Google Scholar]
- 12.Gardes, M. & Bruns, T. D. (1993) Mol. Ecol. 2, 113-118. [DOI] [PubMed] [Google Scholar]
- 13.White, T. J., Bruns, T. D., Lee, S. B. & Taylor, J. W. (1990) in PCR Protocols, eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J. & White, T. J. (Academic, San Diego), pp. 315-322.
- 14.Berbee, M. L., Pirseyedi, M. & Hubbard, S. (1999) Mycologia 91, 964-977. [Google Scholar]
- 15.de Jesus Yanez Morales, M. (2001) Ph.D. thesis (Cornell University, Ithaca, NY).
- 16.Berbee, M. L., Payne, B. P., Zhang, G., Roberts, R. G. & Turgeon, B. G. (2003) Mycol. Res. 107, 169-182. [DOI] [PubMed] [Google Scholar]
- 17.Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. & Higgins, D. G. (1997) Nucleic Acids Res. 24, 4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rambaut, A. (1995) se-al v1.d1 (Department of Zoology, University of Oxford, Oxford).
- 19.Swofford, D. L. (2002) paup*. Phylogenetic Analysis Using Parsimony (*and Other Methods) (Sinauer Associates, Sunderland, MA), Version 4.
- 20.Huelsenbeck, J. P. & Ronquist, F. (2001) Bioinformatics 17, 754-755. [DOI] [PubMed] [Google Scholar]
- 21.Felsenstein, J. (2001) phylip 3.572 (University of Washington, Seattle).
- 22.Strimmer, K. & von Haeseler, A. (1996) Mol. Biol. Evol. 13, 964-969. [Google Scholar]
- 23.Maddison, D. R. & Maddison, W. P. (2001) (Sinauer Associates, Sunderland, MA).
- 24.Câmara, M. P. S., O'Neill, N. R. & van Berkum, P. (2002) Mycologia 94, 660-672. [DOI] [PubMed] [Google Scholar]
- 25.Kondo, N., Nikoh, N., Ijichi, N., Shimada, M. & Fukatsu, T. (2002) Proc. Natl. Acad. Sci. USA 99, 14280-14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avise, J. C. (1994) Molecular Markers, Natural History, & Evolution (Chapman & Hall, New York).
- 27.Glass, N. L., Vollmer, S. J., Staben, C., Grotelueschen, J., Metzenberg, R. L. & Yanofsky, C. (1988) Science 241, 570-573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.