Abstract
All auxiliary α2δ subunits of voltage-gated Ca2+ (CaV) channels contain an extracellular Von Willebrand factor-A (VWA) domain that, in α2δ-1 and -2, has a perfect metal-ion-dependent adhesion site (MIDAS). Modeling of the α2δ-2 VWA domain shows it to be highly likely to bind a divalent cation. Mutating the three key MIDAS residues responsible for divalent cation binding resulted in a MIDAS mutant α2δ-2 subunit that was still processed and trafficked normally when it was expressed alone. However, unlike WT α2δ-2, the MIDAS mutant α2δ-2 subunit did not enhance and, in some cases, further diminished CaV1.2, -2.1, and -2.2 currents coexpressed with β1b by using either Ba2+ or Na+ as a permeant ion. Furthermore, expression of the MIDAS mutant α2δ-2 reduced surface expression and strongly increased the perinuclear retention of CaVα1 subunits at the earliest time at which expression was observed in both Cos-7 and NG108–15 cells. Despite the presence of endogenous α2δ subunits, heterologous expression of α2δ-2 in differentiated NG108–15 cells further enhanced the endogenous high-threshold Ca2+ currents, whereas this enhancement was prevented by the MIDAS mutations. Our results indicate that α2δ subunits normally interact with the CaVα1 subunit early in their maturation, before the appearance of functional plasma membrane channels, and an intact MIDAS motif in the α2δ subunit is required to promote trafficking of the α1 subunit to the plasma membrane by an integrin-like switch. This finding provides evidence for a primary role of a VWA domain in intracellular trafficking of a multimeric complex, in contrast to the more usual roles in binding extracellular ligands in other exofacial VWA domains.
Keywords: integrin, neuron, motif, expression
Voltage-gated Ca2+ (CaV) channels are composed of a poreforming α1 subunit that determines the main biophysical properties of the channel. For the CaV1 and -2 subfamilies, this subunit is associated with an intracellular β subunit (for review, see refs. 1 and 2) and a membrane-anchored, predominantly extracellular α2δ subunit (for review, see ref. 3). Mammalian genes encoding 10 α1, 4 β, and 4 α2δ subunits have been identified (for reviews, see refs. 2 and 4). The topology of the α2δ protein has been determined in detail only for α2δ-1 but is thought to generalize to all 4 α2δ subunits (for review, see ref. 3). All α2δ subunits have predicted N-terminal signal sequences, indicating that the N terminus is extracellular. In early studies of α2δ-1 purified from skeletal and cardiac muscle, it was determined that the α2 subunit is disulfide-bonded to a transmembrane δ subunit, and both subunits are the products of a single gene, encoding the α2δ protein, that is posttranslationally cleaved into α2 and δ (5).
Subsequent to the identification of α2δ subunits as stoichiometric components of skeletal muscle Ca2+ channels, α2δ subunits have also been shown to be associated with native cardiac (L-type) (6) and neuronal N- and P/Q-type channels (7, 8). In coexpression studies where it has been tested, all α2δ subunits enhance all CaV1 and -2 currents. The α2δ subunits also influence the channel's biophysical properties, including inactivation kinetics and voltage-dependence (9); therefore, the effects of the α2δ subunits are not limited to trafficking α1 subunits, but their mechanism of action remains largely unknown.
The α2δ-1 subunit has been shown to bind to extracellular regions, including Domain III on CaVα1 subunits (10, 11). It is unclear what domains of α2δ are involved in these interactions, but all α2δ subunits contain a Von Willebrand factor-A (VWA) domain within the α2 moiety (12). This domain is also present in integrins and is often involved in binding extracellular matrix proteins (12, 13). VWA domains contain a sequence motif representing a metal-ion-dependent adhesion site (MIDAS) that confers divalent metal (usually Mg2+)-dependent binding to the ligand (14). In α2δ-1 and -2, this motif is a perfect MIDAS motif, containing both the DxSxS motif and noncontiguous T and D residues (12), with the T in loop 3 being part of a TDG motif (15), suggesting that α2δ-1 and -2 can both undergo an integrin-like switch and bind ligand in the presence of a divalent cation.
In this study, we have investigated the importance of the VWA domain MIDAS in the functional effects of α2δ-2.
Experimental Procedures
Structural Modeling and CD. Suitable templates for modeling were selected from available structures by using the program fugue (16).
Construction and Heterologous Expression of cDNAs Cell Culture and Immunocytochemistry. Standard molecular biological and cell biological techniques were used, as described in refs. 17 and 18.
Biochemistry and Imaging. Cell-surface proteins in intact cells were biotinylated by using Sulfo-NHS-SS-Biotin (Pierce) for 30 min at room temperature. Standard techniques were used for immunoprecipitation, immunoblotting, and immunocytochemistry. Images were obtained by using a Zeiss LSM confocal microscope and further analyzed with imagej software (National Institutes of Health, Bethesda). The gabapentin-binding assay was performed by using a method similar to that described in ref. 19.
Electrophysiology. Standard techniques were used, essentially as described in ref. 17. Details are given in Supporting Methods, which is published as supporting information on the PNAS web site.
Further details of all methods are given in Supporting Methods. Data are mean (±SEM), and statistical significances were analyzed by using Student's t test for unpaired data.
