Abstract
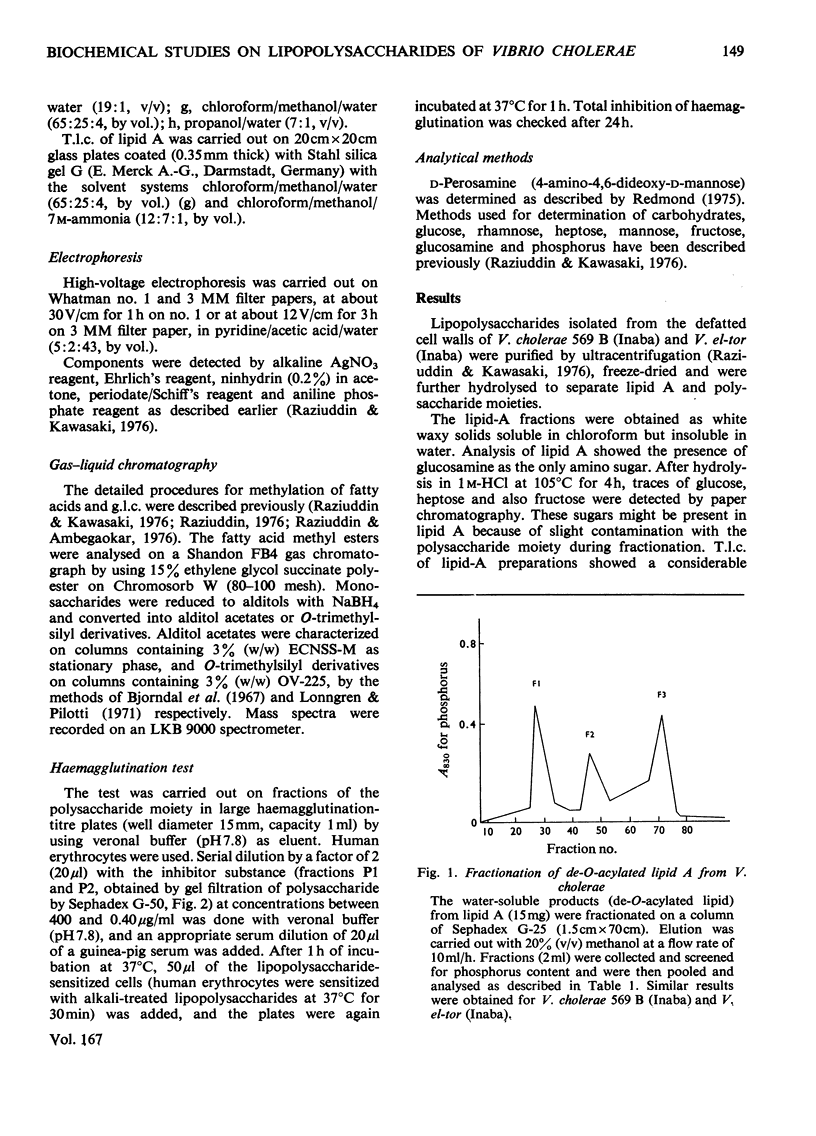
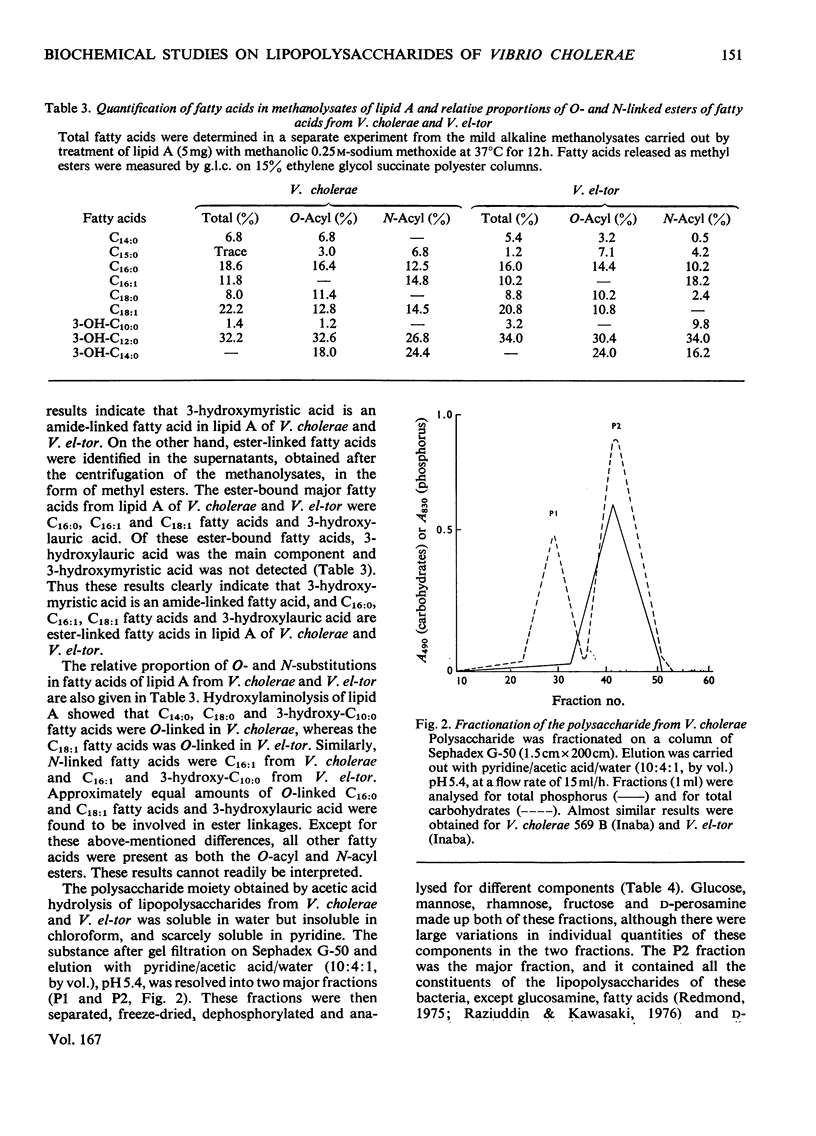
Lipid A and polysaccharide moieties obtained by mild acid hydrolysis of the lipopolysaccharides from Vibrio cholerae 569 B (Inaba) and Vibrio el-tor (Inaba) were characterized. Heterogeneity of lipid A fractions was indicated by t.l.c. and by gel filtration of the de-O-acylated products from mild alkaline methanolysis of the lipids. Presumably lipid A contains a glucosamine backbone, and the fatty acids are probably bound to the hydroxyl and amino groups of glucosamine residues. Approximately equal amounts of fatty acids C16:0, C18:1 and 3-hydroxylauric acid were involved in ester linkages, but 3-hydroxymyristic acid was the only amide-linked fatty acid. Sephadex chromatography of the polysaccharide moiety showed the presence of a high-molecular-weight heptose-free fraction and a low-molecular-weight heptose-containing fraction. Haemagglutination-inhibition assays of these fractions showed the heptose-free fraction to be an O-specific side-chain polysaccharide, whereas the heptose-containing fraction was the core polysaccharide region of the lipopolysaccharides. Identical results were obtained for both organisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Singh P. P. Structural features of lipid A preparations isolated from Escherichia coli and Shigella flexneri. Biochim Biophys Acta. 1970 May 5;202(3):553–555. doi: 10.1016/0005-2760(70)90128-1. [DOI] [PubMed] [Google Scholar]
- Adams G. A., Singh P. P. The chemical constitution of lipid A from Serratia marcescens. Can J Biochem. 1970 Jan;48(1):55–62. doi: 10.1139/o70-010. [DOI] [PubMed] [Google Scholar]
- Armstrong I. L., Redmond J. W. The fatty acids present in the lipopolysaccharide of Vibrio cholerae 569B (Inaba). Biochim Biophys Acta. 1974 May 29;348(2):302–305. doi: 10.1016/0005-2760(74)90242-2. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Hewett M. J., Knox K. W. Biochemical studies on lipopolysaccharides of Veillonella. Eur J Biochem. 1971 Mar 11;19(2):169–175. doi: 10.1111/j.1432-1033.1971.tb01301.x. [DOI] [PubMed] [Google Scholar]