Results
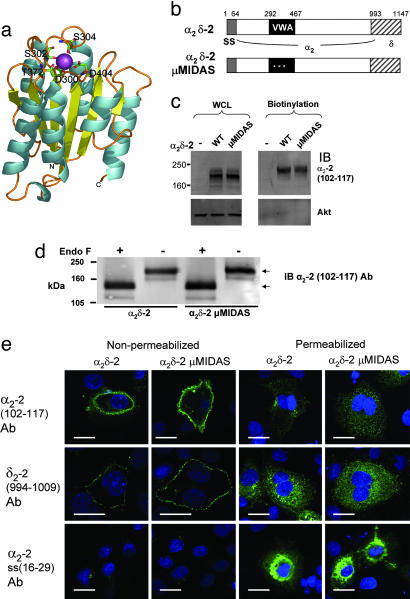
Structural Modeling of the VWA Domain of α2δ-2. Before investigating the function of the predicted VWA domain of α2δ-2, we first determined the likelihood that α2δ-2 is able to fold into a VWA domain. Suitable templates for modeling of α2δ VWA domains (residues 253–430 of α2δ-1 and residues 294–472 of α2δ-2) were selected from available structures. The 1.5-Å crystal structure of the VWA domain from capillary morphogenesis protein 2 (CMG2) in the open (ligand-competent) state and containing a Mg2+ ioninits MIDAS (20), was the best template for both α2δ-1 and -2 VWA domains, with z scores of 15.62 and 18.85, respectively, indicating very high levels of certainty that this is an appropriate template structure. The pairwise sequence identity between each of the α2δ subunits and CMG2 is only ≈16% within the VWA region, but the residues of the MIDAS motif are conserved (see Fig. 6, which is published as supporting information on the PNAS web site). Models (Fig. 1a) were generated according to structural alignments, based on the CMG2 structure. A Mg2+ ion was replaced manually within the MIDAS.
Fig. 1.
Mutation of the MIDAS in α2δ-2 does not prevent its trafficking to the plasma membrane. (a) Model of the α2δ-2 VWA domain. Residues comprising the MIDAS motif are labeled and shown as stick representations. The Mg2+ ion (purple) is coordinated within the MIDAS and is partially exposed at the surface in an appropriate manner for interaction with a second protein. α-helices are colored in cyan and β-strands in yellow. (b) The main domains in the primary sequence of α2δ-2, showing the extent of the VWA domain (black), and the three MIDAS mutations (white asterisks). SS, signal sequence. The amino acids making up the δ subunit are indicated by hatched boxes. (c) Cos-7 cells were transfected with either α2δ-2 or α2δ-2 μMIDAS, as indicated, and cell-surface proteins were biotinylated. (Left) Total expression in whole-cell lysate (WCL). (Right) Pull-down of biotinylated proteins on a 3–8% Tris acetate gel. (Upper) α2-2 (residues 102–117) Ab was used for immunoblotting (IB). (Lower) Anti-Akt Ab was used for IB to show that no intracellular proteins were biotinylated. The percentages of α2δ-2 and α2δ-2 μMIDAS at the cell surface were 10.0% and 9.5%, respectively (representative of three independent experiments; see Fig. 9 legend for calculation details). (d) Biotinylated α2δ-2 (Left) or α2δ-2 μMIDAS (Right), as in c, were treated (+) or not (–) with endoglycosidase F (5 units) and separated on a 7% Tris acetate gel. The upper arrow indicates fully glycosylated α2δ-2, and the lower arrow indicates deglycosylated α2δ-2. (e) Localization of α2δ-2 and α2δ-2 μMIDAS 48 h after transfection in Cos-7 cells that were either nonpermeabilized (Left) or permeabilized (Right) before immunostaining with the primary Abs shown. (Top) α2-2 (residues 102–117) Ab. (Middle) δ2-2(residues 994-1009)Ab. (Bottom) Signal peptide α2-2 (residues 16 –29) Ab. (Scale bar, 20 μm.)
Mutation of the MIDAS Residues of α2δ-2 Do Not Prevent Its Trafficking, Processing Glycosylation, or Expression at the Cell Surface. The results of the modeling study encouraged us to examine the role of the VWA domain in α2δ-2 by mutating to Ala the three divalent metal-coordinating amino acids D300, S302, and S304 in the MIDAS sequence DxSxS (α2δ-2 μMIDAS, Fig. 1b). Mutation of MIDAS motifs in integrins has been used previously to probe the cell-surface role of ligand-binding to these sites (21). We found that mutation of the MIDAS motif did not affect the total expression levels of these constructs, compared with WT α2δ-2, when transfected into tsA-201 (data not shown) or Cos-7 cells (Fig. 1c). In addition, α2δ-2 μMIDAS was expressed at the cell surface in Cos-7 cells to the same extent as WT α2δ-2, as determined by cell-surface biotinylation (Fig. 1c). The biotinylated α2δ-2 and α2δ-2 μMIDAS had the same molecular mass and were reduced in mass to the same extent by endoglycosidase F, suggesting that they are equally glycosylated (Fig. 1d). Cell-surface immunoreactivity in nonpermeabilized cells was observed for both constructs, with not only the anti-peptide α2-2 (102–117) Ab (Fig. 1e) but also an Ab against amino acids in δ-2 (994–1009) (Fig. 1e). No increase in intracellular retention was observed for α2δ-2 μMIDAS, compared with WT α2δ-2 in permeabilized cells (Fig. 1e). Further evidence of the correct processing of α2δ-2 μMIDAS is shown by the cleavage of its N-terminal signal peptide, because no surface staining was observed with the α2-2 (16–29) signal peptide Ab for either construct (Fig. 1e), whereas extensive staining was observed in the perinuclear region in permeabilized cells (Fig. 1e).
It is unlikely, based on previous work on integrins (21), that mutation of MIDAS amino acids will cause the α2δ-2 VWA domain to misfold, and, indeed, we have found that GST-fusion proteins of the WT and MIDAS mutant VWA domains of α2δ-2 expressed and purified from Escherichia coli have very similar CD spectra. The model (Fig. 1a) predicts 40% α-helix, 22% β-sheet, and 38% other structure. The values obtained from CD measurements, after subtraction of the GST spectra, were 32%, 20%, and 48%, respectively [normalized root-mean-square deviation (NRMSD), 0.073], for the WT α2δ-2 VWA domain and 31%, 23%, and 46% (NRMSD, 0.06) for the α2δ-2 μMIDAS VWA domain. Furthermore, WT α2δ-2 and α2δ-2 μMIDAS exhibit similar affinity for binding of [3H]gabapentin (KD of 163.3 ± 8.7 nM and 166.1 ± 1.0 nM, respectively, n = 3 experiments), also indicative of correct folding of the full-length α2δ-2 μMIDAS. The KD for WT α2δ-2 is in agreement with data published in ref. 19.
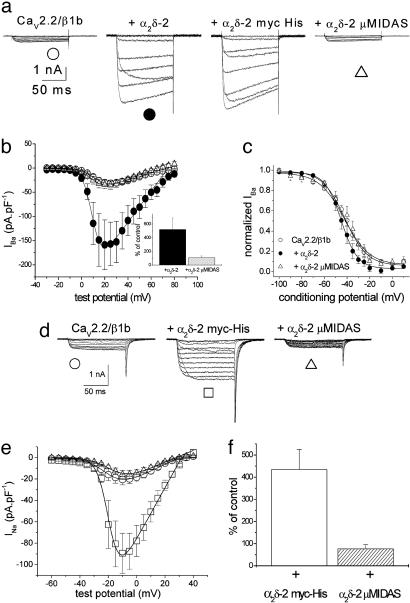
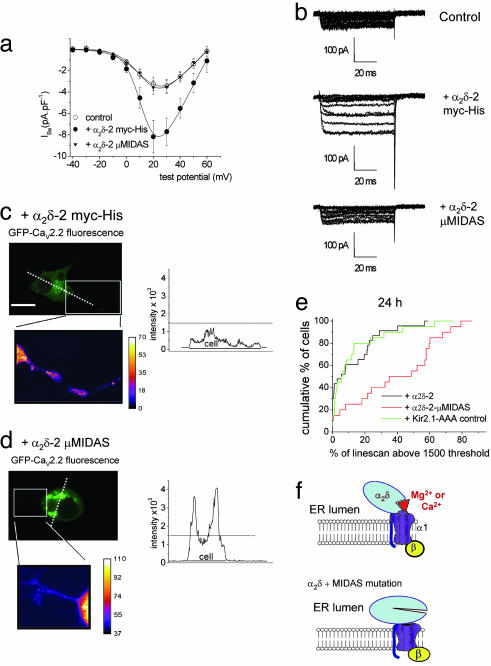
Effect of Mutation of the MIDAS Motif in the VWA Domain on Modulation of Ca2+-Channel Currents by α2δ-2. The amplitude of the very small Ba2+ currents (IBa) through CaV2.2/β1b channels, expressed in tsA-201 cells, was enhanced ≈5-fold by WT α2δ-2 (Fig. 2 a–c) and by α2δ-2 with a C-terminal myc-His tag (Fig. 2a). In contrast, α2δ-2 μMIDAS produced no stimulation of CaV2.2 currents (Fig. 2 a and b). Furthermore, steady-state inactivation was slightly hyperpolarized by α2δ-2, with an increase in voltage-dependence compared with the CaV2.2/β1b combination, but this effect did not occur for α2δ-2 μMIDAS (Fig. 2c). A similar result was obtained by using HA-tagged CaV1.2 (22) (see Fig. 7 a–c, which is published as supporting information on the PNAS web site). In cerebellar Purkinje cells, we have shown that α2δ-2 is the predominant α2δ subtype (17), and, here, the main α1 and β subunits are CaV2.1 and β4 (23, 24). We therefore compared the effect of α2δ-2 and α2δ-2 μMIDAS on CaV2.1/β4 currents. Whereas α2δ-2 enhanced the CaV2.1/β4 current by 6.8-fold ± 0.6 (n = 10), there was no significant effect of α2δ-2 μMIDAS (data not shown).
Fig. 2.
Comparison of the effect of α2δ-2 and the MIDAS mutant construct of α2δ-2 on CaV currents in tsA-201 cells. CaV2.2/β1b was expressed with the various α2δ-2 constructs in tsA-201 cells by using 10 mM Ba2+as charge carrier (see Supporting Methods). (a) Representative current traces elicited between –20 and +40 mV in 10-mV steps from a holding potential (HP) of –90 mV for CaV2.2/β1b (Left), CaV2.2/β1b/α2δ-2 (Center Left), CaV2.2/β1b/α2δ-2 myc-His (Center Right), and CaV2.2/β1b/α2δ-2 μMIDAS (Right). (b) Current–voltage (I–V) relationships for three of the experimental conditions. ○, CaV2.2/β1b (n = 8); •, +α2δ-2 (n = 10); and ▵, +α2δ-2 μMIDAS (n = 11). (Inset) Bar chart at +20 mV (calculated as a percentage of the mean control CaV2.2/β1b currents) for +α2δ-2 (back bar) and +α2δ-2 μMIDAS (hatched bar). The number of determinations is as described above. (c) Steady-state inactivation curves for test pulses to +20 mV from a 15-s conditioning prepulse of between –100 and 0 mV. ○, CaV2.2/β1b (n = 9); •, +α2δ-2 (n = 7); and ▵, +α2δ-2μMIDAS (n = 5). The data were fit with a single Boltzmann equation and the mean voltages at which the channel is 50% available (V50) for inactivation were –42.4, –45.9, and –42.0 mV, respectively, and the slope factors (k) were 11.1, 7.2, and 9.8 mV, respectively. (d) CaV2.2/β1b was expressed with the various α2δ-2 constructs and 100 mM Na+ was used as charge carrier (see Supporting Methods). Representative current traces were elicited between –40 and +15 mV in 5-mV steps from a holding potential of –90 mV for CaV2.2/β1b (Left), CaV2.2/β1b/α2δ-2 myc-His (Center), and CaV2.2/β1b/α2δ-2 μMIDAS (Right). (e) I–V relationships for the three conditions. ○, CaV2.2/β1b (n = 16); □, +α2δ-2 myc-His (n = 17); and ▵, +α2δ-2 μMIDAS (n = 15). (f) Bar chart at –10 mV (calculated as a percentage of the mean control CaV2.2/β1b Na+ currents) for +α2δ-2 myc-His (open bar) and +α2δ-2 μMIDAS (hatched bar). The number of determinations is as in e.