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Drewry D. T., Gray G. W., Wilkinson S. G. Low-molecular-weight solutes released during mild acid hydrolysis of the lipopolysaccharide of Pseudomonas aeruginosa. Biochem J. 1972 Nov;130(1):289–295. doi: 10.1042/bj1300289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewry D. T., Lomax J. A., Gray G. W., Wilkinson S. G. Studies of lipid A fractions from the lipopolysaccharides of Pseudomonas aeruginosa and Pseudomonas alcaligenes. Biochem J. 1973 Jul;133(3):563–572. doi: 10.1042/bj1330563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensom A. H., Gray G. W. The chemical composition of the lipopolyacarideof Pseudomonas aeruginosa. Biochem J. 1969 Sep;114(2):185–196. doi: 10.1042/bj1140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensom A. H., Meadow P. M. Evidence for two regions in the polysaccharide moiety of the lipopolysaccharide of Pseudomonas aeruginosa 8602. FEBS Lett. 1970 Jul 29;9(2):81–84. doi: 10.1016/0014-5793(70)80318-0. [DOI] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O., Kim Y. B., Watson D. W. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972 Dec 4;31(2):230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 6. Investigations on the structure of the lipid A component. Eur J Biochem. 1969 Jan;7(3):370–379. doi: 10.1111/j.1432-1033.1969.tb19618.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Simon M., Lüderitz O. The linkage of phosphate groups and of 2-keto-3-deoxyoctonate to the lipid A component in a Salmonella minnesota lipopolysaccharide. Eur J Biochem. 1971 Aug 16;21(3):355–356. doi: 10.1111/j.1432-1033.1971.tb01476.x. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hancock I. C., Humphreys G. O., Meadow P. M. Characterisation of the hydroxy acids of Pseudomonas aeruginosa 8602. Biochim Biophys Acta. 1970 Mar 10;202(2):389–391. doi: 10.1016/0005-2760(70)90204-3. [DOI] [PubMed] [Google Scholar]
- Hämmerling G., Lehmann V., Lüderitz O. Structural studies on the heptose region of Salmonella lipopolysaccharides. Eur J Biochem. 1973 Oct 18;38(3):453–458. doi: 10.1111/j.1432-1033.1973.tb03079.x. [DOI] [PubMed] [Google Scholar]
- Hämmerling G., Lüderitz O., Westphal O., Mäkelä P. H. Structural investigations on the core polysaccharide of Escherichia coli 0100. Eur J Biochem. 1971 Oct 14;22(3):331–344. doi: 10.1111/j.1432-1033.1971.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Jackson G. D.F., Redmond J. W. Immunochemical studies of the O-antigens of Vibrio cholerae. The constitution of a lipopolysaccharide from V. cholerae 569B (Inaba). FEBS Lett. 1971 Feb 19;13(2):117–120. doi: 10.1016/0014-5793(71)80213-2. [DOI] [PubMed] [Google Scholar]