In Xenopus oocytes, the amplitude of CaV2.2/β1b currents was enhanced ≈3-fold by both WT α2δ-2 and α2δ-2 myc-His (see Fig. 7 d and e). In contrast, after mutation of the MIDAS amino acids, there was no enhancement of the current amplitude, and, indeed, there was a clear reduction, compared with CaV2.2/β1b alone (see Fig. 7 d and e). Furthermore, whereas α2δ-2 hyperpolarized the voltage-dependence of steady-state inactivation, α2δ-2 μMIDAS did not (see Fig. 7f). All data obtained with α2δ-2 μMIDAS could be replicated by using an α2δ-2 construct in which the VWA domain was deleted (data not shown).
We detected no endogenous α2δ subunit protein in Xenopus oocytes (see Fig. 8a, which is published as supporting information on the PNAS web site) or Cos-7 cells (data not shown), whereas endogenous α2δ-1 was detected in tsA-201 cells. The α2δ-1 had the same molecular mass and was glycosylated to the same extent as was α2δ-1 in brain (see Fig. 8b). No endogenous α2δ-2 was observed in any of the cell types used (data not shown).
Does the Effect of Mutation of the MIDAS on Ca2+-Channel Currents Involve Binding of Extracellular Divalent Cations? Because MIDAS motifs bind divalent cations (Fig. 1a), we wished to examine whether the effect of mutation of the MIDAS affected channel permeation. We therefore removed all divalent cations from the external medium and measured Na+ flux through CaV2.2 channels in tsA-201 cells. This protocol produced the same result: the amplitude of the CaV2.2/β1b Na+ currents was enhanced >4-fold by α2δ-2 but not by α2δ-2 μMIDAS, whose currents were slightly smaller than those in the absence of any α2δ-2 (Fig. 2 d–f). Therefore, if Mg2+ (or Ca2+) binding to the MIDAS in α2δ-2 is involved in its function in relation to enhancing currents through CaVα1 channels, it must be during the process of channel assembly or trafficking rather than during the permeation process, once the channels have reached the cell surface.
Does the MIDAS Mutant α2δ-2 Affect Trafficking of CaVα1 Subunits? Given these results, the complete loss of function of the α2δ-2 μMIDAS mutant subunit, despite its normal expression compared with WT α2δ-2, might, therefore, be due to interference with trafficking and a subsequent reduction of plasma-membrane expression of associated CaVα1 subunits. Using cell surface biotinylation, we found a decrease in the percentage of CaV2.2 at the cell surface, when coexpressed with α2δ-2 μMIDAS, compared with WT α2δ-2 by ≈50% (see Fig. 9a, which is published as supporting information on the PNAS web site) and also a 30% decrease in the percentage of α2δ-2 μMIDAS, compared with WT α2δ-2 at the cell surface, when coexpressed with CaV2.2 (see Fig. 10b, which is published as supporting information on the PNAS web site). This result was not the case when the α2δ subunits were expressed alone (Fig. 1c).
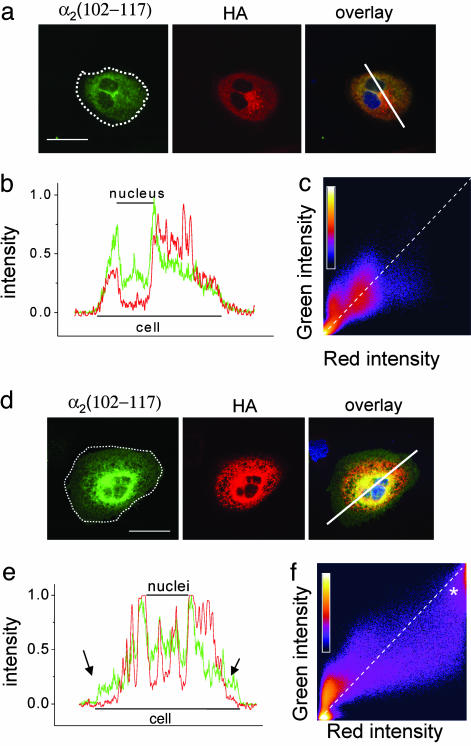
To examine further the basis for the reduction of surface expression of CaVα1 subunits, we determined the subcellular distribution of HA-CaV1.2, expressed with β1b in Cos-7 cells in the presence of either α2δ-2 or α2δ-2 μMIDAS. When HA-CaV1.2 was expressed with WT α2δ-2, its distribution was fairly uniform (Fig. 3a; and see Fig. 10 for an additional example). The immunostaining extended to the periphery of the cell, where it colocalized with α2δ-2, as seen clearly on the line scan (representative of 11 of 11 cells analyzed in three experiments, see Fig. 3b). Further evidence of colocalization was obtained from pixel-intensity-correlation plots (Fig. 3c).
Fig. 3.
Comparison of the effect of α2δ-2 and α2δ-2 μMIDAS on expression of CaV1.2 channels and colocalization with the α2δ construct. (a and d) Localization of α2δ-2 or α2δ-2 μMIDAS (α2 (102–117) Ab (Left), cell outline shown by dotted white line), HA-CaV1.2 (HA Ab, Center), and overlay (Right), including nuclear stain (DAPI, blue) in CaV1.2/β1b/α2δ-2 (a) or α2δ-2 μMIDAS-transfected Cos-7 cells (d). (Scale bar, 50 μm.) (b and e) Normalized pixel intensity of the line scan given on the image. The red line is HA-CaV1.2, and the green line is α2δ-2 (b) or α2δ-2 μMIDAS (e). The arrows in e indicate regions where CaV1.2 immunoreactivity is absent from the periphery of the cell. (c and f) Pixel-intensity-correlation plot for nonzero pixels in the entire image, shown for α2δ-2 (c) or α2δ-2 μMIDAS (f) (green, y axis) vs. CaV1.2 (red, x axis). A pixel-number calibration bar is shown on each plot. * in f indicates an additional region of high-intensity colocalization. The diagonal dotted line indicates theoretical colocalization. (a–c) Cos-7 cells HA-CaV1.2/β1b/α2δ-2. (d–f) HA-CaV1.2/β1b/α2δ-2 μMIDAS.
In contrast, when HA-CaV1.2 was coexpressed with α2δ-2 μMIDAS, areas of marked intracellular retention were observed, most often of both the CaVα1 and the α2δ subunit (Fig. 3d; and see Fig. 10 for an additional example). Furthermore, in 9 of 14 cells examined, HA-CaV1.2 immunoreactivity was observed not to extend as far as α2δ-2 μMIDAS into the periphery of the cell (Fig. 3e). The strong intracellular retention of HA-CaV1.2, together with α2δ-2 μMIDAS, can be seen clearly on the pixel-intensity-correlation plot as an additional peak of high intensity in both red and green fluorescence (Fig. 3f, marked with an asterisk). In agreement with these results, HA-CaV1.2 is able to coimmunoprecipitate with α2δ-2 μMIDAS and WT α2δ-2 (Fig. 9c).
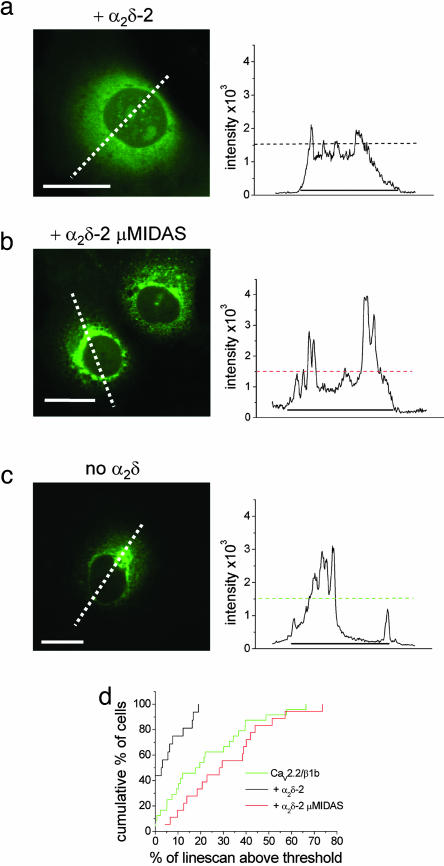
We then used an N-terminal GFP-CaV2.2 construct, which we have previously shown exhibits normal functional properties in Cos-7 cells (25). We examined expression at a very early time point, 24 h after transfection, before the time at which Ca2+ currents could reliably be recorded (data not shown). Compared with coexpression with WT α2δ-2, where the distribution of GFP-CaV2.2 was fairly uniform, when coexpressed with α2δ-2 μMIDAS, there was a significantly greater intracellular accumulation of GFP-CaV2.2, particularly in the perinuclear region (Fig. 4 a, b, and d). The accumulation was also more pronounced than that seen with GFP-CaV2.2, in the absence of any α2δ (Fig. 4 c and d). The same result was observed 48 h after transfection (see Fig. 11, which is published as supporting information on the PNAS web site).
Fig. 4.
Comparison of the effects of α2δ-2 and α2δ-2 μMIDAS on the distribution of GFP-CaV2.2 channels in Cos-7 cells. (Left) Localization of GFP-CaV2.2 in Cos-7 cells when coexpressed with β1b and either α2δ-2 (a) or α2δ-2 μMIDAS (b) and without α2δ (c) 24 h after transfection. White dotted lines correspond to the positions of the line scans shown to the right. (Scale bars, 20 μm.) (Right) Line scans of GFP-CaV2.2 fluorescence were analyzed through the nucleus of the cells shown. The horizontal dotted lines show the 1,500 intensity threshold (12-bit images), and the line beneath the scans shows the extent of the cell. (d) Cumulative frequency histogram of the percentage of the line scan within the cell above the 1,500 intensity threshold for α2δ-2-(n = 26, black line), α2δ-2 μMIDAS-containing cells (n = 32, red line), and in the absence of α2δ (n = 32, green line). The histogram shows the greater proportion of high-intensity GFP-CaV2.2 fluorescence regions in α2δ-2 μMIDAS (mean 33.2 ± 3.1%), compared with both α2δ-2-containing cells (mean 10.1 ± 2.4%; P < 0.0001) and in the absence of α2δ (mean 21.2 ± 3.2%; P < 0.01, compared with α2δ-2 μMIDAS).
Effect of α2δ-2 and α2δ-2 μMIDAS on Expression of Ca2+-Channel Currents in Differentiated NG108–15 Cells. The NG108–15 neuroblastoma–glioma hybrid cell line represents a model for neurons, because it produces extensive neurites and shows high-voltage-activated (HVA) currents, when differentiated. These currents were isolated by maintaining a holding potential of –40 mV. Despite the presence of endogenous α2δ-1 in NG108–15 cells (26), exogenous expression of α2δ-2 enhanced the HVA currents substantially. IBa recorded from differentiated cells transfected with α2δ-2, was increased to 264% of control (Fig. 5 a and b), indicating that the amount of endogenous α2δ is suboptimal for the expression of maximal endogenous Ca2+ currents. In contrast, transfection with α2δ-2 μMIDAS produced no enhancement of IBa (110% of control, Fig. 5 a and b).
Fig. 5.