- Jann B., Jann K., Beyaert G. O. 2-Amino-2,6-dideoxy-d-glucose (D-quinovosamine): a constituent of the lipopolysaccharides of Vibrio cholerae. Eur J Biochem. 1973 Sep 3;37(3):531–534. doi: 10.1111/j.1432-1033.1973.tb03015.x. [DOI] [PubMed] [Google Scholar]
- Kasai N., Nowotny A. Endotoxic glycolipid from a heptoseless mutant of Salmonella minnesota. J Bacteriol. 1967 Dec;94(6):1824–1836. doi: 10.1128/jb.94.6.1824-1836.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacave C., Asselineau J., Serre A., Roux J. Comparaison de la composition chimique d'une fraction lipopolysaccharidique et d'une fraction polysaccharidique isolées de Brucella melitensis. Eur J Biochem. 1969 Jun;9(2):189–198. doi: 10.1111/j.1432-1033.1969.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Lomax J. A., Gray G. W., Wilkinson S. G. Studies of the polysaccharide fraction from the lipopolysaccharide of Pseudomonas alcaligenes. Biochem J. 1974 Jun;139(3):633–643. doi: 10.1042/bj1390633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOWOTNY A. Relation of structure to function in bacterial O antigens. II. Fractionation of lipids present in Boivin-type endotoxin of Serratia marcescens. J Bacteriol. 1963 Feb;85:427–435. doi: 10.1128/jb.85.2.427-435.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raziuddin S. R. Effect of growth temperature and culture age on the lipid composition of Vibrio cholerae 569B (Inaba). J Gen Microbiol. 1976 Jun;94(2):367–372. doi: 10.1099/00221287-94-2-367. [DOI] [PubMed] [Google Scholar]
- Raziuddin S., Ambegaokar S. D. Chemical composition & fatty acid pattern of lipopolysaccharides from Vibrio cholerae. Indian J Biochem Biophys. 1976 Mar;13(1):57–61. [PubMed] [Google Scholar]
- Raziuddin S., Kawasaki T. Biochemical studies on the cell wall lipopolysaccharides (O-antigens) of Vibrio cholerae 569 B (Inaba) and El-tor (Inaba). Biochim Biophys Acta. 1976 Apr 22;431(1):116–126. doi: 10.1016/0005-2760(76)90265-4. [DOI] [PubMed] [Google Scholar]
- Redmond J. W. 4-Amino-4,6-dideoxy-D-mannose (D-perosamine): a component of the lipopolysaccharide of Vibrio cholerae 569B (Inaba). FEBS Lett. 1975 Feb 1;50(2):147–149. doi: 10.1016/0014-5793(75)80476-5. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Palin W. J., Watson D. W. Nature and linkages of the fatty acids present in lipopolysaccharides from Vibrio metchnikovii and Vibrio parahemolyticus. Eur J Biochem. 1973 Aug 1;37(1):116–120. doi: 10.1111/j.1432-1033.1973.tb02965.x. [DOI] [PubMed] [Google Scholar]