Comparison of the effects of α2δ-2 and α2δ-2 μMIDAS on endogenous Ca2+ currents and GFP-CaV2.2 distribution in NG 108–15 cells. (a) The effects of α2δ-2 and α2δ-2 μMIDAS on HVA IBa in NG108–15 cells. Current–voltage relationships for NG108–15 cells transfected with GFP-pRK5 and Kir2.1-AAA (○, control, n = 13), •, α2δ-2 myc-His (n = 12), or ▾, α2δ-2 μMIDAS (n = 13), all in pcDNA3.1. Holding potential = –40 mV. (b) Sample traces for the three conditions shown in a. (Top) Control. (Middle) +α2δ-2. (Bottom) +α2δ-2 μMIDAS. Traces shown are from –40 to +60 mV. (c and d) Localization of GFP-CaV2.2 48 h after transfection, cotransfected with β1b and α2δ-2 myc-His (c) or α2δ-2 μMIDAS (d). (Top Left) GFP fluorescence. (Top Right) Line scan of the dotted line shown on the 12-bit image. (Bottom Left) Enlarged region shown by the box, converted to 8-bit pseudocolor to show intensity in the neurite. The intensity calibration bars are shown on the right. (e) The plots represent a cumulative frequency histogram for all line scans, obtained 24 h after transfection, showing the significantly greater percentage of high-intensity GFP-CaV2.2-fluorescence regions in α2δ-2 μMIDAS-containing cells (red line, mean 42.2 ± 6.1%, n = 20, P < 0.0005) in comparison with α2δ-2-containing cells (black line, 14.6 ± 3.7%, n = 23). An additional control is included, in which a control nonfunctional protein, Kir2.1-AAA, has been included instead of α2δ-2 (green, 15.6 ± 4.8%, n = 20, P < 0.001, compared with α2δ-2 μMIDAS). (f) Proposed role of the MIDAS in α2δ. ER, endoplasmic reticulum.
When NG108–15 cells were transfected with GFP-CaV2.2/β1b and WT α2δ-2 and differentiated, uniform distribution of GFP-CaV2.2 was observed (Fig. 5c). The cells were clearly differentiated with neurites (Fig. 5d, enlarged region). In sharp contrast, coexpression with α2δ-2 μMIDAS produced strong perinuclear retention of GFP-CaV2.2/β1b, although the cells also had a differentiated phenotype, showing neurites (Fig. 5d). Analysis of the amount of signal above an arbitrary threshold on line scans (Fig. 5 c and d) showed that the intracellular retention of GFP-CaV2.2/β1b was significantly greater in the presence of α2δ-2 μMIDAS than in the presence of WT α2δ-2, at both 48 h (data not shown) and 24 h (Fig. 5e). In an additional control experiment, coexpression of GFP-CaV2.2/β1b with a control transmembrane protein Kir2.1-AAA (27), a K+ channel with a mutation in the pore, such that it does not conduct current, does not produce any intracellular retention of CaV2.2/β1b (Fig. 5e). We also showed that GFP-CaV2.2 was expressed similarly under all conditions (see Fig. 12, which is published as supporting information on the PNAS web site).
Discussion
The Functional Interaction of CaVα1/β Channels with α2δ-2. Previous in vitro studies have shown that all α2δ subunits increase the maximum conductance of a number of expressed Ca2+-channel α1/β subunit combinations at the whole-cell level (1, 17, 28–30). We have previously investigated the effect of α2δ-2 on the CaV2.1/β4 Ca2+-channel subunit combination and showed that it had no effect on single-channel conductance (17, 18). This finding implies that α2δ-2 probably has its main effect on the lifetime of the channel complex in the plasma membrane, by either enhancing forward trafficking or reducing turnover of the channel once inserted in the plasma membrane.
All integrin β subunits contain a VWA domain with a MIDAS motif (13). Based on structural studies, the inactive form of the integrin dimer is bent in a loose hairpin. When activated, the integrin dimers alter conformation and bind their ligands in the presence of divalent cations (usually Mg2+, see Fig. 1a; for review, see ref. 31). The single particle EM studies of Ca2+-channel complexes have also shown the associated α2δ subunit to be bent (32–34). It was recently hypothesized that all VWA domains that contain a perfect MIDAS motif (DxSxS and a coordinating T and D) will both bind Mg2+ and subsequently undergo an integrin-like switch (15). It is tempting to speculate that the CaVα1 subunit represents an endogenous ligand for the α2δ MIDAS and that an integrin-like switch is required for trafficking the channel complex.
Mechanism of Inhibition of Trafficking by the MIDAS Mutant of α2δ-2. One hypothesis for the role of α2δ subunits is that they act as vestibules to increase Ca2+ availability at the mouth of the pore (35), and, therefore, mutating the MIDAS so that this motif is nonfunctional might reduce the conductance. If this were the mechanism, the effect should be lost when using Na+ as the charge carrier. However, we have shown that exactly the same effect of α2δ-2 μMIDAS occurs under these conditions as in the presence of divalent cations.
This finding leaves two further hypotheses. First, correct binding of a divalent cation to the α2δ MIDAS in the lumen of the endoplasmic reticulum or other intracellular compartment may be essential for the trafficking of CaVα1 subunits to the plasma membrane. Several pieces of our evidence support this hypothesis, pointing to a defect in CaVα1-subunit trafficking in the presence of α2δ-2 μMIDAS, caused by an intracellular interaction between the two proteins. We observed marked intracellular retention of HA-CaV1.2 and intracellular colocalization with α2δ-2 μMIDAS, whereas when α2δ-2 μMIDAS is expressed alone, it is processed and trafficked to the cell membrane normally. A similar phenomenon of intracellular retention was observed for GFP-CaV2.2inlive Cos-7 and NG108–15 cells. We have also observed less CaV2.2 at the plasma membrane by cell-surface biotinylation when α2δ-2 μMIDAS is coexpressed, compared with WT α2δ-2. There was also a smaller proportion of α2δ-2 μMIDAS at the cell surface when this subunit is coexpressed with CaV2.2, compared with when it is expressed alone, whereas this was not the case for WT α2δ-2.
The second hypothesis would be that the CaVα1/β heterodimers and the α2δ subunits are trafficked separately to the plasma membrane, where they then interact, allowing the α2δ to increase the lifetime of the channel at the plasma membrane. A prediction based on this hypothesis is that there would be no interaction in the case of α2δ-2 μMIDAS. Evidence against this hypothesis is (i) that the colocalization and coimmunoprecipitation of α2δ-2 μMIDAS and CaVα1 subunits indicates an interaction, albeit an abortive one, and (ii) that the intracellular retention of GFP-CaV2.2 was more marked in the presence of α2δ-2 μMIDAS than in the absence of any α2δ subunit, before the time at which Ca2+ currents could be recorded, indicating that an interaction must occur early in the process of assembly and trafficking. Thus, even in the absence of a functional MIDAS in α2δ-2, an interaction occurs, presumably through the mutated VWA domain and, possibly, through other sites in α2δ-2, but this interaction does not result in trafficking. We propose that the interaction between CaVα1 and an α2δ subunit requires the high divalent cation concentration within the endoplasmic reticulum (ER) to bind to the MIDAS in α2δ and allow forward trafficking of the complex through an integrin-like switch (Fig. 5F). This switch may serve as a trafficking checkpoint, so that, if the CaVα1 subunit is not folded correctly, both α1 and α2δ will be retained within the ER.
Mechanism of Suppression of CaVα1 Currents by MIDAS Mutant α2δ-2. There is a strong suppressive effect of α2δ-2 μMIDAS in Xenopus oocytes, whereas in NG108–15 cells and tsA 201 cells, only a lack of up-regulation of the endogenous HVA IBa by α2δ-2 μMIDAS is seen. The clear difference that we have found between these expression systems is the presence of endogenous α2δ-1 in NG108–15 cells (26) and in tsA 201 cells, whereas no endogenous α2δ protein was detected in the Xenopus oocytes used in this study (Fig. 7). We therefore suggest that, in Xenopus oocytes, in the absence of any endogenous α2δ subunits, α2δ-2 μMIDAS interacts with, and causes intracellular retention of, expressed CaVα1/β complexes, which otherwise produce substantial currents in this system, although not in mammalian cells, for reasons unknown (e.g., Fig. 2 a–c, compared with Fig. 7 d and e). In contrast, the channels underlying the very small CaVα1/β IBa observed in tsA 201 cells may reach the plasma membrane only because they have an associated endogenous α2δ. Furthermore, in NG108–15 cells, there is evidence that the up-regulation of endogenous Ca2+ currents that occurs upon differentiation results, at least in part, from the stabilization of preformed channels (36), and, thus, the channels making up the endogenous HVA current in NG108–15 cells are likely to already have an associated endogenous α2δ-1. These endogenous channels would, therefore, be protected from association with the MIDAS mutant of α2δ-2 and, thus, from further suppression by intracellular retention.
Conclusions
Our finding that α2δ-2 μMIDAS increased the internal retention of both CaV1 and CaV2 channels, whereas it is normally processed and trafficked itself when expressed alone, indicates that the MIDAS motif of the VWA domain has its action on trafficking of the CaVα1 subunit. Our results suggest that α2δ subunits normally interact, through the intact MIDAS, with the CaVα1 subunit at a very early time point in their maturation, to enhance trafficking. This finding provides evidence for a primary role of a VWA domain in the intracellular trafficking of a multimeric complex, in contrast to their more usual role in extracellular interactions.
Supplementary Material
Acknowledgments
We thank Dr. E. Bourinet (Institut de Génétique Humaine–Centre National de la Recherche Scientifique, Montpellier, France) for HA-tagged CaV1.2 cDNA and Dr. W. J. Frith for help with image processing. This work was supported, in part, by the Medical Research Council (United Kingdom) and the Wellcome Trust.
Author contributions: C.C., I.F., F.H., M.W.R., K.M.P., A.D., and A.C.D. designed research; C.C., M.N.-R., I.F., F.H., J.W., M.W.R., J.H., K.M.P., L.D., A.D., and A.C.D. performed research; C.C., I.F., F.H., M.W.R., J.H., K.M.P., A.D., and A.C.D. analyzed data; and A.C.D. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CaV, voltage-gated Ca2+; HVA, high-voltage-activated; IBa, Ba2+ current; MIDAS, metal-ion-dependent adhesion site; VWA, Von Willebrand factor-A.