- Rooney S. A., Goldfine H. Isolation and characterization of 2-keto-3-deoxyoctonate-lipid A from a heptose-deficient mutant of Escherichia coli. J Bacteriol. 1972 Aug;111(2):531–541. doi: 10.1128/jb.111.2.531-541.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S. A., Goldfine H., Sweeley C. C. The identification of trans-2-tetradecenoic acid in hydrolysates of lipid A from Escherichia coli. Biochim Biophys Acta. 1972 Jul 7;270(3):289–295. doi: 10.1016/0005-2760(72)90192-0. [DOI] [PubMed] [Google Scholar]
- Russa R., Lorkiewicz Z. Fatty acids present in the lipopolysaccharide of Rhizobium trifolii. J Bacteriol. 1974 Sep;119(3):771–775. doi: 10.1128/jb.119.3.771-775.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Jann B., Jann K. Immunochemistry of R lipopolysaccharides of Escherichia coli. Different core regions in the lipopolysaccharides of O group 8. Eur J Biochem. 1969 Oct;10(3):501–510. doi: 10.1111/j.1432-1033.1969.tb00717.x. [DOI] [PubMed] [Google Scholar]
- Weckesser J., Drews G., Fromme I. Chemical analysis of and degradation studies on the cell wall lipopolysaccharide of Rhodopseudomonas capsulata. J Bacteriol. 1972 Mar;109(3):1106–1113. doi: 10.1128/jb.109.3.1106-1113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbraith L., Lightfoot G. A. Cell walls, lipids, and lipopolysaccharides of Pseudomonas species. Eur J Biochem. 1973 Feb 15;33(1):158–174. doi: 10.1111/j.1432-1033.1973.tb02666.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Wober W., Alaupović P. Studies on the protein moiety of endotoxin from gram-negative bacteria. Characterization of the protein moiety isolated by acetic acid hydrolysis of endotoxin from Serratia marcescens 08. Eur J Biochem. 1971 Apr;19(3):357–367. doi: 10.1111/j.1432-1033.1971.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Wober W., Alaupović P. Studies on the protein moiety of endotoxin from gram-negative bacteria. Characterization of the protein moiety isolated by phenol treatment of endotoxin from Serratia marcescens 08 and Escherichia coli 0 141:K85(B). Eur J Biochem. 1971 Apr;19(3):340–356. doi: 10.1111/j.1432-1033.1971.tb01323.x. [DOI] [PubMed] [Google Scholar]