References
- 1.Walker, D. & De Waard, M. (1998) Trends Neurosci. 21, 148–154. [DOI] [PubMed] [Google Scholar]
- 2.Dolphin, A. C. (2003) J. Bioeng. Biomembr. 35, 599–620. [DOI] [PubMed] [Google Scholar]
- 3.Arikkath, J. & Campbell, K. P. (2003) Curr. Opin. Neurobiol. 13, 298–307. [DOI] [PubMed] [Google Scholar]
- 4.Catterall, W. A. (2000) Annu. Rev. Cell Dev. Biol. 16, 521–555. [DOI] [PubMed] [Google Scholar]
- 5.Jay, S. D., Sharp, A. H., Kahl, S. D., Vedvick, T. S., Harpold, M. M. & Campbell, K. P. (1991) J. Biol. Chem. 266, 3287–3293. [PubMed] [Google Scholar]
- 6.Chang, F. C. & Hosey, M. M. (1988) J. Biol. Chem. 263, 18929–18937. [PubMed] [Google Scholar]
- 7.Witcher, D. R., De Waard, M., Sakamoto, J., Franzini-Armstrong, C., Pragnell, M., Kahl, S. D. & Campbell, K. P. (1993) Science 261, 486–489. [DOI] [PubMed] [Google Scholar]
- 8.Liu, H., De Waard, M., Scott, V. E. S., Gurnett, C. A., Lennon, V. A. & Campbell, K. P. (1996) J. Biol. Chem. 271, 13804–13810. [PubMed] [Google Scholar]
- 9.Canti, C., Davies, A. & Dolphin, A. C. (2003) Curr. Neuropharmacol. 1, 209–217. [Google Scholar]
- 10.Felix, R., Gurnett, C. A., De Waard, M. & Campbell, K. P. (1997) J. Neurosci. 17, 6884–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurnett, C. A., Felix, R. & Campbell, K. P. (1997) J. Biol. Chem. 272, 18508–18512. [DOI] [PubMed] [Google Scholar]
- 12.Whittaker, C. A. & Hynes, R. O. (2002) Mol. Biol. Cell 13, 3369–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphries, M. J. (2000) Biochem. Soc. Trans. 28, 311–340. [PubMed] [Google Scholar]
- 14.Plow, E. F., Haas, T. K., Zhang, L., Loftus, J. & Smith, J. W. (2000) J. Biol. Chem. 275, 21785–21788. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya, A. A., Lupher, M. L., Jr., Staunton, D. E. & Liddington, R. C. (2004) Structure (Cambridge, U.K.) 12, 371–378. [DOI] [PubMed] [Google Scholar]
- 16.Shi, J., Blundell, T. L. & Mizuguchi, K. (2001) J. Mol. Biol. 310, 243–257. [DOI] [PubMed] [Google Scholar]
- 17.Barclay, J., Balaguero, N., Mione, M., Ackerman, S. L., Letts, V. A., Brodbeck, J., Canti, C., Meir, A., Page, K. M., Kusumi, K., et al. (2001) J. Neurosci. 21, 6095–6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodbeck, J., Davies, A., Courtney, J.-M., Meir, A., Balaguero, N., Canti, C., Moss, F. J., Page, K. M., Pratt, W. S., Hunt, S. P., et al. (2002) J. Biol. Chem. 277, 7684–7693. [DOI] [PubMed] [Google Scholar]
- 19.Marais, E., Klugbauer, N. & Hofmann, F. (2001) Mol. Pharmacol. 59, 1243–1248. [DOI] [PubMed] [Google Scholar]
- 20.Lacy, D. B., Wigelsworth, D. J., Scobie, H. M., Young, J. A. & Collier, R. J. (2004) Proc. Natl. Acad. Sci. USA 101, 6367–6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen, J., Salas, A. & Springer, T. A. (2003) Nat. Struct. Biol. 10, 995–1001. [DOI] [PubMed] [Google Scholar]
- 22.Altier, C., Dubel, S. J., Barrère, C., Jarvis, S. E., Stotz, S. C., Spaetgens, R. L., Scott, J. D., Cornet, V., De Waard, M., Zamponi, G. W., et al. (2002) J. Biol. Chem. 277, 33598–33603. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher, C. F., Lutz, C. M., O'Sullivan, T. N., Shaughnessy, J. D., Jr., Hawkes, R., Frankel, W. N., Copeland, N. G. & Jenkins, N. A. (1996) Cell 87, 607–617. [DOI] [PubMed] [Google Scholar]
- 24.Burgess, D. L., Jones, J. M., Meisler, M. H. & Noebels, J. L. (1997) Cell 88, 385–392. [DOI] [PubMed] [Google Scholar]
- 25.Raghib, A., Bertaso, F., Davies, A., Page, K. M., Meir, A., Bogdanov, Y. & Dolphin, A. C. (2001) J. Neurosci. 21, 8495–8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyatt, C. N., Page, K. M., Berrow, N. S., Brice, N. L. & Dolphin, A. C. (1998) J. Physiol. (London) 510, 347–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tinker, A., Jan, Y. N. & Jan, L. Y. (1996) Cell 87, 857–868. [DOI] [PubMed] [Google Scholar]
- 28.Mori, Y., Friedrich, T., Kim, M.-S., Mikami, A., Nakai, J., Ruth, P., Bosse, E., Hofmann, F., Flockerzi, V., Furuichi, T., et al. (1991) Nature 350, 398–402. [DOI] [PubMed] [Google Scholar]
- 29.Klugbauer, N., Lacinova, L., Marais, E., Hobom, M. & Hofmann, F. (1999) J. Neurosci. 19, 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hobom, M., Dai, S., Marais, E., Lacinova, L., Hofmann, F. & Klugbauer, N. (2000) Eur. J. Neurosci. 12, 1217–1226. [DOI] [PubMed] [Google Scholar]
- 31.Carman, C. V. & Springer, T. A. (2003) Curr. Opin. Cell Biol. 15, 547–556. [DOI] [PubMed] [Google Scholar]
- 32.Wang, M.-C., Collins, R., Ford, R. C., Berrow, N. S., Dolphin, A. C. & Kitmitto, A. (2004) J. Biol. Chem. 279, 7159–7168. [DOI] [PubMed] [Google Scholar]
- 33.Wolf, M., Eberhart, A., Glossmann, H., Striessnig, J. & Grigorieff, N. (2003) J. Mol. Biol. 332, 171–182. [DOI] [PubMed] [Google Scholar]
- 34.Wang, M. C., Dolphin, A. & Kitmitto, A. (2004) FEBS Lett. 564, 245–250. [DOI] [PubMed] [Google Scholar]
- 35.Wang, M.-C., Berrow, N. S., Ford, R. C., Dolphin, A. C. & Kitmitto, A. (2002) J. Mol. Biol. 323, 85–98. [DOI] [PubMed] [Google Scholar]
- 36.Passafaro, M., Clementi, F. & Sher, E. (1992) J. Neurosci. 12, 3372–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.